Abstract
Interactions between MEK1/2 inhibitors and the dual Abl/Src kinase inhibitor dasatinib (BMS-354825) were examined in chronic myeloid leukemia (CML) cell lines and primary specimens. Cotreatment of K562 or LAMA cells with subtoxic or marginally toxic concentrations of PD184352 (or U0126) and dasatinib synergistically potentiated mitochondrial damage, caspase activation, and apoptosis. Similar interactions were observed in CD34+ cells from one CML patient–derived but not in a normal human CD34+ bone marrow cell specimen. These interactions were associated with multiple perturbations in survival signaling pathways, including inactivation of Bcr/Abl, STAT5, and ERK1/2; down-regulation of Bcl-xL and Mcl-1; and dephosphorylation/activation of Bim. They were also associated with BAX/BAK conformational change, mitochondrial dysfunction, and caspase activation. Bim knockdown by shRNA suppressed BAX and BAK conformational change and protected cells from dasatinib/PD184352 lethality. Conversely, K562 cells ectopically expressing Mcl-1 or Bcl-xL were significantly less susceptible to dasatinib/PD184352 toxicity. Notably, the dasatinib/PD184352 regimen was active against leukemic cells exhibiting various forms of imatinib mesylate resistance, including Bcr/Abl overexpression, Lyn activation, and several Bcr/Abl kinase domain mutations (eg, E255K, M351T), but not T315I. Together, these findings suggest that strategies combining dasatanib with MEK1/2 inhibitors warrant further investigation in Bcr/Abl+ malignancies, particularly in the setting of imatinib mesylate–resistant disease.
Introduction
Chronic myelogenous leukemia (CML) is a stem-cell disease characterized in 95% of cases by the reciprocal translocation of the long arms of chromosomes 9 and 22, resulting in a chimeric fusion protein with constitutively active tyrosine kinase activity (Bcr/Abl).1,2 Bcr/Abl signals downstream to multiple survival pathways, including STAT5, Bcl-xL, ERK1/2 (extracellular signal regulated kinase 1/2), and NF-κB, among others, which collectively confer a survival advantage on CML cells compared with their normal counterparts.2,3 The therapy of CML has changed dramatically with the introduction of imatinib mesylate (Gleevec), a tyrosine kinase inhibitor that inhibits Bcr/Abl as well as other kinases including c-Kit.4,5 Despite the success of imatinib mesylate in CML patients, it is less effective in patients with more advanced disease (eg, accelerated or blast phase).6–8 In addition, patients who initially respond eventually become refractory to imatinib due to the development of increased expression of Bcr/Abl, or more commonly, the appearance of mutations in the kinase domain that prevent drug binding and inhibitory activity.9–11 For these reasons, attempts to circumvent or overcome imatinib mesylate resistance represent the focus of intense interest.
One approach to this problem involves combining imatinib mesylate with other signaling inhibitors, and combination studies involving agents such as flavopiridol,12 farnesyltransferase inhibitors,13,14 histone deacetylase inhibitors,15,16 and Akt inhibitors17 have been described. Another strategy involves the design of second-generation Bcr/Abl kinase inhibitors that are more active than imatinib mesylate and/or able to kill Bcr/Abl+ cells that have become resistant to imatinib mesylate. An example of such agents is BMS-354825 (dasatinib), a dual Bcr/Abl and Src kinase inhibitor that is active against Bcr/Abl+ cells when administered at nanomolar concentrations.18,19 Notably, dasatinib is active against cells exhibiting certain Bcr/Abl mutations (eg, E255K, M351T), but is relatively ineffective against cells with T315I mutation, which occupies a “gatekeeper” position in the Bcr/Abl kinase region.18,20 The relative contribution of Bcr/Abl and Src kinase inhibition in the lethality of dasatinib remains to be fully elucidated. Recent preclinical studies suggest potential benefit for combining imatinib mesylate with Bcr/Abl kinase inhibitors such as dasatinib.21
The Raf1/MEK1/2/ERK1/2 pathway is an important survival signaling cascade involved in cell proliferation, differentiation, and transformation.22–24 It has also been implicated in the antiapoptotic actions of Bcr/Abl.2 While MEK activity appears restricted to only one class of substrates, ERK activates more than 70 substrates including nuclear transcription factors.22–25 For this reason, several pharmacologic MEK1/2 inhibitors have recently entered the clinic, and have been shown to inhibit their targets (ie, ERK1/2 phosphorylation) when administered at well-tolerated doses.26,27
Previously, we reported that MEK1/2 inhibitors markedly enhanced the lethality of imatinib mesylate in Bcr/Abl+ leukemia cells, including some that were resistant to imatinib due to increased Bcr/Abl expression.28 In view of such findings, it would be clearly of interest to determine whether MEK1/2 inhibitors might similarly enhance the activity of dasatinib. To address this issue, the effects of combined exposure of Bcr/Abl+ leukemia cells to dasatinib and a clinically relevant MEK1/2 inhibitor have been examined in CML cells sensitive and resistant to imatinib. Our results indicate that these agents interact in a highly synergistic manner to induce mitochondrial injury and apoptosis in such cells in association with multiple perturbations in survival signaling pathways, including inactivation of Bcr/Abl, EKR1/2, and Stat5; down-regulation of Bcl-xL; and dephosphorylation of Bim. Significantly, this regimen is very effective in triggering apoptosis in imatinib (IM)–resistant cells, including those overexpressing Lyn or Bcr/Abl as well as expressing certain mutant forms of Bcr/Abl (eg, E255K, M351T), but not the T315I mutation. Together, these findings suggest that strategies combining dasatanib with MEK1/2 inhibitors warrant further investigation in Bcr/Abl+ malignancies, particularly in the setting of imatinib mesylate–resistant disease.
Material and methods
All studies have been sanctioned by the institutional review board of Virginia Commonwealth University (IRB approval no. 3321 and no. 3340).
Cells
LAMA-84 cells were purchased from the German Collection of Microorganisms and Cell Cultures (Braunschweig, Germany). K562 cells (originally obtained from ATCC, Rockville, MD) exhibiting an increase in Bcr/Abl protein expression were obtained by in vitro passaging of K562 in progressively increasing doses of imatinib. K562/Lyn cells that lost Bcr/Abl and overexpressed Lyn kinase were described in our previous study.29 Cells were cultured in RPMI supplemented with sodium pyruvate, nonessential amino acid, l-glutamate, penicillin, streptomycin, and 10% heat-inactivated FCS (Hyclone, Logan, UT). BaF/3 cells expressing wild-type and mutant forms of Bcr/Abl (eg, E255K, M351T, and T315I) were obtained as previously described.30
Bone marrow was obtained with informed consent, in accordance with the Declaration of Helsinki, from a patient with chronic-phase CML undergoing a routine diagnostic bone marrow aspiration and from a patient with a nonmyeloid hematologic disorder (iron deficiency). Mononuclear cells were isolated by Ficoll-Hypaque (Sigma-Aldrich, St Louis, MO) density gradient separation and enriched for CD34+ cells using a Miltenyi microbead separation system (Miltenyi BioTech, Auburn, CA) according to the manufacturer's protocol. The CD34+ cells were diluted into RPMI medium containing 10% fetal calf serum at a concentration of 6 × 105 cells/mL and exposed to drugs.
Reagents
Dasatinib (BMS-354825) was provided by the Cancer Treatment and Evaluation Program, National Cancer Institute (Bethesda, MD) and was dissolved in 10 mM in DMSO. U0126 was purchased from Alexis Biochemicals (San Diego, CA) and dissolved in 50 mM in DMSO. PD184352 was chemically synthesized in-house based on the published structure of the drug, and stored as a powder in a dry atmosphere under light-protected condition at −80°C and dissolved in 100 mM in DMSO. All materials were stored frozen under light-protected conditions at −80°C. Z-VAD-FMK was purchased from MP Biomedicals (Irvine, CA).
Assessment of apoptosis
Briefly, cells were washed once with phosphate-buffered saline (PBS), and were stained with annexin-V–FITC (BD Pharmingen, San Diego, CA) and 5 μg/mL propidium iodide in 1 × binding buffer (10 mM HEPES/NaOH [pH 7.4], 140 mM NaOH, and 2.5 mM CaCl2) for 25 minutes at room temperature in the dark. The apoptotic cells were determined using a Becton Dickinson FACScan cytofluorometer (Mansfield, MA) with Cell Quest software (also from Becton Dickinson). Both early apoptotic (annexin-V positive, PI negative) and late apoptotic (annexin-V positive, PI positive) cells were included in cell death determinations.
Western blot analysis
Western blot analysis was performed as previously described.31 The following were used as primary antibodies: cleaved caspase-3, phospho-STAT5 (Tyr694), Lyn, phospho-Lyn (Tyr507), phospho-Mek1/2 (Ser217/221), phospho-p44/42 MAP kinase (Thr202/Tyr204), phosphor-Src (Tyr527), p-Bad (Ser112), p-Bad (Ser136), and phospho-Akt (Ser473) (all from Cell Signaling Technology, Beverly, MA); antiphosphotyrosine (4G10; Upstate, Lake Placid, NY); Bim (Calbiochem, San Diego, CA); total ERK1/2, Raf-1, c-Src, Bid, STAT5, Abl, phosphor-JNK, and Bcl-xL (all from Santa Cruz Biotechnology, Santa Cruz, CA); Mcl-1 (BD Pharmingen); caspase-8 (Alexis, San Diego, CA); poly(ADP-ribose) polymerase (Biomol Research Laboratories, Plymouth Meeting, PA); and actin (Sigma-Aldrich). The blots were stripped and reprobed with actin antibodies to ensure equal loading and transfer of proteins.
Bax and Bak conformational change
Cells were lysed in 1% CHAPS buffer as described previously.31 Subsequently, 300 μg protein lysates was subjected to immunoprecipitation using anti-Bax (6A7; Sigma-Aldrich) or anti-Bak (Ab-1; Calbiochem), which recognize only Bax and Bak protein, respectively, that have undergone conformational changes.32 Immunoprecipitates were then subjected to Western blot analysis with anti-Bax (BD Pharmingen) and anti-Bak (Upstate).
Analysis of cytosolic cytochrome c
Cells were lysed by incubating in digitonin lysis buffer (75 mM NaCl, 8 mM Na2HPO4, 1 mM NaH2PO4, 1 mM EDTA, and 350 μg/mL digitonin) for 10 minutes at room temperature. The lysate was centrifuged at 1000g for 10 minutes. The supernatant was collected and further centrifuged at 50 000g for 1 hour at 4°C. The resulting supernatant was considered as cytosolic fraction. The cytosolic fraction was quantified and prepared in a final concentration in 1 × NuPAGE LDS sample buffer (Invitrogen, Frederick, MD) and subjected to Western blot. Cytochrome c and AIF antibodies (Santa Cruz Biotechnology) were used as primary antibodies.
Generation of stably transfected cell lines
pSR-BIM and pSR-control constructs were described previously.33,34 Transfection of K562 cells was carried out using an Amaxa Nucleofector apparatus (Cologne, Germany) according to the manufacturer's protocol. Briefly, 2 million cells were suspended in 100 μL transfection reagent of kit V with 1 μg DNA and electroporated protocol (T-16). Transfected cells were immediately transferred to regular medium. Cells were selected in regular medium in the presence of puromycine (2 μg/mL). K562 cells ectopically overexpressing Mcl-1 and Bcl-xL were obtained by stable transfection as described in our previous report.31
Statistical analysis
The significance of differences between experimental conditions was determined using the 2-tailed Student t test. Characterization of synergistic and antagonistic interactions in cells exposed to a range of dasatinib and PD184352 concentrations administered at a fixed ratio was performed using median dose effect analysis in conjunction with a commercially available software program (CalcuSyn; Biosoft, Ferguson, MO).
Results
Dasatinib interacts synergistically in a concentration- and time-dependent manner with MEK1/2 inhibitors in Bcr/Abl+ human leukemia cells
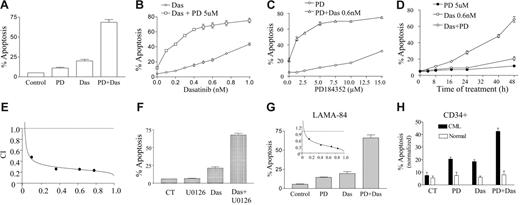
Interactions between MEK1/2 inhibitors and dasatinib were first examined in Bcr/Abl+ human leukemia cells. Individual exposure (48 hours) to 5 μM PD184352 or 0.6 nM dasatinib minimally or only modestly induced apoptosis in these cells (Figure 1A). However, combined treatment resulted in a pronounced increase in apoptosis (ie, ∼ 65% of cells). In addition, coexposure to an essentially nontoxic concentration of PD184352 (eg, 5 μM) significantly increased the lethality of dasatinib concentrations as low as 0.1 nM, although the extent of apoptosis increased as the concentration of dasatinib increased, approaching plateau levels at dasatinib concentrations of 0.5 to 0.6 nM (Figure 1B). The PD184352 dose-response curve shown in Figure 1C revealed that PD184352 concentrations as low as 1 μM significantly increased the toxicity of 0.6 nM dasatinib, whereas near-maximal potentiation was observed at a concentration of 5 μM. Time-course analysis indicated that simultaneous exposure to 0.6 nM dasatinib and 5 μM PD184352 resulted in only a small increase in apoptosis after 16 hours, which increased by 24 hours and was very extensive at later intervals (Figure 1D). Median dose effect analysis of apoptosis induction, in which K562 cells were exposed to a range of dasatinib and PD184352 concentrations, alone and in combination, at a fixed concentration ratio, yielded combination index (CI) values less than 1.0, consistent with a synergistic interaction (Figure 1E). Similar interactions were observed when dasatinib was combined with a nontoxic concentration of another MEK1/2 inhibitor, U0126 (Figure 1F). Synergistic interactions between dasatinib and PD184352 were observed in another Bcr/Abl+ human leukemia cell type, LAMA-84 (Figure 1G). Lastly, PD184352 (2.5 μM) or dasatinib (1 nM) administered individually (48 hours) was modestly toxic to CD34+ mononuclear cells isolated from the bone marrow of an imatinib mesylate–refractory CML patient, whereas combined treatment resulted in a further increase in lethality. In contrast, the same treatment exerted little toxicity toward normal CD34+ bone marrow mononuclear cells from a patient with a nonmyeloid hematologic disorder (iron deficiency) (Figure 1H). Collectively, these findings indicate that nontoxic or minimally toxic concentrations of MEK1/2 inhibitors markedly potentiate the lethality of extremely low concentrations of dasatinib in Bcr/Abl+ leukemia cells.
Coadministration of dasatinib and MEK1/2 inhibitors markedly increases apoptosis in Bcr/Abl+ human leukemia cells and CML CD34+ cells. (A) K562 cells were exposed to 5 μM PD184352 (PD) and 0.6 nM dasatinib (Das) alone or in combination for 48 hours, after which the percentage of apoptotic cells was determined by annexin-V/PI analysis as described in “Materials and methods.” (B) K562 cells were exposed for 48 hours to the designated concentration of dasatinib alone or in conjunction with 5 μM PD, after which the percentage of apoptotic cells was determined. (C) K562 cells were exposed to the designated concentration of PD alone or in combination with 0.6 nM dasatinib for 48 hours, after which apoptosis was determined. (D) K562 cells were treated with PD184352 (5 μM) or dasatinib (0.6 nM) individually or in combination for the indicated intervals, after which the extent of cell death was determined. (E) K562 cells were exposed to varying concentrations of PD184352 and dasatinib at a fixed ratio; then combination index (CI) values were determined in relation to the fractional effect using a commercially available software program as described in “Materials and methods.” Combination index values less than 1.0 correspond to a synergistic interaction. (F) K562 cells were exposed to the MEK1/2 inhibitor U0126 (20 μM) and dasatinib (0.6 nM) alone or in combination for 48 hours, after which the percentage of apoptosis was determined. (G) LAMA-84 cells were exposed to 1 μM PD and 0.5 nM dasatinib alone or in combination for 48 hours, after which the percentage of apoptotic cells was determined. CI value was determined in LAMA-84 cells as the cells were exposed to varying concentrations of PD and dasatinib (inset). All values represent the means ± SD for duplicate determinations performed on 3 separate occasions. In all cases, apoptosis was determined by annexin-V/PI staining and flow cytometry. (H) CD34+ cells isolated from the bone marrow of a patient with chronic-phase CML and a patient with a nonmyeloid hematologic disorder (iron deficiency) were exposed to PD184352 (2.5 μM) with or without dasatinib (1 nM) alone and in combination for 48 hours. At the end of this period, the percentage of apoptotic cells was determined by annexin-V/PE.
Coadministration of dasatinib and MEK1/2 inhibitors markedly increases apoptosis in Bcr/Abl+ human leukemia cells and CML CD34+ cells. (A) K562 cells were exposed to 5 μM PD184352 (PD) and 0.6 nM dasatinib (Das) alone or in combination for 48 hours, after which the percentage of apoptotic cells was determined by annexin-V/PI analysis as described in “Materials and methods.” (B) K562 cells were exposed for 48 hours to the designated concentration of dasatinib alone or in conjunction with 5 μM PD, after which the percentage of apoptotic cells was determined. (C) K562 cells were exposed to the designated concentration of PD alone or in combination with 0.6 nM dasatinib for 48 hours, after which apoptosis was determined. (D) K562 cells were treated with PD184352 (5 μM) or dasatinib (0.6 nM) individually or in combination for the indicated intervals, after which the extent of cell death was determined. (E) K562 cells were exposed to varying concentrations of PD184352 and dasatinib at a fixed ratio; then combination index (CI) values were determined in relation to the fractional effect using a commercially available software program as described in “Materials and methods.” Combination index values less than 1.0 correspond to a synergistic interaction. (F) K562 cells were exposed to the MEK1/2 inhibitor U0126 (20 μM) and dasatinib (0.6 nM) alone or in combination for 48 hours, after which the percentage of apoptosis was determined. (G) LAMA-84 cells were exposed to 1 μM PD and 0.5 nM dasatinib alone or in combination for 48 hours, after which the percentage of apoptotic cells was determined. CI value was determined in LAMA-84 cells as the cells were exposed to varying concentrations of PD and dasatinib (inset). All values represent the means ± SD for duplicate determinations performed on 3 separate occasions. In all cases, apoptosis was determined by annexin-V/PI staining and flow cytometry. (H) CD34+ cells isolated from the bone marrow of a patient with chronic-phase CML and a patient with a nonmyeloid hematologic disorder (iron deficiency) were exposed to PD184352 (2.5 μM) with or without dasatinib (1 nM) alone and in combination for 48 hours. At the end of this period, the percentage of apoptotic cells was determined by annexin-V/PE.
PD184352 enhances dasatinib-mediated mitochondrial damage and caspase activation in Bcr/Abl+ leukemia cells
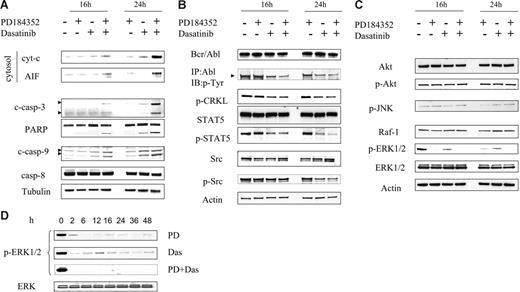
Effects of these agents, administered alone and in combination, were then examined with respect to mitochondrial integrity and activation of the caspase cascade. Studies were performed at 16 hours, when apoptosis was minimal, and at 24 hours, when the extent of cell death was only modest (∼ 25%) (Figure 1D) to reduce the possibility that changes represented a consequence of the cell death process itself. As shown in Figure 2A, dasatinib and PD184352 individually had little effect on release of the proapoptotic mitochondrial proteins cytochrome c and AIF (apoptosis-inducing factor) in K562 cells, whereas the effects of combined exposure were pronounced. Consistent with these findings, cotreatment with both agents markedly induced procaspase-3 and -9 activation and PARP cleavage, whereas the effects of individual treatment were negligible or modest. Little change in caspase-8 activation was noted with any treatment. Together, these findings indicate that combined exposure of Bcr/Abl+ leukemia cells results in a marked increase in activation of the mitochondrial pathway.
Combined treatment with PD184352 and dasatinib results in a marked increase in mitochondrial injury and caspase activation, and diminishes expression/activation of Bcr/Abl, STAT5, and ERK1/2. (A) K562 cells were treated with PD (5 μM) and dasatinib (0.6 nM) alone or in combination for 16 hours and 24 hours, after which mitochondria-free cytosolic fractions were obtained as described in “Materials and methods” and subjected to Western blot to monitor release of cyt-c and AIF. Alternatively, whole-cell lysates were obtained and subjected to Western blot analysis to monitor activation of caspases and PARP. (B-C) K562 cells were exposed to PD and dasatinib alone or in combination for 16 hours and 24 hours, after which protein lysates were prepared and subjected to Western blot (WB) using the indicated primary antibodies. (D) p-ERK1/2 expression was monitored in cells exposed to either dasatinib or PD184352 alone or in combination. Each lane was loaded with 25 μg protein. Blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer of protein. Two additional experiments yielded equivalent results.
Combined treatment with PD184352 and dasatinib results in a marked increase in mitochondrial injury and caspase activation, and diminishes expression/activation of Bcr/Abl, STAT5, and ERK1/2. (A) K562 cells were treated with PD (5 μM) and dasatinib (0.6 nM) alone or in combination for 16 hours and 24 hours, after which mitochondria-free cytosolic fractions were obtained as described in “Materials and methods” and subjected to Western blot to monitor release of cyt-c and AIF. Alternatively, whole-cell lysates were obtained and subjected to Western blot analysis to monitor activation of caspases and PARP. (B-C) K562 cells were exposed to PD and dasatinib alone or in combination for 16 hours and 24 hours, after which protein lysates were prepared and subjected to Western blot (WB) using the indicated primary antibodies. (D) p-ERK1/2 expression was monitored in cells exposed to either dasatinib or PD184352 alone or in combination. Each lane was loaded with 25 μg protein. Blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer of protein. Two additional experiments yielded equivalent results.
Combined treatment with PD184352/dasatinib results in a modest reduction in p-Bcr/Abl and -Stat5 expression, and a marked inactivation of ERK1/2
Effects of combined treatment on the status of various signaling pathways in Bcr/Abl+ leukemia cells were then investigated. Treatment of K562 cells with dasatinib alone for 16 hours or 24 hours displayed a reduction in expression of p-Bcr/Abl, while coadministration of PD184352 resulted in a slight further decline (Figure 2B). Consistent with this finding, expression of p-Crkl and p-Stat5, downstream targets of Bcr/Abl,35,36 was reduced by dasatinib alone and to a slightly greater extent when dasatinib was combined with PD184352. In addition, dasatinib administered alone or in combination with PD184352 modestly reduced the expression of p-Src (Figure 2B).
Parallel studies were performed to investigate the effects of these agents on MAP kinase and related pathways. Dasatinib and PD184352, alone or in combination, had almost no effect on expression of phospho-Akt, -p38 MAPK, or -JNK (Figure 2C). However, results were quite different when the ERK1/2 pathway was examined. Dasatinib alone reduced the expression of p-ERK1/2 at early intervals, although reactivation occurred at 16 hours, followed by a decline. PD184352 administered alone substantially reduced ERK1/2 activation at intervals of 6 or more hours. However, combined treatment with dasatinib and PD184352 essentially abrogated ERK1/2 activation as early as 2 hours after drug exposure (Figure 2C-D). No changes in levels of total ERK1/2 were noted with any treatment. Collectively, these findings indicate that combined exposure of Bcr/Abl+ leukemia cells to dasatinib and PD184352 caused a modest further inactivation of Bcr/Abl and STAT5 and the complete abrogation of ERK1/2 activation.
Combined treatment of PD184352 and dasatinib reduces expression of Bcl-xL, Mcl-1, and p-BadSer112 and induces dephosphorylation of Bim
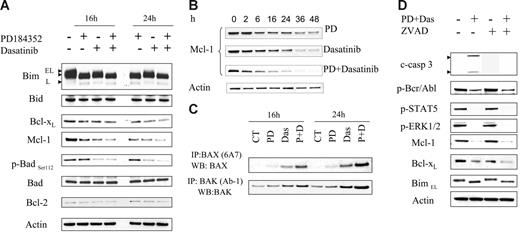
The effects of exposure of cells to PD184352 with or without dasatinib were examined in relation to expression of various Bcl-2 family members. K562 cells predominantly expressed the BimEL form of Bim, and to a much lesser extent, BimL (Figure 3A). Exposure of cells to PD184352 (with or without dasatinib) for 16 or 24 hours resulted in enhanced migration of bands on sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels, corresponding to dephosphorylation of both BimEL and BimL.34 In addition, expression of Bcl-xL declined in cells exposed to either PD184352 or dasatinib alone, but expression was essentially abrogated following exposure to PD184352 plus dasatinib, particularly at the 24-hour interval (Figure 3A). A very similar response pattern was noted in the case of the antiapoptotic protein Mcl-1 (Figure 3A). A more detailed time-course study was then carried out that revealed that combined treatment resulted in a more rapid and complete down-regulation of Mcl-1 with combined drug treatment (Figure 3B). Dasatinib alone or in combination with PD184352 decreased levels of p-BadSer112, the ERK1/2 regulatory site (Figure 3A), but not p-BadSer136, the Akt phosphorylation site (data not shown). Levels of total Bad did not change noticeably with any treatment. Finally, levels of other Bcl-2 family proteins such as Bid and Bcl-2 did not change appreciably with all treatments.
Effects of PD184352/dasatinib on expression of Bcl-2 family members. (A) K562 cells were treated with 5 μM PD with or without 0.6 nM dasatinib for 16 hours and 24 hours, after which cells were lysed and subjected to WB to monitor the expression of Bcl-2 family members. (B) Mcl-1 expression was monitored in cells exposed to either dasatinib or PD184352 alone or in combination. (C) K562 cells were exposed to PD or dasatinib individually or in combination for 16 hours and 24 hours, after which cells were lysed and subjected to immunoprecipitation to monitor Bax and Bak conformational change as described in “Materials and methods.” (D) K562 cells were pretreated with 20 μM ZVAD-fmk for 2 hours followed by PD/dasatinib as described above for 24 hours. At the end of this period, the cells were lysed and subjected to WB to monitor p-Bcr/Abl, p-STAT5, Bcl-xL, Mcl-1, and p-ERK1/2. Each lane was loaded with 25 μg protein. Blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer of protein. Two additional experiments yielded equivalent results.
Effects of PD184352/dasatinib on expression of Bcl-2 family members. (A) K562 cells were treated with 5 μM PD with or without 0.6 nM dasatinib for 16 hours and 24 hours, after which cells were lysed and subjected to WB to monitor the expression of Bcl-2 family members. (B) Mcl-1 expression was monitored in cells exposed to either dasatinib or PD184352 alone or in combination. (C) K562 cells were exposed to PD or dasatinib individually or in combination for 16 hours and 24 hours, after which cells were lysed and subjected to immunoprecipitation to monitor Bax and Bak conformational change as described in “Materials and methods.” (D) K562 cells were pretreated with 20 μM ZVAD-fmk for 2 hours followed by PD/dasatinib as described above for 24 hours. At the end of this period, the cells were lysed and subjected to WB to monitor p-Bcr/Abl, p-STAT5, Bcl-xL, Mcl-1, and p-ERK1/2. Each lane was loaded with 25 μg protein. Blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer of protein. Two additional experiments yielded equivalent results.
Combined treatment of Bcr/Abl+ cells with dasatinib and PD184352 results in a marked increase in conformationally changed Bax and Bak
Following the appropriate noxious stimuli, the multidomain proapoptotic Bcl-2 family members BAK and BAX undergo conformational changes resulting in the mitochondrial outer membrane permeability (MOMP) transition and release of cytochrome c, AIF, and Smac/DIABLO.37–39 Consequently, the effects of PD184352 and dasatinib were examined in relation to effects on Bax and Bak conformation. Treatment of K562 cells for 16 or 24 hours with dasatinib induced a very modest conformational change in Bax and Bak, whereas PD184352 by itself was ineffective (Figure 3C). However, combined treatment with both agents resulted in a major conformational change in both Bax and Bak at both intervals. These findings suggest that conformational changes in Bax and Bak induced by dasatinib/PD184352 may underlie the dramatic increase in apoptosis induced by this regimen.
Inactivation of Bcr/Abl, Stat5, and ERK1/2 and down-regulation of Mcl-1 and Bcl-xL represent caspase-independent events
To determine whether the signaling perturbations described above might be secondary to caspase activation, K562 cells were exposed to the pan-caspase inhibitor fmk-ZVAD (20 μM) 2 hours before administered dasatinib/PD184352. As anticipated, fmk-ZVAD blocked caspase-3 activation in this regimen (Figure 3D). In contrast, ZVAD had little or no effect on dasatinib/PD184352-mediated inactivation of Bcr/Abl or Stat5; down-regulation of Mcl-1 and Bcl-xL; or dephosphorylation of Bim (Figure 3D), indicating that these events are more likely to represent causes than consequences of activation of the caspase cascade.
Inactivation of Bim plays a functional role in the lethality of the dasatinib/PD184352 regimen
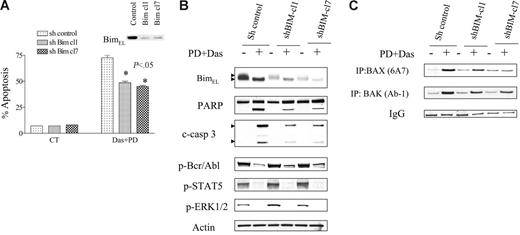
To assess the functional significance of dasatinib/PD184352-mediated Bim dephosphorylation/activation in the lethality of this regimen, we transfected a shBim construct into K562 cells to diminish endogenous expression of Bim. Cells were also transfected with a scrambled, nonspecific shRNA construct to serve as a control. Two clones (shBim-cl1 and shBim-cl7), both of which displayed a marked decrease in the endogenous expression of Bim (including the phosphorylated and dephosphorylated forms of BimEL), were used (Figure 4B). As shown in Figure 4A, both clones exhibited partial but significant protection from dasatinib/PD184352-mediated apoptosis compared with controls (P < .05 in each case). Consistent with this finding, a clear decrease in caspase-3 cleavage and PARP degradation was also observed in cells in which Bim was knocked down (Figure 4B). In contrast, there was no diminution in Bcr/Abl, Stat5, or ERK1/2 inactivation, or Bcl-xL down-regulation, suggesting that these events do not stem from Bim activation. Significantly, shBim-cl1 and shBim-cl7 cells displayed a clear reduction in dasatinib/PD184352-mediated Bax and Bak conformational change compared with controls (Figure 4C). These findings raise the possibility that Bim activation contributes to the lethality of the dasatinib/PD184352 regimen, possibly by interacting with the proapoptotic multidomain proteins Bax and Bak to promote mitochondrial injury.
Knockdown of BIM by shRNA protects cells from PD/dasatinib-mediated lethality. K562 cells were stably transfected with shRNA-Bim or shRNA-control constructs in a pSUPER.retro vector as described in “Materials and methods.” Two clones displaying reduced endogenous expression of Bim were isolated and used in subsequent experiments. (A) K562 cells with sh-control, sh-Bim-cl1, or sh-Bim-cl7 were exposed to PD/Das for 48 hours, after which the percentage of apoptosis was determined by annexin-V/PI. * denotes significantly less than values obtained for empty vector cells (P < .05). All values represent the means ± SD for duplicate determinations performed on 3 separate occasions. (B) Cells were exposed to PD/Das for 24 hours, after which the cells were subjected to Western blot analysis using the indicated antibodies. Each lane was loaded with 25 μg protein. Blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer of protein. Two additional experiments yielded equivalent results. (C) Cells were lysed, Bax and Bak were immunoprecipitated, and Western blot analysis was used to detect Bax and Bak conformational changes using 6A7 and Ab-1 detection antibodies as described in “Materials and methods.” Values represent 3 separate experiments.
Knockdown of BIM by shRNA protects cells from PD/dasatinib-mediated lethality. K562 cells were stably transfected with shRNA-Bim or shRNA-control constructs in a pSUPER.retro vector as described in “Materials and methods.” Two clones displaying reduced endogenous expression of Bim were isolated and used in subsequent experiments. (A) K562 cells with sh-control, sh-Bim-cl1, or sh-Bim-cl7 were exposed to PD/Das for 48 hours, after which the percentage of apoptosis was determined by annexin-V/PI. * denotes significantly less than values obtained for empty vector cells (P < .05). All values represent the means ± SD for duplicate determinations performed on 3 separate occasions. (B) Cells were exposed to PD/Das for 24 hours, after which the cells were subjected to Western blot analysis using the indicated antibodies. Each lane was loaded with 25 μg protein. Blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer of protein. Two additional experiments yielded equivalent results. (C) Cells were lysed, Bax and Bak were immunoprecipitated, and Western blot analysis was used to detect Bax and Bak conformational changes using 6A7 and Ab-1 detection antibodies as described in “Materials and methods.” Values represent 3 separate experiments.
Down-regulation of Mcl-1 and Bcl-xL plays a functional role in dasatinib/PD184352-mediated lethality
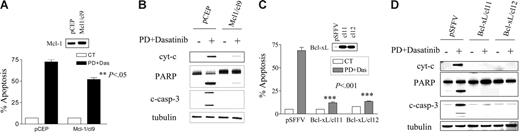
As noted previously, the decline in expression of Mcl-1 and Bcl-xL was more rapid and extensive in cells exposed to dasatinib/PD184352 compared with cells exposed to the agents individually (Figure 3C-D). To determine the functional significance of Mcl-1 and Bcl-xL down-regulation in dasatinib/PD184352-mediated lethality, K562 cells ectopically expressing Mcl-1 or Bcl-xL were used. As shown in Figure 5A, K562/Mcl-1-cl9 cells were significantly more resistant to dasatinib/PD184352-mediated apoptosis than their empty vector counterparts (P < .05). Cells ectopically expressing Mcl-1 were also less susceptible than controls to cytochrome c release, caspase-3 cleavage, and PARP degradation (Figure 5B). Similarly, K562/Bcl-xL-cl11 and -cl12 cells were very significantly more resistant to dasatinib/PD184352-mediated apoptosis than their empty vector counterparts (Figure 5C; P < .001 in each case). Consistent with these findings, cells ectopically expressing Bcl-xL were markedly less sensitive to dasatinib/PD184352-mediated cytochrome c release, caspase-3 cleavage, and PARP degradation compared with controls (Figure 5D). However, ectopic expression of both Mcl-1 and Bcl-xL failed to prevent inactivation of Bcr/Abl, Stat5, and ERK1/2, or dephosphorylation of Bim (data not shown), suggesting the latter events operate upstream of Mcl-1 and Bcl-xL. Collectively, these findings are consistent with the notion that decreased Mcl-1 and Bcl-xL expression contributes functionally, at least in part, to PD184352/dasatinib-mediated lethality.
Enforced expression of Mcl-1 or Bcl-xL protects cells from PD/dasatinib-mediated lethality. (A) K562/pCEP and K562/Mcl-1/cl9 cells were exposed to PD (5 μM) and dasatinib (0.6 nM) for 48 hours, after which the percentage of apoptotic cells was determined by annexin-V/PI. ** denotes significantly less than values obtained for empty vector cells (P < .05). (B) Cells were exposed to PD/dasatinib for 24 hours, and then the whole cells were lysed and subjected to WB analysis using the indicated primary antibodies. (C) Similarly, K562/pSFFV, K562/Bcl-xL/cl-11, and K562/Bcl-xL/cl-12 cells were exposed to PD/dasatinib for 48 hours, after which the percentage of apoptosis was determined. *** denotes significantly less than values obtained for empty vector cells (P < .001). (D) Cells were exposed to PD/dasatinib for 24 hours, after which the cytosolic fraction was extracted and WB used to monitor cyt-c release. Alternatively, whole cells were lysed and subjected to WB analysis using the indicated primary antibodies. For all studies, each lane was loaded with 25 μg protein; blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer of protein. For all studies, 2 additional experiments yielded equivalent results.
Enforced expression of Mcl-1 or Bcl-xL protects cells from PD/dasatinib-mediated lethality. (A) K562/pCEP and K562/Mcl-1/cl9 cells were exposed to PD (5 μM) and dasatinib (0.6 nM) for 48 hours, after which the percentage of apoptotic cells was determined by annexin-V/PI. ** denotes significantly less than values obtained for empty vector cells (P < .05). (B) Cells were exposed to PD/dasatinib for 24 hours, and then the whole cells were lysed and subjected to WB analysis using the indicated primary antibodies. (C) Similarly, K562/pSFFV, K562/Bcl-xL/cl-11, and K562/Bcl-xL/cl-12 cells were exposed to PD/dasatinib for 48 hours, after which the percentage of apoptosis was determined. *** denotes significantly less than values obtained for empty vector cells (P < .001). (D) Cells were exposed to PD/dasatinib for 24 hours, after which the cytosolic fraction was extracted and WB used to monitor cyt-c release. Alternatively, whole cells were lysed and subjected to WB analysis using the indicated primary antibodies. For all studies, each lane was loaded with 25 μg protein; blots were stripped and reprobed with antitubulin antibody to ensure equal loading and transfer of protein. For all studies, 2 additional experiments yielded equivalent results.
The dasatinib/PD184352 regimen potently induces apoptosis in imatinib mesylate–resistant cells overexpressing Lyn or Bcr/Abl and in some, but not all, Bcr/Abl mutants
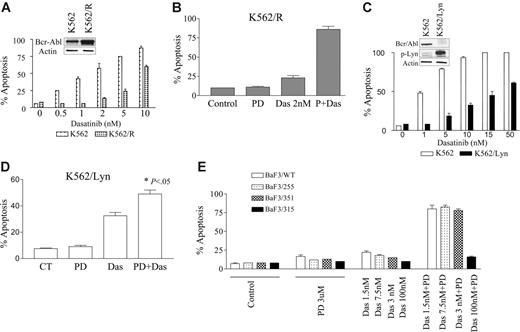
Finally, attempts were made to determine whether this strategy would be effective in Bcr/Abl+ cells resistant to imatinib mesylate through various mechanisms. To this end, K562/R cells, which displayed approximately a 4- to 5-fold increase in Bcr/Abl protein expression, were used (Figure 6A inset). K562/R cells were significantly less sensitive than their wild-type counterparts to dasatinib (Figure 6A). Notably, while exposure to 2 nM dasatinib (48 hours) was only minimally toxic to K562R cells, coadministration of a subtoxic concentration of 5 μM PD184352 dramatically increased apoptosis (eg, to ∼ 75% of cells; Figure 6B). Thus, the interaction was, at least qualitatively, similar to that observed in the case of wild-type imatinib mesylate–sensitive cells. Next, we evaluated the effects of dasatinib/PD184352 regimen in K562/Lyn cells that we and others have shown exhibit a reduction in Bcr/Abl expression and activation of Lyn29 (Figure 6C inset). K562/Lyn cells are highly resistant to imatinib29 but remain sensitive to dasatinib although at much higher concentrations than control cells (Figure 6C). Notably, coadministration of a subtoxic concentration of PD184352 (5 μM) with dasatinib (10 nM) significantly increased apoptosis in K562/Lyn cells (ie, from 36% to 50%; P < .05; Figure 6D).
Effect of PD184352/dasatinib in imatinib-resistant Bcr/abl+ leukemia cells. (A) K562 and K562/R cells, which display increased expression of Bcr/Abl (inset), were cultured in the presence of varying concentrations of imatinib or dasatinib for 48 hours. (B) K562/R cells were treated with 2 nM dasatinib with or without 5 μM PD for 48 hours, after which apoptosis was determined by annexin-V/PI analysis. (C) K562 and K562/Lyn cells, which display increased expression of p-Lyn and diminished expression of Bcr/Abl (inset), were cultured in the presence of the indicated concentrations of dasatinib for 48 hours, after which apoptosis was determined. (D) K562/Lyn cells were exposed to 5 μM PD184352 with or without 10 nM dasatinib for 48 hours, after which apoptosis was monitored by annexin-V/PI analysis (* denotes significant increase compared with drug treated alone; P < .05.) (E) Wild-type and mutant Brc-Abl/BaF3 cells were exposed to 3 μM PD184352 with or without 1.5 nM to 100 nM dasatinib alone or in combination for 24 hours, after which the percentage of apoptotic cells was determined by annexin-V/PI analysis. All values represent the means ± SD for duplicate determinations performed on 3 separate experiments.
Effect of PD184352/dasatinib in imatinib-resistant Bcr/abl+ leukemia cells. (A) K562 and K562/R cells, which display increased expression of Bcr/Abl (inset), were cultured in the presence of varying concentrations of imatinib or dasatinib for 48 hours. (B) K562/R cells were treated with 2 nM dasatinib with or without 5 μM PD for 48 hours, after which apoptosis was determined by annexin-V/PI analysis. (C) K562 and K562/Lyn cells, which display increased expression of p-Lyn and diminished expression of Bcr/Abl (inset), were cultured in the presence of the indicated concentrations of dasatinib for 48 hours, after which apoptosis was determined. (D) K562/Lyn cells were exposed to 5 μM PD184352 with or without 10 nM dasatinib for 48 hours, after which apoptosis was monitored by annexin-V/PI analysis (* denotes significant increase compared with drug treated alone; P < .05.) (E) Wild-type and mutant Brc-Abl/BaF3 cells were exposed to 3 μM PD184352 with or without 1.5 nM to 100 nM dasatinib alone or in combination for 24 hours, after which the percentage of apoptotic cells was determined by annexin-V/PI analysis. All values represent the means ± SD for duplicate determinations performed on 3 separate experiments.
Lastly, the effects of the dasatinib/PD184352 regimen were examined in Ba/F3 cells transfected with wild-type Bcr/Abl or Bcr/Abl expressing either of 3 clinically relevant mutations (eg, E255K, M351T, and T315I). Individual exposure of cells to 3 μM PD184252 or dasatinib concentrations ranging from 1.5 to 7.5 nM exerted little toxicity to BaF3/WT, BaF3/E255K, or BaF3/M351T cells. Combined treatment resulted in a dramatic increase in lethality in wild-type, E255K, and M351T cells (Figure 6E). However, the combination of dasatinib/PD184352 did not result in enhanced toxicity toward BaF3/T315I cells, consistent with previous reports indicating that the T315I mutation confers a high degree of dasatinib resistance.18 Collectively, these studies indicate that the dasatinib/PD184352 regimen effectively induces apoptosis in leukemic cells exhibiting several clinically relevant forms of imatinib mesylate resistance, including Bcr/Abl overexpression as well as certain Bcr/Abl kinase domain mutations. They also suggest, albeit indirectly, that resistance of the T315I Bcr/Abl mutation to dasatinib may be responsible for the relative lack of activity of the dasatinib/PD184352 regimen in this line.
Discussion
The present results demonstrate that the novel Abl/Src kinase inhibitor dasatinib interacts in a highly synergistic manner with clinically relevant MEK1/2 inhibitor to induce apoptosis in Bcr/Abl+ leukemia cells, including those both sensitive and resistant to imatinib mesylate. Furthermore, these events occur in association with multiple perturbations in survival signaling pathways, including inactivation of Bcr/Abl, Crkl, EKR1/2, and Stat5; down-regulation of Bcl-xL and Mcl-1; and dephosphorylation/activation of Bim. The development of imatinib mesylate resistance prompted the development of second-generation kinase inhibitors such as dasatinib, a dual Bcr/Abl and Src kinase inhibitor that is a significantly more potent inhibitor of Bcr/Abl on a molar basis than imatinib mesylate.40 In contrast to the latter agent, which primarily binds and traps Bcr/Abl in an closed, inactive conformation, dasatinib binds to both the inactive and open, active conformation.18,19 Dasatinib, an orally available drug, is currently undergoing clinical trials in patients with CML or Ph-positive acute lymphoblastic leukemia (ALL) who could not tolerate or who are resistant to imatinib. Early data indicate that dasatinib induces hematologic and cytogenetic response in many of these imatinib mesylate–resistant patients.20 However, acquired resistance to dasatinib may eventually occur, as already observed in the case of some patients with blast crisis or Ph-positive ALL,20 as well as in preclinical studies.41 Consequently, new strategies are needed to overcome or circumvent the problem of drug resistance.
Previously, we reported that MEK1/2 inhibitors interacted synergistically with imatinib mesylate to induce apoptosis in Bcr/Abl-expressing cells.28 While there are certain similarities between this and the present study (eg, the observed down-regulation of Stat5, Mcl-1, and Bcl-xL), there are also a number of differences. For example, in the earlier study, cells exposed to imatinib mesylate displayed in a marked compensatory activation of the MEK1/2/ERK1/2 pathway after initial suppression.28 While cells exposed to dasatinib also exhibited early ERK1/2 inactivation followed by a slight rebound at later time points (Figure 2D), the latter phenomenon was substantially less than that observed in cells exposed to imatinib. This differential response may reflect the capacity of dasatinib to inhibit Src kinases, as well as cross-talk that exists between the MEK1/2/ERK1/2 module and Src family kinases.42,43
The present findings suggest that alterations in Bim, a proapoptotic protein that played an important role in apoptosis, are involved in antileukemic synergism between dasatinib and MEK1/2 inhibitors.44,45 The Bcl-2 family consists of (a) BH3 multidomain members that either suppress apoptosis (eg, Bcl-xL, Bcl-2, and Mcl-1) or trigger it (eg, Bax and Bak), and (b) BH3-only domain members that promote apoptosis (eg, Bim, Bad, Bid, Noxa, and Puma).37,46 Recently, the function of Bim has been examined in relation to leukemia cell survival, particularly in the case of CML.44,45 The results of recent reports suggest that the ERK1/2-dependent phosphorylation of Bim (eg, on residues S55, S65, and S100) in response to survival factors inhibits interactions between Bim and Bax, thereby promoting survival.34 In support of this concept, cells expressing a nonphosphorylatable form of Bim demonstrated enhanced interactions with Bax and a lower threshold for apoptosis.34 It is noteworthy that in Bcr/Abl+ leukemia cells, dephosphorylation of Bim (eg, by PD184352) was associated with enhanced conformational change in Bax following dasatinib exposure (Figure 3B). Such findings are consistent with previous reports suggesting that Bim acts, at least in part, by triggering a conformational change in Bax.47 Significantly, knockdown of Bim by shRNA suppressed both BAX conformational change as well as sensitivity of cells to the combination treatment, arguing that Bim modifications play a functional role in lethality. It is also important to note that Bim knockdown also suppressed BAK conformational change in dasatinib/PD184352-treated cells (Figure 4). It has been suggested that the activation of BAK may occur indirectly (ie, through the actions of BAX or other mitochondrial protein32,48 ), and it is therefore possible that the observed consequences of Bim dephosphorylation may also involve this proapoptotic protein.
Treatment of cells with dasatinib with or without PD184352 resulted in pertubations in the expression/activation of multiple survival signaling proteins, and it is likely that the combined effects of these events contributed to lethality. For example, the dasatinib/PD184352 regimen diminished expression of the antiapoptotic protein Bcl-xL, which is known to be regulated by both STAT549,50 and ERK1/2.51,52 Consequently, the combination of dasatinib and PD184352 may have reduced Bcl-xL expression by disrupting each of these pathways. In addition, expression of Mcl-1 is known to be regulated by MEK1/2/ERK1/252,53 and Stat5,36 which may account for the observed down-regulation of Mcl-1 in dasatinib/PD184352-treated cells. Furthermore, recent studies suggest that Mcl-1 cooperates with Bcl-xL to inactivate Bak.54 Thus, the limited disruption of both Mcl-1 and Bcl-xL expression in cells exposed to this regimen could release BAK, thereby triggering mitochondrial dysfunction culminating in cell death (Figure 3). Expression of p-BadSer112, which is primarily regulated by protein kinase A (PKA) and ERK1/2/RSK,55,56 was also reduced in cells exposed to dasatinib with or without PD184352. Of interest, p-BadSer112 was down-regulated by dasatinib but not PD184352 (MEK1/2 inhibitor), raising the possibility that dasatinib may inactivate BadSer112 via PKA. In this context, the nonphosphorylated (active) form of BAD heterodimerizes with antiapoptotic Bcl-2 family protein such as Bcl-xL or Bcl-2 and promotes cell death.57 Collectively, the present findings raise the possibility that a broad constellation of perturbations in survival and signaling pathways may contribute to the pronounced lethality of this combination regimen.
Coadministration of PD184352 increased dasatinib lethality in imatinib mesylate–resistant Bcr/Abl+ leukemia cells, including those displaying increased expression of Bcr/Abl or loss of Bcr/Abl accompanied by activation of the Src kinase Lyn.29,58,59 It is also noteworthy that this regimen was very effective in triggering apoptosis in cells expressing certain clinically relevant mutant forms of Bcr/Abl (eg, E255K and M351T).60,61 Of interest, Ba/F3-T315I cells, which contain a mutation in the “gatekeeper” region and are largely resistant to dasatinib and AMN107,20,40,62 were also resistant to the combination of PD184352 and dasatinib. This finding suggests that inhibition of Bcr/Abl activation by dasatinib, although modest, is critical for synergistic interactions with PD184352. The possible selection of cells bearing the T315I or related Bcr/abl mutations receiving dasatinib or AMN10720,40,62 has prompted the search for agents or regimens active in this situation. Recently, it has been reported that the aurora kinase inhibitor VX-680 effectively inhibits Bcr/abl displaying the T315I mutation.63 In view of the present findings, it would be interesting to determine what effect, if any, MEK1/2 inhibition might have on the response of such cells to this agent.
It is commonly believed that the RAS/RAF-1/MEK1/2/ERK1/2 signaling pathway mediates survival signaling in diverse transformed cell types, including Bcr/Abl+ leukemia cells.2 Such findings have prompted the clinical development of several pharmacologic MEK1/2 inhibitors including PD184352 (or CI-1040), PD032591, and AZD6244 (ARRY142886).26,27,64,65 Results of early clinical trials indicate that it is feasible to achieve the desired pharmacodynamic effect (eg, ERK1/2 inactivation) at well-tolerated MEK inhibitors doses.26 These considerations, in conjunction with evidence that interrupting the MEK1/2/ERK1/2 pathway dramatically sensitizes Bcr/Abl+ leukemia cells, including those resistant to imatinib mesylate, to extremely low concentrations of dasatinib through multiple mechanisms make this strategy an attractive one to pursue. In this regard, additional studies will be required to determine whether such a strategy selectively targets leukemic cells. Finally, it has been proposed that the failure to eradicate leukemic stem cells may be responsible for disease relapse.66,67 In this regard, both imatinib mesylate and dasatinib have been shown to be relatively ineffective against Bcr/abl+ leukemic stem cells (LSCs).66,67 Consequently, the utility of combining dasatinib with MEK1/2 inhibitors may ultimately depend upon whether improved LSC killing occurs. Accordingly, studies designed to address this question are currently under way.
Authorship
Contribution: T.K.N. designed experiments, performed the research, managed the database, and helped to write the paper; M.R. contributed vital tools and helped in the research design; H.H. contributed vital tools; P.D. helped in the research design and preparation of the paper; S.G. designed and supervised the research, managed the database, contributed vital reagents, and helped to write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Steven Grant, Division of Hematology/Oncology, Virginia Commonwealth University/Massey Cancer Center, Goodwin Research, 401 College St, VA, 23298; e-mail: stgrant@hsc.vcu.edu.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by awards CA63753, CA93738, CA100866, CA88906, and CA72955 from the NIH; a Translational Research Award from the Leukemia and Lymphoma Society of America (6045-03); an award from the Department of Defense (DAMD-17-03-1-0209); an award from the V Foundation; and the Universal Professorship (P.D.).

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal