Abstract
The recruitment and activation of neutrophils at infected tissues is essential for host defense against invading microorganisms. However, excessive neutrophil recruitment or activation can also damage the surrounding tissues and cause unwanted inflammation. Hence, the responsiveness of neutrophils needs to be tightly regulated. In this study, we have investigated the functional role of tumor suppressor PTEN in neutrophils by using a mouse line in which PTEN is disrupted only in myeloid-derived cells. Chemoattractant-stimulated PTEN−/− neutrophils displayed significantly higher Akt phosphorylation and actin polymerization. A larger fraction of these neutrophils displayed membrane ruffles in response to chemoattractant stimulation. In addition, chemoattractant-induced transwell migration and superoxide production were also augmented. Single-cell chemotaxis assays showed that PTEN−/− neutrophils have a small (yet statistically significant) defect in directionality. However, these neutrophils also showed an increase in cell speed. As a result, overall chemotaxis, which depends on speed and directionality, was not affected. Consistent with the increased responsivenessof PTEN−/− neutrophils, the in vivo recruitment of these cells to the inflamed peritoneal cavity was significantly enhanced. Thus, as a physiologic-negative regulator, PTEN should be a promising therapeutic target for modulating neutrophil functions in various infectious and inflammatory diseases.
Introduction
Neutrophils are the most abundant cell type among circulating white blood cells. The recruitment and activation of neutrophils are important components of the innate immune system. In response to inflammatory stimuli, neutrophils migrate from the blood to infected tissues, where they protect their host by engulfing, killing, and digesting invading bacterial and fungal pathogens. Conversely, excessive neutrophil accumulation or hyperresponsiveness of neutrophils can also be detrimental to the system. The toxic reactive oxygen species and granule enzymes (eg, proteases) released by neutrophils can damage surrounding tissues and cause unwanted and exaggerated tissue inflammation. Hence, the response of neutrophils to inflammatory stimuli needs to be well controlled.
Neutrophils get recruited to the site of infection by responding to a variety of chemokines, leukotrienes, complement peptides, and some chemicals released by bacteria directly, such as peptides bearing the N-formyl group (formyl-peptides).1–3 All these responses are mediated by heterotrimeric guanine nucleotide-binding regulatory proteins (G proteins)–coupled receptors (GPCRs). One essential downstream target of GPCRs is PtdIns(3,4,5)P3, an inositol phospholipid which has been implicated in a variety of neutrophil functions such as polarization, chemotaxis, and superoxide generation.4–6 PtdIns(3,4,5)P3 exerts its function by mediating protein translocation via pleckstrin homology (PH) domains on the protein.7,8 This membrane translocation is crucial for these proteins to fulfill their functions in various cellular processes such as cell survival, proliferation, growth, differentiation, polarization, chemotaxis, cytoskeletal rearrangement, and membrane trafficking.9–12 PH domain membrane translocation is dependent on concentrations of PtdIns(3,4,5)P3 in the plasma membrane and the amount of 2 inositol phosphates, InsP7 and Ins(1,3,4,5)P4 which can compete for PH domain binding with PtdIns(3,4,5)P3, in the cytosol.13
Although PtdIns(3,4,5)P3 is synthesized by the enzyme PI3 kinase (PI3K), its level can also be regulated by PTEN (phosphatase and tensin homologue deleted on chromosome 10). PTEN was first identified as a tumor suppressor gene located at 10q2314,15 and then subsequently shown to be a phosphatidylinositol 3′-phosphatase that dephosphorylates PtdIns(3,4,5)P3 to PtdIns(4,5)P2.16 It has been reported that PTEN's intracellular localization and activity can be regulated by chemoattractants in both Dictyostelium discoideum17,18 and neutrophils.19,20 In chemoattractant-stimulated Dictyostelium, PI3K is localized at the front of the chemotaxing cells, and PTEN is excluded from the cell front and localized at the uropod (cell back). This reciprocal front/back localization of PI3K and PTEN confines PtdIns(3,4,5)P3 production to the leading edge of the cell and thereby mediates directional actin polymerization, pseudopod formation, and chemotaxis.18,21 Chemoattractant stimulation of PTEN−/−Dictyostelium resulted in an enhanced and prolonged PtdIns(3,4,5)P3 production and actin polymerization. A similar uropod localization of PTEN has also been reported in chemoattractant-stimulated neutrophils.19,20 However, the exact role of PTEN in neutrophil function has not yet been clearly defined. This is partially due to the embryonic lethality of PTEN knockout mice.
In this work, we have investigated the role of PTEN in the regulation of neutrophil function by using a myeloid-specific PTEN knockout mouse.
Materials and methods
Mice
The conditional PTEN knockout mouse (PTENloxP/loxP) and the myeloid-specific Cre mouse were purchased from the Jackson Laboratories (Bar Harbor, ME). The experimental myeloid-specific PTEN knockout mice were generated as previously described.22 In all the experiments performed with the knockout mice we always used corresponding littermates (PTENwt/wt;Cre+/+ or PTENwt/wt;Cre+/−) as wild-type controls. All procedures involving mice were approved and monitored by the Children's Hospital Animal Care and Use Committee. Mouse bone marrow neutrophils were prepared as described by Zhu et al.22
Ruffling assay
Mouse neutrophils were cultured for 1 hour in RPMI/10% FCS, washed once with RPMI/2% FCS, and then resuspended at a density of 1 × 106/mL in the same medium. Cells (200 μL) were allowed to settle down for 5 minutes on Labtek chambered coverglass coated with 30 μg/mL bovine fibronectin (Sigma, St Louis, MO). Nomarski/DIC (differential interference contrast) images were captured using a 40× oil immersion objective on an Olympus IX-71 microscope (Melville, NY). Images were acquired every 10 seconds for 10 minutes using a CCD camera controlled by IPLab imaging software (IPLab, Rockville, MD). Cells were stimulated with 200 μL of 2 × concentrated fMLP or IL-8 (Sigma) and the field imaged was changed every 6 to 7 frames to capture as many cells as possible. The percentage of neutrophils extending pseudopods or ruffling was calculated from fields captured 4 to 8 minutes after chemoattractant stimulation.
Chemotaxis assay
Freshly prepared murine neutrophils were plated on glass-bottomed dishes (precoated with 10 μg/mL fibronectin) and allowed to adhere for 20 minutes in a 37°C/5% CO2 incubator. Cells were then exposed to a micropipette filled with 10 μM fMLP, and images were captured every 20 seconds for 20 minutes. Outlines of migrating cells were traced using DIAS software (Solltech, Oakdale, IA), and tracks were generated using centroid-based methods. Chemotactic parameters were then analyzed from the cell tracks using an in-house Matlab program. A description of the chemotactic parameters are described (Figure S4).
Quantification of F-actin levels
Murine neutrophils were starved during preparation (0.25% BSA used instead of FCS) and resuspended at a density of 5 × 106/mL in RPMI/0.25% BSA. Cells (5 × 105) were stimulated with 100 μL of 2 μM fMLP in RPMI/0.25% BSA for 1, 3, or 5 minutes; fixed with 200 μL 8% formaldehyde; and incubated on ice for 20 minutes. After preblocking overnight at 4°C with 5% nonfat dry milk, cells were stained for 30 minutes with 0.13 μg/mL fluorescien phalloidin (Sigma) in PBS containing 0.1% Triton X-100 and 5% milk. Intensity of phalloidin staining was analyzed using a FACSCalibur machine.
Transwell migration assay
Neutrophils were resuspended in RPMI/2% FCS at a density of 2 to 3 × 106/mL. Bottom wells of the Transwell device (Chemotx, Neuroprobe, Gaithersburg, MD) were filled with 29 μL chemoattractant (fMLP, IL-8, or C5a) in RPMI/2% FCS. The filter plate was then carefully aligned over the bottom wells, and 25 μL of the cell suspension was added on top of the filters. The chemotaxis system was then incubated in a humidified 37°C/5% CO2 incubator for 60 minutes. The number of cells that had migrated into the bottom well was determined using a hemacytometer. WT and PTEN−/− neutrophils were run in parallel.
Superoxide production
Murine neutrophils were resuspended at a density of 1 × 107/mL in HBSS and kept on ice until use. A reaction mixture containing 20 μL of 0.5 mM isoluminol, 10 μL of 80 U/mL horseradish peroxidase (Type XII; Sigma), 40 μL cells, and 110 μL HBSS was added into each well of a 96-well Maxisorp plate (Nunc, Rochester, NY) and allowed to equilibrate to 37°C for 4 minutes in a 1420 Wallac Victor2 multilabel counter (Wallac, Waltham, MA). Chemoattractant (20 μL) was then added to the reaction mixture via the injection port of the luminometer, and luminiscence was recorded (for 2 seconds) at fixed time intervals. For the SOD inhibitable cytochrome-c assay, a 200-μL reaction mixture containing 4 × 105 cells was incubated with 1.5 mg/mL cytochrome-c (from horse heart; Sigma) (with or without 100 U/mL SOD) in HBSS and then stimulated with fMLP (or buffer only). After 5 minutes at RT, cytochrome-c reduction in each sample was detected by spinning down cells and reading absorbance (at 550 nm) of the supernatant in a spectrophotometer. The difference between absorbance of the sample with and without SOD was recorded.
Mouse peritonitis model
PTEN−/− or wild-type mice (n = 3) were left uninjected or intraperitoneally injected with either 1 mL of 3% thioglycollate (TG) (Sigma) in distilled water or 200 μL of 6 × 107Escherichia coli (strain 19138; ATCC, Manassas, VA) in 0.9% saline. At various times after injection, the mice were killed, and peritoneal exudate cells were harvested by 3 successive washes with 10 mL HBSS containing 0.2% BSA and 20 mM EDTA. The exudate cell count was determined using a hemacytometer and the differential cell count by microscopic analysis of Wright-Giemsa–stained cytospins. Total number of neutrophils was then determined accordingly.23
Surface expression of fMLP receptors
Wild-type and PTEN−/− neutrophils (0.5 × 106) were first preblocked on ice for 45 minutes in HBSS (supplemented with 1% BSA/0.02% sodium azide). Cells were then incubated with 3 nM, 6 nM, or 12 nM FITC-fMLP (Invitrogen, Carlsbad, CA) in the same medium (for 1 hour on ice), in the presence or absence of 10 μM unlabeled fMLP. Cells were then washed once and immediately analyzed by flow cytometry. Neutrophils were gated using their characteristic forward/side scatter. Specific binding of FITC-fMLP was calculated by subtracting geometric mean fluorescence intensity in the presence of 10μM unlabeled fMLP from the geometric mean fluorescence intensity in the absence of 10 μM unlabeled fMLP.
Statistical analysis
Analysis of statistical significance for indicated data sets was performed using the Student t test capability on Microsoft Excel (Redman, WA).
Results
Myeloid-specific PTEN knockout mice
Conventional PTEN−/− mice die in early embryonic stage.24 To assess the physiologic role of PTEN in neutrophils, we generated a conditional myeloid-specific PTEN knockout mouse by crossing a PTEN-floxed mouse with a myeloid-specific Cre line. In this Cre line, the expression of Cre recombinase gene is under the control of lysozyme promoter which is activated only in the myeloid linage, including monocytes, mature macrophages, and neutrophils. Western blotting analysis showed that the expression of wild-type PTEN protein is completely abolished in neutrophils isolated from either Cre+/−;PTENloxP/loxP or Cre+/+;PTENloxP/loxP mice (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article; also data not shown). Cre-mediated deletion of loxP-flanked PTEN gene in myeloid cells was highly efficient. The amount of Cre recombinase expressed from only one copy of the Cre gene was enough to initiate PTEN deletion. The homozygous mice (Cre+/−;PTENloxP/loxP and Cre+/+;PTENloxP/loxP) were viable, fertile, normal in size and did not display any gross physical or behavioral abnormalities. The peripheral blood count was also normal in these mice (Figure S2). Microscopic examination of blood smears did not show any morphologic abnormality in neutrophil or macrophage (data not shown).
PtdIns(3,4,5)P3 signaling is elevated in PTEN−/− neutrophils
Uniform treatment of neutrophils with chemokines or fMLP elicits instant and transient elevation of PtdIns(3,4,5)P3 in the plasma membrane. We first examined whether loss of the phosphatase PTEN leads to the up-regulation of fMLP-elicited PtdIns(3,4,5)P3 production. We monitored PtdIns(3,4,5)P3 levels by measuring phosphorylation of Akt. Akt contains a PtdIns(3,4,5)P3-specific PH domain that can be recruited to the plasma membrane via its specific binding to PtdIns(3,4,5)P3. Only the Akt molecules on the plasma membrane can be phosphorylated and get activated.11,25 We examined PtdIns(3,4,5)P3-dependent phosphorylation of Akt in fMLP-stimulated WT and PTEN−/− neutrophils (Figure 1A). Before chemoattractant stimulation, Akt phosphorylation was virtually undetectable in both wild-type and PTEN−/− neutrophils. On stimulation, WT neutrophils showed maximum Akt phosphorylation at 3 minutes, which then declined marginally by 5 minutes (Figure 1B). In contrast, PTEN−/− neutrophils displayed a more enhanced Akt phosphorylation than WT neutrophils. Nearly twice the Akt phosphorylation was achieved by 3 minutes. Even at 5 minutes after stimulation the level of phospho-Akt was much higher in PTEN−/− than in WT. These results are consistent with the role of PTEN as a PtdIns(3,4,5)P3 phosphatase and suggests that PTEN is a major negative regulator of chemoattractant-induced PtdIns(3,4,5)P3 synthesis.
Akt phosphorylation and actin polymerization in PTEN−/− neutrophils. (A-B) Suspensions of wild-type and PTEN−/− neutrophils (0.8 × 106 cells) were stimulated with 1 μM fMLP and lysed at 0, 60, 180, and 300 seconds. Protein lysates from 0.16 × 106 cells were resolved on SDS–polyacrylamide gel electrophoresis (PAGE). Phosphorylated and total Akt were detected by Western blotting analysis using anti–Phospho-Akt (Ser473) (1:5000) and anti-Akt (1:1000) antibodies (Cell Signaling, Beverly, MA), respectively. (A) Results of the Western blots. (B) Results of densitometry. Akt phosphorylation expressed as ratio of phospho-Akt and total Akt. (C-D) Wild-type and PTEN−/− neutrophils (0.5 × 106 cells) were stimulated in suspension with 1 μM fMLP for 0, 60, 180, and 300 seconds; fixed; and stained with 0.13 μg/mL fluorescien phalloidin. Stained neutrophils were then analyzed by fluorescence-activated cell sorting (FACS). (C) Kinetics of F-actin polymerization. Phalloidin staining was represented as mean fluorescence at specified time normalized to mean fluorescence of unstimulated cells. The original FACS data were presented in Figure S3. (D) Decline in F-actin level was represented as the ratio of phalloidin fluorescence at 60 seconds (maximum) to fluorescence at 300 seconds. Data in panels C and D are represented as mean ± SD.
Akt phosphorylation and actin polymerization in PTEN−/− neutrophils. (A-B) Suspensions of wild-type and PTEN−/− neutrophils (0.8 × 106 cells) were stimulated with 1 μM fMLP and lysed at 0, 60, 180, and 300 seconds. Protein lysates from 0.16 × 106 cells were resolved on SDS–polyacrylamide gel electrophoresis (PAGE). Phosphorylated and total Akt were detected by Western blotting analysis using anti–Phospho-Akt (Ser473) (1:5000) and anti-Akt (1:1000) antibodies (Cell Signaling, Beverly, MA), respectively. (A) Results of the Western blots. (B) Results of densitometry. Akt phosphorylation expressed as ratio of phospho-Akt and total Akt. (C-D) Wild-type and PTEN−/− neutrophils (0.5 × 106 cells) were stimulated in suspension with 1 μM fMLP for 0, 60, 180, and 300 seconds; fixed; and stained with 0.13 μg/mL fluorescien phalloidin. Stained neutrophils were then analyzed by fluorescence-activated cell sorting (FACS). (C) Kinetics of F-actin polymerization. Phalloidin staining was represented as mean fluorescence at specified time normalized to mean fluorescence of unstimulated cells. The original FACS data were presented in Figure S3. (D) Decline in F-actin level was represented as the ratio of phalloidin fluorescence at 60 seconds (maximum) to fluorescence at 300 seconds. Data in panels C and D are represented as mean ± SD.
Augmented actin polymerization in PTEN−/− neutrophils
One important downstream effect of chemoattractant-induced PtdIns(3,4,5)P3 production in neutrophils is actin polymerization.17,26 Previous studies in Dictyostelium discoideum17 and Jurkat T cells26 have shown that loss of PTEN elevates F-actin levels. We have examined whether this up-regulation is preserved in our mouse knockout system as well. As expected, PTEN−/− neutrophils showed a more exaggerated and prolonged F-actin response compared with WT neutrophils. The level of F-actin in unstimulated PTEN−/− and WT neutrophils was nearly the same (Figure S3). On stimulation with 1 μM fMLP, F-actin levels increased dramatically in both WT and PTEN−/− neutrophils within 1 minute (Figure 1C). However, the increase was more pronounced in PTEN−/− neutrophils than in WT neutrophils. As time progressed, F-actin levels in WT neutrophils declined significantly (∼ 0.6 times maximum) by 5 minutes. In contrast, PTEN−/− neutrophils exhibited a more prolonged biphasic response, with sustained F-actin even at 5 minutes after stimulation (Figure 1D).
Enhanced ruffling in PTEN−/− neutrophils
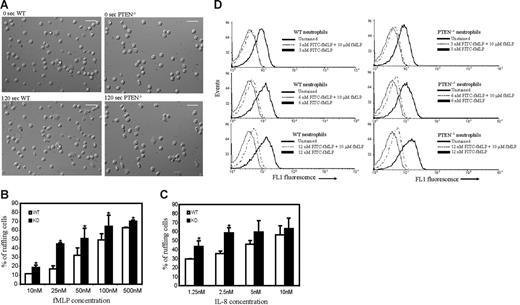
We next investigated the morphologic changes of neutrophils in response to chemoattractants. We found that both WT and PTEN−/− neutrophils were predominantly round before stimulation (Figure 2; Movies S1 and S2). When uniformly stimulated with fMLP, both WT and PTEN−/− neutrophils displayed membrane ruffles and polarized, forming distinct pseudopods and uropods. When we quantified the fraction of ruffling neutrophils, we found that more PTEN−/− neutrophils (Figure 2; Movie S1) ruffled in comparison to WT cells (Figure 2; Movie S2). When we performed a sensitivity analysis using different concentrations of fMLP, we found that this difference was apparent even at low concentrations of fMLP (< 25nM). As the concentration of fMLP was increased, PTEN−/− neutrophils always showed higher activity than WT neutrophils. However, this difference became progressively smaller as the dose applied was increased (Figure 2B). Similar results were observed when IL-8 was used as chemoattractant (Figure 2C).
Increased chemoattractant-induced ruffling in PTEN−/− neutrophils. Neutrophils (2 × 105) purified from WT or PTEN−/− mice were plated on Labtek chamber slides and uniformly stimulated with fMLP or IL-8. (A) WT (left) and PTEN−/− (right) neutrophils before (top) and 2 minutes after stimulation (bottom) with 100 nM fMLP. Scale bar, 25 μm. (B-C) Sensitivity analysis for chemoattractant-induced neutrophil ruffling. Neutrophils were uniformly stimulated with the indicated concentration of fMLP (B) or IL-8 (C), and the percentage of cells that ruffled or extended pseudopods was calculated from images captured 4 to 8 minutes after stimulus was added. Data are mean ± SD collected from (n = 3) separate preparations of neutrophils. *P < .05 versus wild-type neutrophils. Two videos of this experiment are included (Movie S1 and S2). (D-E) fMLP receptor levels in WT and PTEN−/− neutrophils. Bone marrow–derived wild-type and PTEN−/− neutrophils (0.5 × 106) in HBSS/1%BSA/0.02% sodium azide were incubated with 3 nM, 6 nM, or 12 nM FITC-fMLP for 1 hour on ice, in the presence or absence of 10 μM unlabeled fMLP. Cells were then washed once and immediately analyzed by flow cytometry. Neutrophils were gated by using their characteristic forward/side scatter. (D) FACS analysis histograms of wild-type (left) and PTEN−/− neutrophils (right).
Increased chemoattractant-induced ruffling in PTEN−/− neutrophils. Neutrophils (2 × 105) purified from WT or PTEN−/− mice were plated on Labtek chamber slides and uniformly stimulated with fMLP or IL-8. (A) WT (left) and PTEN−/− (right) neutrophils before (top) and 2 minutes after stimulation (bottom) with 100 nM fMLP. Scale bar, 25 μm. (B-C) Sensitivity analysis for chemoattractant-induced neutrophil ruffling. Neutrophils were uniformly stimulated with the indicated concentration of fMLP (B) or IL-8 (C), and the percentage of cells that ruffled or extended pseudopods was calculated from images captured 4 to 8 minutes after stimulus was added. Data are mean ± SD collected from (n = 3) separate preparations of neutrophils. *P < .05 versus wild-type neutrophils. Two videos of this experiment are included (Movie S1 and S2). (D-E) fMLP receptor levels in WT and PTEN−/− neutrophils. Bone marrow–derived wild-type and PTEN−/− neutrophils (0.5 × 106) in HBSS/1%BSA/0.02% sodium azide were incubated with 3 nM, 6 nM, or 12 nM FITC-fMLP for 1 hour on ice, in the presence or absence of 10 μM unlabeled fMLP. Cells were then washed once and immediately analyzed by flow cytometry. Neutrophils were gated by using their characteristic forward/side scatter. (D) FACS analysis histograms of wild-type (left) and PTEN−/− neutrophils (right).
This study suggests that PTEN−/− neutrophils are generally more sensitive to chemoattractant than WT neutrophils. The difference in responsiveness was not due to a developmental defect in PTEN−/− versus WT neutrophils, because both WT and PTEN−/− neutrophils were equally capable of responding to high concentrations of chemoattractant. However, this difference in responsiveness could occur because of enhanced fMLP receptor expression in PTEN−/− neutrophils. To examine whether this was the case, we measured receptor expression in both wild-type and PTEN−/− neutrophils. Our results showed that nearly the same amount of fMLP receptor was expressed in PTEN−/− and wild-type neutrophils (Figure 2D; Table 1). Hence, the increased ruffling in PTEN−/− neutrophils should be a direct consequence of elevated PtdIns(3,4,5)P3 levels.
FITC-fMLP bound to the fMLP receptors, at various concentrations of FITC-fMLP
| FITC-fMLP concentration, nM . | FITC-fMLP bound to the fMLP receptors, arbitrary fluorescence units . | |
|---|---|---|
| WT . | PTEN−/− . | |
| 3 | 3.88 | 3.98 |
| 6 | 5.27 | 5.08 |
| 12 | 5.82 | 6.96 |
| FITC-fMLP concentration, nM . | FITC-fMLP bound to the fMLP receptors, arbitrary fluorescence units . | |
|---|---|---|
| WT . | PTEN−/− . | |
| 3 | 3.88 | 3.98 |
| 6 | 5.27 | 5.08 |
| 12 | 5.82 | 6.96 |
Specific binding was determined by subtracting geometric mean fluorescence intensity in the presence of 10 μM unlabeled fMLP from the geometric mean fluorescence intensity in the absence of 10 μM unlabeled fMLP. Data shown are representative from one of multiple experiments with similar results.
Formation of multiple pseudopodia in PTEN−/− neutrophils
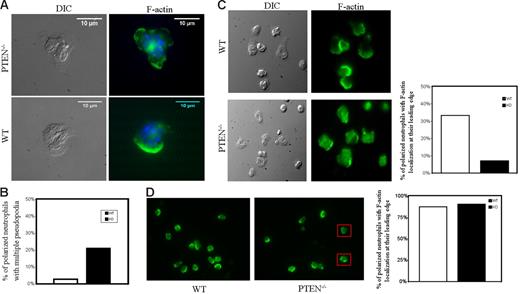
To take a closer look at the morphologic polarity in PTEN−/− neutrophils, we stained F-actin using FITC-phalloidin. Interestingly, we observed that a considerable fraction of polarized PTEN−/− neutrophils (∼ 20%) showed multiple pseudopodia (usually 2 pseudopods, together on one side) (Figure 3A). In comparison, WT neutrophils showed multiple pseudopodia less frequently (Figure 3B). Multiple pseudopodia were also apparent in PTEN−/− neutrophils that were stimulated in suspension (Figure 3D), suggesting that this was not a cell adhesion-mediated phenomenon. Time lapse movies of migrating PTEN−/− neutrophils (Movie S3) showed that the multiple pseudopodia were transiently extended and dynamic in nature. These pseudopods were clearly not a persistent morphologic feature of PTEN−/− neutrophils.
Formation of multiple pseudopodia in PTEN−/− neutrophils. WT and PTEN−/− neutrophils (4 × 105) were plated on glass-bottomed dishes and stimulated with 100 nM fMLP for 5 minutes. Cells were then fixed with 4% formaldehyde, permeabilized with 0.2% Triton-X 100, and stained with fluorescein phalloidin (for F-actin) and Hoechst dye (nuclear stain). (A) Representative DIC/Nomarski and phalloidin (green)/Hoechst (blue) stained images of PTEN−/− neutrophils showing multiple pseudopodia (top). Images were captured using a 96×/1.42 NA oil objective (Olympus). Most WT neutrophils show a single pseudopod (bottom). White arrows indicate pseudopods. (B) Percentage of polarized PTEN−/− and WT neutrophils showing multiple pseudopodia 5 minutes after stimulation with 100 nM fMLP; 50 cells were evaluated for each case. (C-D) F-actin localization in adhered versus suspended PTEN−/− and WT neutrophils. (C) WT and PTEN−/− neutrophils were plated on fibronectin precoated dishes, stimulated with 100 nM fMLP for 5 minutes, fixed with 4% formaldehyde, permeabilized with 0.2% Triton-X-100, and stained with 0.13 mg/mL phalloidin. Images were captured using a 60×/1.42 oil objective (Olympus). WT neutrophils (top, left) show distinct F-actin folds at their leading edges, whereas PTEN−/− neutrophils (bottom, left) are more spread out and do not have as intense F-actin localization at their leading edges. Polarized WT and PTEN−/− neutrophils (n = 51) were scored for clear F-actin localization at the leading edge (with no staining at cell tail or body) and represented in bar graph (right). (D) When neutrophils were stimulated in suspension, the difference in F-actin localization at the leading edge was not apparent. Both WT and PTEN−/− neutrophils intensely localized phalloidin at their leading edges. PTEN−/− neutrophils with multiple pseudopodia are shown in red boxes. Polarized WT and PTEN−/− neutrophils (n = 43) were scored for F-actin localization at their leading edges (with no staining at cell tail or body) and represented in bar graph (right). Images were captured using a 40×/1.35 NA oil objective (Olympus).
Formation of multiple pseudopodia in PTEN−/− neutrophils. WT and PTEN−/− neutrophils (4 × 105) were plated on glass-bottomed dishes and stimulated with 100 nM fMLP for 5 minutes. Cells were then fixed with 4% formaldehyde, permeabilized with 0.2% Triton-X 100, and stained with fluorescein phalloidin (for F-actin) and Hoechst dye (nuclear stain). (A) Representative DIC/Nomarski and phalloidin (green)/Hoechst (blue) stained images of PTEN−/− neutrophils showing multiple pseudopodia (top). Images were captured using a 96×/1.42 NA oil objective (Olympus). Most WT neutrophils show a single pseudopod (bottom). White arrows indicate pseudopods. (B) Percentage of polarized PTEN−/− and WT neutrophils showing multiple pseudopodia 5 minutes after stimulation with 100 nM fMLP; 50 cells were evaluated for each case. (C-D) F-actin localization in adhered versus suspended PTEN−/− and WT neutrophils. (C) WT and PTEN−/− neutrophils were plated on fibronectin precoated dishes, stimulated with 100 nM fMLP for 5 minutes, fixed with 4% formaldehyde, permeabilized with 0.2% Triton-X-100, and stained with 0.13 mg/mL phalloidin. Images were captured using a 60×/1.42 oil objective (Olympus). WT neutrophils (top, left) show distinct F-actin folds at their leading edges, whereas PTEN−/− neutrophils (bottom, left) are more spread out and do not have as intense F-actin localization at their leading edges. Polarized WT and PTEN−/− neutrophils (n = 51) were scored for clear F-actin localization at the leading edge (with no staining at cell tail or body) and represented in bar graph (right). (D) When neutrophils were stimulated in suspension, the difference in F-actin localization at the leading edge was not apparent. Both WT and PTEN−/− neutrophils intensely localized phalloidin at their leading edges. PTEN−/− neutrophils with multiple pseudopodia are shown in red boxes. Polarized WT and PTEN−/− neutrophils (n = 43) were scored for F-actin localization at their leading edges (with no staining at cell tail or body) and represented in bar graph (right). Images were captured using a 40×/1.35 NA oil objective (Olympus).
It appears that disruption of PTEN also affects F-actin localization at the pseudopodia. When cells were plated on fibronectin-coated coverslips and then stimulated with fMLP, WT neutrophils displayed intensely stained, well-formed, F-actin localization at their leading edges (Figure 3A,C, top). Whereas F-actin in PTEN−/− neutrophils was more diffuse and did not show exclusive localization at the leading edge or pseudopodia (Figure 3A,C, bottom). A large fraction of these neutrophils showed increased phalloidin staining throughout the remainder of the cell. Interestingly, this difference in F-actin localization was not observed in neutrophils stimulated in suspension (Figure 3D). Hence, signals elicited by cell adhesion might affect F-actin localization in PTEN−/− neutrophils.
Increased and prolonged superoxide production in PTEN−/− neutrophils
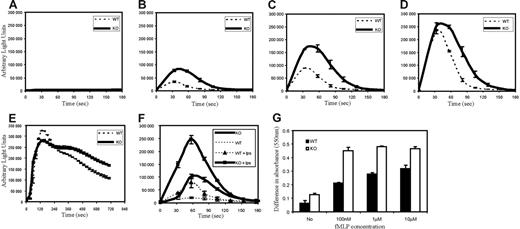
PtdIns(3,4,5)P3 is known to be an important regulator of chemoattractant-induced NADPH oxidase activation and superoxide production. Accordingly, we investigated whether PTEN deficiency had any effect on fMLP-stimulated superoxide production. Neutrophils from WT and PTEN−/− mice were stimulated with fMLP, and superoxide products were detected using isoluminol chemiluminescence. We found a significant difference in both amplitude and time scale of superoxide production in PTEN−/− neutrophils (Figure 4A-D). When stimulated with 500 nM fMLP (Figure 4B), PTEN−/− neutrophils showed nearly 3 times higher (maximum) superoxide production than WT neutrophils. The peak production of superoxide was delayed by about 10 seconds in PTEN−/− neutrophils. In addition, we also observed a more prolonged response in PTEN−/− neutrophils than in WT neutrophils. Wild-type neutrophils took about 90 seconds to return to their prestimulus level, whereas PTEN−/− neutrophils took nearly 140 seconds (Figure 4B). Similar results were obtained with neutrophils stimulated with 1 μM fMLP (Figure 4C). Interestingly, at high concentrations of fMLP (10 μM), both populations showed a similar peak response, indicating that WT neutrophils were equally capable of responding to fMLP. However, the sustained response observed in PTEN−/− neutrophils was still apparent (Figure 4D).
Increased and prolonged superoxide production in PTEN−/− neutrophils. (A-E) Bone marrow–derived neutrophils (4 × 105) from WT and PTEN−/− mice were treated (A) with buffer containing no chemoattractant, (B) 500 nM fMLP, (C) 1 μM fMLP, (D) 10 μM fMLP, (E) 500 nM PMA, or (F) with (or without) 1 μg/mL LPS, 1 hour, 37°C, then 1 μM fMLP. ROS production was monitored in the presence of 50 μM isoluminol and 0.8 U HRP in a luminometer at 37°C. Chemiluminiscence (arbitrary light units) was recorded (for 2 seconds) at indicated time points. WT and PTEN−/− neutrophils were assayed in parallel. Data are mean ± SD from 1 experiment representative of 3. (G) Bone marrow neutrophils (4 × 105) from WT and PTEN−/− mice were incubated with 1.5 mg/mL cytochrome-c (with or without 100 U/mL SOD) and stimulated with no chemoattractant, 100 nM fMLP, 1 μM fMLP or 10 μM fMLP. After 5 minutes, cytochrome-c reduction was detected by spinning down cells and reading absorbance (at 550 nm) of the supernatant in a spectrophotometer. Data are represented as the difference in absorbance between samples with and without SOD; mean ± SD from (n = 3) independent stimulations.
Increased and prolonged superoxide production in PTEN−/− neutrophils. (A-E) Bone marrow–derived neutrophils (4 × 105) from WT and PTEN−/− mice were treated (A) with buffer containing no chemoattractant, (B) 500 nM fMLP, (C) 1 μM fMLP, (D) 10 μM fMLP, (E) 500 nM PMA, or (F) with (or without) 1 μg/mL LPS, 1 hour, 37°C, then 1 μM fMLP. ROS production was monitored in the presence of 50 μM isoluminol and 0.8 U HRP in a luminometer at 37°C. Chemiluminiscence (arbitrary light units) was recorded (for 2 seconds) at indicated time points. WT and PTEN−/− neutrophils were assayed in parallel. Data are mean ± SD from 1 experiment representative of 3. (G) Bone marrow neutrophils (4 × 105) from WT and PTEN−/− mice were incubated with 1.5 mg/mL cytochrome-c (with or without 100 U/mL SOD) and stimulated with no chemoattractant, 100 nM fMLP, 1 μM fMLP or 10 μM fMLP. After 5 minutes, cytochrome-c reduction was detected by spinning down cells and reading absorbance (at 550 nm) of the supernatant in a spectrophotometer. Data are represented as the difference in absorbance between samples with and without SOD; mean ± SD from (n = 3) independent stimulations.
When cells were treated with phorbol-12-myristate-13-acetate (PMA), a protein kinase C (PKC) activator, superoxide production in PTEN−/− neutrophils was not very different from wild-type neutrophils (Figure 4E), suggesting that the enhanced superoxide production is specific for GPCR-mediated signals. We have also tested whether neutrophil priming could further elevate superoxide production in fMLP-stimulated PTEN−/− neutrophils. Neutrophil priming is an important feature of neutrophil inflammatory response. In this process, pretreatment with inflammatory factors such LPS and TNFα dramatically augments superoxide production in response to chemoattractants such as C5a and fMLP.27–29 This phenomenon allows neutrophils to tightly control time and amplitude of superoxide production. fMLP-stimulated PTEN−/− neutrophils displayed enhanced superoxide production after LPS priming. However, the enhancement in wild-type neutrophils (after priming) was nearly 2-fold higher than that observed in PTEN−/− neutrophils (Figure 4F).
We also performed cytochrome-c reduction assays to confirm the increased production of superoxide anions (O2−) in PTEN−/− neutrophils (Figure 4G). Similar to what was observed in the isoluminol chemiluminescence assay, superoxide production was always enhanced in PTEN−/− neutrophils when compared with wild-type neutrophils. We detected more superoxide production in PTEN−/− neutrophils even in the absence of any stimuli, highlighting that PTEN might be important for preventing unwarranted superoxide production.
Enhanced transwell chemotaxis of PTEN−/− neutrophils
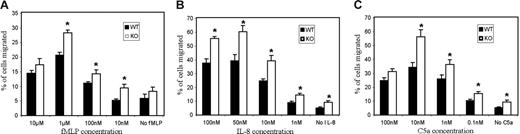
The recruitment of circulating effector leukocytes, including neutrophils, monocytes, and effector T cells, to the sites of injury or infection is mediated by a process called chemotaxis, in which cells sense and move up a gradient of molecules (chemoattractants such as chemokines, formyl-peptides, complement peptides C5a and C3a). A pathway mediated by PtdIns(3,4,5)P3 has been established as one of the essential chemotactic signals.5,30,31 Hence, we investigated chemotaxis of PTEN−/− neutrophils in a transwell migration system. Cells were plated on transwell filters and induced to migrate in response to the chemoattractant added to wells beneath the filters. The migration of neutrophils to these lower wells requires 2-dimensional chemotaxis on top of the filter (toward the holes), followed by migration through the holes into the bottom well of chemoattractant. The number of cells in the bottom well was then used to calculate the percentage of cells migrated. More PTEN−/− neutrophils migrated into the lower wells (in comparison to wild type), even when only medium (without chemoattractant) was added to the lower well (Figure 5). As fMLP concentration in the lower well was varied, both populations of neutrophils exhibited typical bell-shaped migration curves that peaked at 1 μM fMLP (Figure 5A). Very high concentrations of fMLP (10 μM) induced similar migration in PTEN−/− and wild-type neutrophils. However, at lower fMLP concentrations (1 μM), more PTEN−/− neutrophils were able to migrate into the lower well in comparison to wild type. Similarly, other chemoattractants such as IL-8 and C5a also induced enhanced migration in PTEN−/− neutrophils compared with wild type (Figure 5B-C). These results are consistent with higher sensitivity and responsiveness of PTEN−/− neutrophils.
Enhanced transwell migration in PTEN−/− neutrophils. WT and PTEN−/− neutrophils (0.5 × 104) were allowed to migrate in response to the indicated concentration of (A) fMLP, (B) IL-8, and (C) C5a in transwell systems. Percentage of cells that migrated into the bottom well was recorded. Data shown are mean ± SD of 3 wells, from 1 experiment representative of 3. *P < .05 versus wild-type neutrophils.
Enhanced transwell migration in PTEN−/− neutrophils. WT and PTEN−/− neutrophils (0.5 × 104) were allowed to migrate in response to the indicated concentration of (A) fMLP, (B) IL-8, and (C) C5a in transwell systems. Percentage of cells that migrated into the bottom well was recorded. Data shown are mean ± SD of 3 wells, from 1 experiment representative of 3. *P < .05 versus wild-type neutrophils.
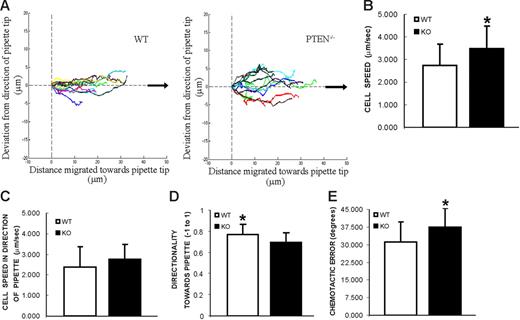
Directional sensing in PTEN−/− neutrophils
In the transwell migration assay, the final readout depends on the ability of neutrophils to respond to chemoattractant, their migration speeds, and their directional sensing ability. We cannot categorically conclude which of these factors are responsible for the enhanced transwell migration in PTEN−/− cells. In fact, previously published data suggest that depletion of PTEN leads to a defect in directional sensing.17–19,32 Thus, we further investigated this by performing single-cell chemotaxis assays using PTEN−/− neutrophils. In this assay, cells were plated on coverslips and exposed to a point source of chemoattractant (a micropipette filled with 10 μM fMLP). Both PTEN−/− (Movie S4) and wild-type (Movie S5) neutrophils were able to successfully chemotax toward the micropipette tip. However, when cell tracks of these neutrophils were aligned in the direction of the pipette tip and overlaid on each other (Figure 6A), we found that the WT neutrophils seemed slightly more efficient than the PTEN−/− neutrophils. WT neutrophils moved straight up the direction of the pipette tip (Figure 6A, left), whereas PTEN−/− neutrophils wavered along their path to the pipette tip (Figure 6A, right). Consistent with this inference, we found a subtle difference in average directionality (P < .05) toward the pipette tip (Figure 6D) (ratio of distance moved in the direction of the pipette tip and actual distance moved; see Figure S4 for description of chemotaxis parameters). The Chemotactic error (average angular deviation from the direction of the pipette tip) was also slightly higher (P < .05) in PTEN−/− neutrophils than WT neutrophils (Figure 6E). However, when we compared average cell speed, we found that PTEN−/− (3.49 ± 0.95 μm/min) neutrophils moved significantly faster (P < 0.05) than WT neutrophils (2.74 ± 0.95 μm/min) (Figure 6B). Therefore, PTEN−/− neutrophils are generally more active and move faster than WT neutrophils but seem to waste some of their energy in the wrong direction. Most importantly, a parameter that represents overall chemotaxis, average cell speed in the direction of the pipette tip (distance moved in the direction of the pipette divided by time) was similar (P > .05) in wild-type and PTEN−/− neutrophils (Figure 6C). Hence, our results indicate that PTEN−/− neutrophils are equally capable of chemotaxing and reaching their final target, when compared with wild-type neutrophils.
Directional sensing in PTEN−/− neutrophils. Chemotaxis in response to a point source of chemoattractant. Neutrophils from WT or PTEN−/− mice were plated on coverslips and exposed to a micropipette filled with 10 μM fMLP. Trajectories of migrating cells were tracked from frames taken every 20 seconds. Two videos of the experiment described in this figure are included (Movies S4 and S5). (A) Cell tracks are plotted in reference to each cell's starting point and aligned with respect to the direction of the pipette tip (black arrow, positive x-axis). (B) Average cell speed is calculated from the trajectories of migrating cells. *P < .05 versus wild-type neutrophils. (C) Average cell speed in the direction of the pipette tip (± μm/minutes). (D) Average directionality toward pipette tip (−1 to 1). *P < .05 versus PTEN−/− neutrophils. (E) Chemotactic error, calculated as the average angle the cell deviates from the direction of the micropipette tip (degrees). *P < .05 versus wild-type neutrophils. A detailed explanation of all these parameters can be found in Figure S4. Results are mean ± SD of 10 to 13 cells from 3 different movie sequences.
Directional sensing in PTEN−/− neutrophils. Chemotaxis in response to a point source of chemoattractant. Neutrophils from WT or PTEN−/− mice were plated on coverslips and exposed to a micropipette filled with 10 μM fMLP. Trajectories of migrating cells were tracked from frames taken every 20 seconds. Two videos of the experiment described in this figure are included (Movies S4 and S5). (A) Cell tracks are plotted in reference to each cell's starting point and aligned with respect to the direction of the pipette tip (black arrow, positive x-axis). (B) Average cell speed is calculated from the trajectories of migrating cells. *P < .05 versus wild-type neutrophils. (C) Average cell speed in the direction of the pipette tip (± μm/minutes). (D) Average directionality toward pipette tip (−1 to 1). *P < .05 versus PTEN−/− neutrophils. (E) Chemotactic error, calculated as the average angle the cell deviates from the direction of the micropipette tip (degrees). *P < .05 versus wild-type neutrophils. A detailed explanation of all these parameters can be found in Figure S4. Results are mean ± SD of 10 to 13 cells from 3 different movie sequences.
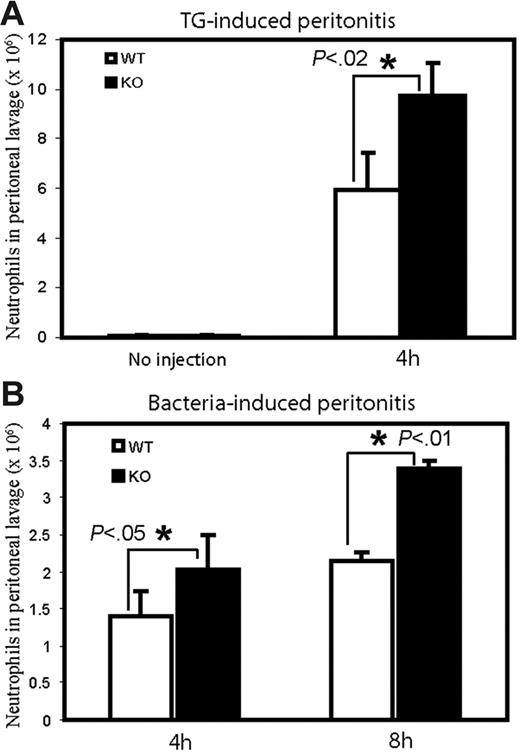
Elevated neutrophil recruitment at sites of inflammation in a mouse peritonitis model
We next investigated whether this enhanced responsiveness could lead to elevated neutrophil recruitment at sites of inflammation in live PTEN−/− mice. Several animal models, such as pneumonia, peritonitis, and pleurisy, have been developed to study neutrophil accumulation at sites of inflammation. We have used a mouse peritonitis model to examine this process (Figure 7). Inflammation was induced by intraperitoneal injection of thioglycollate (TG) (Figure 7A) or E coli (Figure 7B). Thioglycollate is a polysaccharide mixture that has been widely used for decades to induce a mild peritonitis, yielding information about the early events of inflammation. Very few peritoneal neutrophils (∼ 4-6 × 104) were found in wild-type and PTEN−/− mice that were unchallenged (Figure 7A). After induction of inflammatory reactions, the number of neutrophils in the wild-type peritoneal exudate reached nearly 6 × 106 4 hours after TG injection. In contrast PTEN−/− mice showed a dramatic increase in TG-induced neutrophil recruitment; nearly 10 × 106 neutrophils were recruited to the peritoneal cavity 4 hours after induction (Figure 7A). A similar effect was observed when bacteria were injected. At both 4 and 8 hours after E coli injection, the number of neutrophils in the peritoneal exudates was significantly higher in PTEN−/− in comparison to wild-type mice (Figure 7B).
Enhanced neutrophil recruitment to sites of inflammation in PTEN−/− mice. WT and PTEN−/− mice were (A) left unchallenged (left), intraperitoneally injected with 1 mL 3% thioglycollate in distilled water (right) or (B) intraperitoneally injected with 200 μL of 6 × 107E coli in 0.9% saline. At the indicated times mice were killed, and peritoneal lavage fluid was collected. Total neutrophil content in the lavage fluid was then calculated by microscopic examination of Wright-Giemsa–stained cytospins. Data shown at each time point are mean ± SD of 3 mice.
Enhanced neutrophil recruitment to sites of inflammation in PTEN−/− mice. WT and PTEN−/− mice were (A) left unchallenged (left), intraperitoneally injected with 1 mL 3% thioglycollate in distilled water (right) or (B) intraperitoneally injected with 200 μL of 6 × 107E coli in 0.9% saline. At the indicated times mice were killed, and peritoneal lavage fluid was collected. Total neutrophil content in the lavage fluid was then calculated by microscopic examination of Wright-Giemsa–stained cytospins. Data shown at each time point are mean ± SD of 3 mice.
Discussion
PtdIns(3,4,5)P3 has been established as one of the essential signals that mediates various GPCR-initiated cellular processes in neutrophils, such as polarization (pseudopod extension and uropod formation), chemotaxis, and superoxide formation.4,5,30,31,33,34 Because tumor suppressor PTEN is a phosphatidylinositol 3′-phosphatase that degrades PtdIns(3,4,5)P3 to PtdIns(4,5)P2, it emerged as an ideal candidate for negatively modulating neutrophil functions. Recent work has demonstrated that chemoattractants can in fact activate PTEN via the Rho family of GTPases.19 Nevertheless, the exact role of PTEN in neutrophils has not yet been revealed. In this study, we deleted PTEN specifically in myeloid-derived cells using a lysozyme M (lyzs) promoter-driven Cre-loxP system. A similar myeloid-specific knockout has been used to study PTEN function in macrophages.35 These mice were viable and fertile, and their blood parameters (neutrophils, monocytes, lymphocytes) seemed relatively normal. This observation is interesting in the context that PTEN has been shown to be critical for hematopoietic regulation and leukemia prevention.36,37 Mice with PTEN disrupted in hematopoietic stem cells developed myeloproliferative disorder and acute myeloid/lymphoid leukemia. We did not observe any such expansion of myeloid cells in our myeloid-specific PTEN knockout mice. This is probably because the lyzs promoter-driven Cre recombinase expression occurs only during later stages of myeloid differentiation. It has also been shown that rapamycin, a specific inhibitor of mTOR kinase, was able to prevent leukemia that was caused by PTEN deletion in hematopoietic stem cells.37 In our studies, rapamycin did not affect the enhanced sensitivity observed in PTEN−/− neutrophils (data not shown). Other downstream effectors of PI(3,4,5)P3 such as Akt, Prex1, DOCK2, or PDK1 may play a role in mediating sensitivity to the chemoattractant.
As expected, chemoattractant stimulation of PTEN−/− neutrophils resulted in increased PtdIns(3,4,5)P3 synthesis and Akt phosphorylation. PTEN−/− neutrophils also displayed exaggerated and sustained F-actin polymerization. This difference in actin polymerization in PTEN−/− cells has also been reported in other systems such as Dictyostelium17 and Jurkat T cells.26 In addition, we found that PTEN−/− neutrophils were more sensitive to chemoattractant stimulation than WT neutrophils. For the same dose of chemoattractant, more PTEN−/− neutrophils ruffled, extended pseudopods, and polarized in comparison to WT neutrophils, suggesting that PTEN−/− neutrophils were more capable of mitigating the threshold required for transition from basal to activated state. At high concentrations of chemoattractant the difference between PTEN−/− and WT neutrophils was less apparent, indicating that the loss of PTEN might not change the ability of the cell to respond to chemoattractant but only the threshold for the response.
PtdIns(3,4,5)P3 has been implicated in chemoattractant-mediated NADPH oxidase activation.38–40 Hence, a loss of PTEN could up-regulate superoxide production. Consistent with this hypothesis, we found that PTEN−/− neutrophils produced nearly 3 times more superoxide than WT neutrophils. Although the superoxide production was transient in both PTEN−/− and WT neutrophils, PTEN−/− exhibited a more prolonged response in comparison to WT. At saturating concentrations of chemoattractant, the maximal response observed in both WT and PTEN−/− was similar, showing that both populations were equally capable of generating superoxide. However, even at these high chemoattractant concentrations, the prolonged response was still apparent in PTEN−/− neutrophils. Interestingly, superoxide production was enhanced even in unstimulated PTEN−/− neutrophils, suggesting that PTEN might play a role in preventing unwarranted superoxide production at the basal state. We also tested the ability of these neutrophils to display enhanced chemoattractant-induced superoxide production after “priming” with proinflammatory factors such as LPS.27–29 Priming was able to enhance superoxide production in both WT and PTEN−/− neutrophils. However, when compared with the unprimed control, LPS priming induced a larger increase in superoxide in WT neutrophils than in PTEN−/−. This is probably because the stimulated superoxide production in unprimed PTEN−/− neutrophils is already close to the saturation regime; priming can only enhance activity within the saturable limit.
PTEN has been shown to be an essential component of the gradient-sensing machinery in Dictyostelium.17,18,21,41 On exposure to a gradient of chemoattractant, PI3K gets recruited to the leading edge and PTEN dissociates from the front and localizes to the back and sides of the cell. The localization of these enzymes confines PtdIns(3,4,5)P3 production to the leading edge of the cell, which in turn induces actin polymerization and pseudopod formation. Dictyostelium cells depleted of PTEN persistently formed multiple pseudopodia, displayed defects in directional migration, and decrease in cell speed.32 The role of PTEN in mammalian cell chemotaxis has been investigated in several laboratories, and their results have been somewhat contradicting.19,26,42 This could be caused by different experimental systems (eg, the type and source of cells) and conditions (eg, chemoattractant concentrations) used in each study. In our study, we show that PTEN−/− mouse neutrophils also displayed multiple pseudopodia. However, this was not a persistent morphologic defect but only a transient state that probably occurs as a consequence of increased PtdIns(3,4,5)P3 in the PTEN−/− cells. This transient morphologic feature is clearly not as serious a defect as the persistent formation of multiple pseudopodia observed in PTEN-deficient Dictyostelium cells.17 In our single-cell micropipette assay, both WT and PTEN−/− neutrophils migrated successfully toward the pipette tip. PTEN−/− neutrophils were generally more active but seemed to spend a bit more effort in extending pseudopods that were not perfectly oriented in the right direction. These cells showed a small (yet statistically significant) defect in directionality toward the pipette but an increase in cell migration speed. Hence, overall chemotaxis was not affected. We conclude that PTEN plays only a small role (or at least is not the only negative regulator required) in neutrophil directional sensing but has significant effects on threshold for activation and sensitivity in response to chemoattractants. Directional sensing in neutrophils might be mediated by other negative regulators such as a SHIP143,44 or IP3KB (Y.J. and J.L., unpublished data, September 2006).
Consistent with the increased responsiveness of PTEN−/− neutrophils, we also observed enhanced transwell migration in PTEN−/− neutrophils. Loss of PTEN caused enhanced migration even in the absence of chemoattractant stimulation. This could be caused by chemoattractant-independent activation of neutrophils. For example, cell adhesion and subsequent integrin activation can induce cell polarization/migration, and this process might also be PtdIns(3,4,5)P3/PTEN mediated. Another possibility is that the PTEN−/− neutrophils have a lower threshold for activation and therefore are more susceptible to activation by stimuli such as growth factors that are present in FCS or chemoattractants secreted by neutrophils themselves. This in fact highlights that PTEN is necessary at the basal state to prevent unwarranted activation of neutrophils. We currently do not know the indicators that mark the elevated basal state of PTEN−/− neutrophils. It is possible that these cells have higher basal PI(3,4,5)P3 levels, which in turn could promote Rac activation and cytochrome b recruitment to the membrane, in the absence of chemoattractant.
Neutrophil recruitment and subsequent activation is essential for the killing and clearance of pathogens. However, hyperactivation of neutrophils can also damage the surrounding tissues. Neutrophils encounter various signals that can potentially elicit their responses. More than 50 chemokines have been identified in humans, and many of them are continuously present in blood or tissues. However, neutrophils will not chemotax or generate superoxide in response to a chemoattractant stimulation unless the stimulation is strong and stable enough, suggesting the presence of intracellular inhibitory factors that can suppress the positive signals elicited by a weak or unstable chemoattractant stimuli.30,45 These intracellular inhibitors establish a threshold for neutrophil responses. Neutrophils respond only when they receive a stimulation that can overcome the negative effect of the inhibitors. PTEN−/− neutrophils display more enhanced sensitivity to chemoattractant stimulation, suggesting that PTEN might be one such inhibitory regulator. Because more neutrophils are recruited to the site of inflammation in PTEN knockout mice, PTEN and other components of the PtdIns(3,4,5)P3 signaling pathway may be used as therapeutic targets for modulating neutrophil responses, which need to be enhanced to facilitate the pathogen-killing capability or suppressed to aid the resolution of inflammation.
Authorship
Contribution: K.K.S., Y.J., B.T.S., H.J., H.H., J.P.M. and H.R.L. designed the experiments; D.Z. and J.Y. generated and maintained the transgenic mice; K.K.S. performed the experiments and analyzed the data; K.K.S. and H.R.L. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Hongbo R. Luo, Department of Pathology, Harvard Medical School, Department of Lab Medicine and Joint Program in Transfusion Medicine, Children's Hospital Boston, Karp Family Research Bldg, Rm 10214, Boston, MA 02115; e-mail: hongbo.luo@childrens.harvard.edu.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
We thank Peter Devreotes, Henry Bourne, Dan Wu, Miho Iijima, Ulrich von Andrian, Leslie Silberstein, James Campbell, John Manis, and Li Cai for helpful discussions.
This work was supported by the National Institutes of Health (NIH) grants NS052200 and GM076084 (H.R.L.), NIH grants HL068153 and HL079392 (J.M.), NIH training grant HL066987 (D.Z.), and by a Research Scholar Grant from American Cancer Society (H.R.L.).


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal