Umbilical cord blood (UCB) has been used as a potential source of various kinds of stem cells, including hematopoietic stem cells, mesenchymal stem cells, and endothelial progenitor cells (EPCs), for a variety of cell therapies. Recently, EPCs were introduced for restoring vascularization in ischemic tissues. An appropriate procedure for isolating EPCs from UCB is a key issue for improving therapeutic efficacy and eliminating the unexpected expansion of nonessential cells. Here we report a novel method for isolating EPCs from UCB by a combination of negative immunoselection and cell culture techniques. In addition, we divided EPCs into 2 subpopulations according to the aldehyde dehydrogenase (ALDH) activity. We found that EPCs with low ALDH activity (Alde-Low) possess a greater ability to proliferate and migrate compared to those with high ALDH activity (Alde-High). Moreover, hypoxia-inducible factor proteins are up-regulated and VEGF, CXCR4, and GLUT-1 mRNAs are increased in Alde-Low EPCs under hypoxic conditions, while the response was not significant in Alde-High EPCs. In fact, the introduction of Alde-Low EPCs significantly reduced tissue damage in ischemia in a mouse flap model. Thus, the introduction of Alde-Low EPCs may be a potential strategy for inducing rapid neovascularization and subsequent regeneration of ischemic tissues.
Introduction
Endothelial progenitor cells (EPCs) were originally identified as a population of stem cells in human peripheral blood (PB) and characterized by the expression of CD34, KDR (VEGFR-2), and CD133 markers.1,–3 Subsequently, EPCs have been isolated from other sources, such as bone marrow (BM), fetal liver, and umbilical cord blood (UCB).4,–6 Recent studies have shown that EPCs are a potential tool for therapeutic angiogenesis in the treatment of patients suffering from severe limb ischemia or myocardial infarction.7 EPCs have been identified as contributors to vessel development in both normal physiological processes such as wound healing and pathological processes such as cancer.8
The definition of an EPC has been controversial and hence the method to isolate EPCs has been variable among investigators.9 Several studies have demonstrated that there are 2 distinct types of EPCs, the so-called early and late EPCs, which appear sequentially.10 Early EPCs, likely originating from monocytic/dendritic cells, are characterized by the expression of CD45 and CD14, together with some endothelial cell (EC) markers, and have a short lifespan of 3 to 4 weeks. On the other hand, late EPCs rapidly grow out from mononuclear cells with a cobblestone-like morphology and are characterized by EC markers such as CD31, CD34, VEGFR2, and VE-cadherin, but are negative for myeloid markers.10 However, Yoder et al have recently demonstrated that progeny of the CD45+CD14+ cells that coexpress EC markers are not endothelial progenitor cells but hematopoietic-derived myeloid progenitor cells.11 Alternatively, Ingram et al divided ECs into several subpopulations according to their clonogenic and proliferative potential.12 They identified a population of highly proliferative endothelial potential–colony-forming cells (HPP-ECFCs), which form secondary and ternary colonies in human UCB. Given the therapeutic usefulness of EPCs, the effective isolation of highly proliferative EPCs is centrally important for the generation of reliable and safe cell-based therapy.
Aldehyde dehydrogenase (ALDH) is an enzyme responsible for oxidizing intercellular aldehydes.13 This enzyme plays an important role in ethanol, vitamin A,14,15 and cyclophosphamide metabolism.16 Hematopoietic progenitor cells, including stem cells17 and intestinal crypt stem cells,16 express high levels of ALDH. Although ALDH is a cytosolic protein, its expression level can be monitored by flow cytometry using the fluorescent aldehyde called dansyl aminoacetaldehyde (DAAA, also known as Aldefluor).18 In recent studies, human hematopoietic stem cells (HSCs) with a high ALDH expression level were isolated from UCB based on Aldefluor intensity.19,20 Of interest, the high ALDH-expressing HSCs showed a better hematopoietic progenitor function and repopulation activity compared to HSCs with low ALDH levels.
In the present study, we isolated 2 populations of EPCs from UCB according to their ALDH activity (Alde-Low and Alde-High) and examined their surface markers and functions. We found that Alde-High and Alde-Low EPCs differ significantly in their abilities to proliferate and respond to induction by hypoxia both in vitro and in vivo. To our surprise, we found that EPCs with low ALDH activity (Alde-Low) are highly proliferative and migratory compared with those with high ALDH activity (Alde-High), contrary to the case of HSCs. Moreover, Alde-Low EPCs were more responsive to hypoxia in inducing the up-regulation of HIF (hypoxia-inducible factor) protein levels which cause the induction of target genes, such as, VEGF, CXCR4, and GLUT-1 mRNAs, while the response to hypoxia is not significant in Alde-High EPCs. In fact, introducing Alde-Low EPCs significantly repaired ischemic tissue in a mouse flap model. Therefore, our procedure for isolating EPCs using Aldefluor offers a technique for purifying EPCs with a significant ability to regenerate tissue.
Materials and methods
Isolation of adherent cells from UCB
Human full-term UCB samples were collected from umbilical cord veins with permission from the local ethics authorities at the University of Tsukuba. The UCB samples were diluted (1:4) in phosphate-buffered saline (PBS) and the cells were mixed with HetaSep solution (StemCell Technologies, Vancouver, BC) at a ratio of 4:1, then centrifuged at 50g for 5 minutes at 4°C to deplete erythrocytes. The upper layer was collected and added to a mixture of monoclonal antibodies against the cell surface markers CD3, CD14, CD19, CD38, CD66b, and glycophorin A (RosetteSep solution, catalog no. 15168; StemCell Technologies) at a ratio of 20:1 to crosslink unwanted cells to erythrocytes according to the manufacturer's instructions. The treated cells were laid onto a density gradient buffer (Histopaque 1083 g/cm3; Sigma-Aldrich, St Louis, MO) and centrifuged at 250g for 20 minutes at room temperature. The cell layer between the plasma and density buffer was collected and analyzed using a FACSCalibur (BD Biosciences, San Jose, CA). Those cells were then counted using a hemocytometer and plated onto a 25-cm2 culture flask (Sumitomo Bakelite, Tokyo, Japan). The culture medium consisted of IMDM (Invitrogen, Carlsbad, CA) with 10% FBS (Hyclone, South Logan, UT), 2 mg/mL l-glutamine (Invitrogen), 10 ng/mL hb-FGF (Peprotech, London, United Kingdom), and 0.1% (vol/vol) penicillin-streptomycin (100 U/mL penicillin, 0.1 mg/mL streptomycin; Invitrogen). The cells were maintained in a 25-cm2 culture flask (Sumitomo Bakelite) at 37°C in 5% CO2 and in a humidified atmosphere. The culture medium was replaced with fresh medium once a week. Human umbilical vein endothelial cells (HUVECs) were purchased from Cambrex (Walkersville, MD), and maintained in endothelial cell basal medium 2 (EBM-2; Cambrex) supplemented with EGM-2 SingleQuots containing 2% FBS, VEGF, FGF-2, EGF, IGF-1, hydrocortisone, heparin, and ascorbic acid. At subconfluency, adherent cells were harvested with 0.05% (vol/vol) trypsin-EDTA (Invitrogen) and replated at a ratio from 1:4 to 1:5.
The clusters formed in the flask were analyzed by the uptake of PE-conjugated low-density lipoprotein from human plasma acetylated, DiI complex (DiI-Ac-LDL; Molecular Probes, Eugene, OR). Cells were visualized with an Olympus IX71 microscope (Olympus, Tokyo, Japan) under UPlan FI (×4).
Frozen cell stocks were prepared using Cell Banker (ZENOAQ, Koriyama, Japan) solution and stored in liquid nitrogen for further experiments.
All experiments were performed using EPCs from at least 2 distinct sources of UCB and results were reproducible.
Antibodies
The antibodies used in this study were as follows. Fluorescein isothiocyanate (FITC)–labeled HLA-A,B,C (W6/32), phycoerythrin (PE)–labeled anti-CD31 (WM59) and anti–c-kit (104D2), and allophycocyanine (APC)–labeled anti-CD45 (HI30) (BioLegend, San Diego, CA); FITC-labeled anti-CD105 (SN6) (Serotec, Oxford, United Kingdom); PE-labeled anti–VE-cadherin (TEA/31) (Beckman Coulter, France); PE-labeled anti-CD166 (3A6), anti-CD14 (M5E2), APC-labeled anti-CD34 (581), and anti–mouse IgG (BD Biosciences). Purified anti–VEGFR-2 (KDR) antibody was used as previously reported.21 After staining the cells with fluorochrome-conjugated antibodies, cells were sorted using FACSVantageSE (BD Biosciences) as previously described.22
Analysis of aldehyde dehydrogenase activity in EPCs
ALDH activity was analyzed with Aldefluor reagent (StemCell Technologies) according to the manufacturer's specifications and a previous report.19 Aldefluor substrate (0.625 μg/mL) was added to 1 to 2 × 106 EPCs in the Aldefluor assay buffer and incubated for 30 minutes at 37°C to induce the conversion of Aldefluor substrate to the fluorescent product. For each experiment, an aliquot of Aldefluor-stained cells was immediately quenched with 5 μL of 1.5 mM diethylaminobenzaldehyde (DEAB), a specific ALDH inhibitor. After treatment with Aldefluor reagent, cells were stained with fluorochrome-conjugated antibodies and sorted using FACSVantage (BD Biosciences).
Growth curve
HUVECs or EPCs were plated at a density of 2 × 104 cells per 35-mm dish (Sumitomo Bakelite). Cell culture medium was changed to fresh one every third day. Dead cells were excluded by the use of trypan-blue staining solution (Invitrogen) and the numbers of live cells in triplicate dishes were scored using a hemocytometer at 24-hour intervals for 10 days.
Analysis of endothelial tube formation in Matrigel
Analysis of capillary formation in Matrigel (BD Biosciences) was performed as specified in the manufacturer's protocol. Matrigel (300 μL) was aliquoted into a 4-well plate (Nalge Nunc; Nunc, Rochester, NY) and incubated at 37°C for 30 minutes. After trypsinization, 5 × 104 HUVECs or EPCs were suspended with 200 μL culture medium and plated onto the preincubated Matrigel as previously described.22 After 8 to 12 hours of incubation under normoxia or hypoxia (5% O2), tube formation in the Matrigel was observed under a microscope and the number of capillary tubes in a random field of each well was quantitated. The average number of tubes obtained from 3 wells was given.
Analysis of HIF protein levels and the expression of hypoxia-inducible genes
HUVECs or EPCs were plated at a density of 1 × 105 cells per 6-cm dish (Sumitomo Bakelite). Culture medium was replaced with fresh one at 80% confluency, and the cells were subsequently exposed to oxygen concentration of 20%, 5%, or 1% for 6 to 8 hours. To evaluate the gene expression of hypoxia-inducible genes by reverse-transcription–polymerase chain reaction (RT-PCR), total RNA was isolated using the RNAeasy mini kit (Qiagen, Hilden, Germany) as described before.23 To analyze HIF protein levels, nuclear extracts were prepared as described previously.24
RT-PCR
Total RNA (1 μg) was reverse transcribed using an RT-PCR kit (BD Biosciences). The cDNAs were amplified by the GeneAmp PCR System (Applied Biosystems, Foster City, CA) for 22 to 36 cycles of 95°C for 5 seconds and 68°C for 30 seconds. The primers used for the PCR reactions were as follows: VEGF164 (5′-GAAGACCCTGCACTCAATCAAGAAGTT; 3′-CTGCATGGTGATGTTGGACTCCTCAGT), KDR (5′-AGTGTGGAGGACTTCCAGGGAGGAAAT; 3′-GGCCAAGCTTGTACCATGTGAGGTTCT), FLT-1 (5′-CCGGGAGAGACTTAAACTGGGCAAATC; 3′-TTAGGCTCCATGTGTAGTGCTGCATCC), CXCR4 (5′-CTGTGACCGCTTCTACCCCAATGACTT; 3′-CCAAGGAAAGCATAGAGGATGGGGTTC), GLUT-1 (5′-ATCTCATCGAAGGTTCGGCCTTTGG; 3′-CCTTGGATGTCCTATCTGAGCATCG), β-actin (5′-GTGCGTGACATTAAGGAGAAGCTGTGC; 3′-GTACTTGCGCTCAGGAGGAGCAATGAT).
Western blot analysis
Nuclear extracts (30 μg) were electrophoresed on 8.5% sodium dodecyl sulfate–polyacrylamide gel electrophoresis (SDS-PAGE) gels. Rabbit anti–HIF-2α antibody25 and anti–nHIF-1α antibody (Novus, Littleton, CO) were used for immunoblot assay. HRP-conjugated goat anti–rabbit IgG (Invitrogen) or goat anti–mouse IgG (Invitrogen) was used as secondary antibody, and enhanced chemiluminescence (GE Healthcare Bio-Science, Hampshire, United Kingdom) was used for detection. Goat anti–Lamin B antibody (Santa Cruz, Santa Cruz, CA) was used to monitor protein loading and transfer.
In vivo EPC function assay
Adult mice with a mixed C57/BL6 and ICR genetic background were used in the study. Immunosuppression was performed by intraperitoneal injection of cyclosporin-A (Wako, Osaka, Japan) at 20 mg/kg body weight 2 days before the assay. The injection of cyclosporin-A was continued daily for the entire period of the assay.26 Adult mice with BALB/c nu/nu genetic background were also used in this study without the treatment of cyclosporin-A.
A flap ischemia model was made in mouse back skin as previously described.27 A peninsular shaped incision (3 cm by 2 cm) was made on the dorsal surface for single side circulation and an ischemia gradient.
Cultured HUVECs, Alde-High EPCs, and Alde-Low EPCs were infected with MSCV-IRES-EGFP as described before,23 and GFP-labeled HUVECs and EPCs were purified using FACSVantage. Following surgery, mice received an injection of GFP-labeled HUVECs or EPCs (5 × 105 cells/mouse) in the tail vein. An in vivo migration assay was carried out 24 hours after the EPC injection, while dorsal skin repair after generating the incision was assessed 7 days subsequent to the injection. On day 7, mice were injected with 200 μL of Banderiraea simplicifolia lectin I-TRITC (0.1 mg/mL) (Sigma-Aldrich) from tail vein, and after 5 minutes the mice were killed and analyzed as described before.28 Tissues were visualized with an Olympus BX51 microscope (Olympus) under UPlan SApo (×10, ×20, ×40).
Migration assay
Migration assay was performed as described previously.27 HUVECs or EPCs (5 × 104 cells) were seeded onto transwells (6.5 mm, 8-μm pore; BD Biosciences) in IMDM supplemented with 0.5% FBS. IMDM/0.5% FBS with recombinant human SDF-1 (200 ng/mL; R&D Systems, Minneapolis, MN) was added to lower chamber. The assays were performed under different oxygen tensions (20% or 5%) for 6 hours, and nonmigrating cells were wiped away from the top surface of the membrane. Adherent cells to the undersurface of the membrane were stained with Diff-Quik staining solution (International Reagents, Kobe, Japan) and scored the number under an inverted microscopy.
Statistical analysis
Statistical evaluations of data were conducted using the Student t test for per-comparison analysis. Data are presented as means ± SD.
Results
Isolation of UCB-derived EPCs
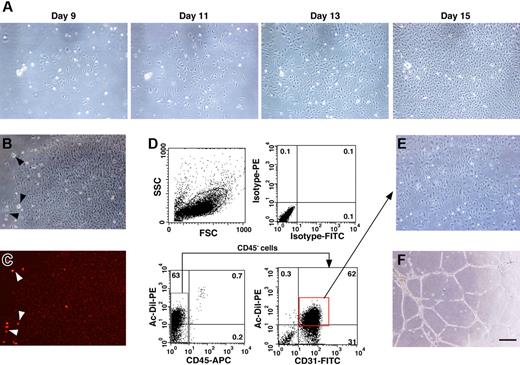
In order to isolate EPCs from UCB, mononuclear cells were separated by density gradient centrifugation after the depletion of hematopoietic cells. It has been reported that EPCs derived from UCB are CD45−/CD31+/CD105+.12 Therefore, we examined the frequency of these markers in the mononuclear cell population and found that CD45−/CD31+/CD105+ cells appeared at a frequency of 2.7% ± 1.0% (n = 4). We cultured the isolated mononuclear cells in IMDM/10% FBS with hb-FGF in a flask without specific surface coating. Adherent cells started to grow about 7 days after culturing and then grew rapidly to form colonies with a tightly compact morphology (Figure 1A). We then examined the incorporation of Dil-Ac-LDL in the adherent cells (Figure 1B-C). The incorporation of Dil-Ac-LDL is a hallmark for both ECs and macrophages. Almost all of the adherent cells were weakly positive for DiI-Ac-LDL incorporation, while some cells with a distinct morphology, possibly macrophages, showed a greater incorporation of DiI-Ac-LDL (Figure 1B-C arrowheads). We scored the number of cobblestone-like colonies and found that 16.6 ± 8.9 DiI-Ac-LDL–positive colonies per flask (surface area of 25 cm2, n = 6) had developed by day 21 of culturing.
EPC colonies derived from human UCB. (A) Cobblestone-like clusters start to appear around 1 week after plating the mononuclear cells in a flask. Cell morphology was photographed on days 9, 11, 13, and 15 after seeding. (B-C) Adherent colonies were analyzed for the incorporation of PE-conjugated DiI-Ac-LDL (B, bright field; C, dark field). Note the strong incorporation of DiI-Ac-LDL into cells that were possibly macrophages based on their morphology (arrowheads). (D) Adherent cells were examined for the expression of CD31 and the incorporation of DiI-Ac-LDL by FACS. (E-F) CD45−/CD31+/DiI-Ac-LDL–positive cells were sorted and expanded for further experiments and these cells formed capillary tube–like structures on Matrigel. Bar indicates 50 μm.
EPC colonies derived from human UCB. (A) Cobblestone-like clusters start to appear around 1 week after plating the mononuclear cells in a flask. Cell morphology was photographed on days 9, 11, 13, and 15 after seeding. (B-C) Adherent colonies were analyzed for the incorporation of PE-conjugated DiI-Ac-LDL (B, bright field; C, dark field). Note the strong incorporation of DiI-Ac-LDL into cells that were possibly macrophages based on their morphology (arrowheads). (D) Adherent cells were examined for the expression of CD31 and the incorporation of DiI-Ac-LDL by FACS. (E-F) CD45−/CD31+/DiI-Ac-LDL–positive cells were sorted and expanded for further experiments and these cells formed capillary tube–like structures on Matrigel. Bar indicates 50 μm.
After the DiI-Ac-LDL incorporation assay, the cells were trypsinized and immunostained with fluorochrome conjugated CD31 and CD45 antibodies. In order to eliminate contamination with hematopoietic cells, the CD45− cells were gated and the DiI-Ac-LDL–positive/CD31+ cells were sorted (Figure 1D). The DiI-Ac-LDL–positive macrophages/monocytes are excluded at this process, because these cells are positive for CD45. The culturing of sorted cells was continued in IMDM/10% FBS supplemented with hb-FGF. These cells displayed EC-like morphology and formed capillary tube–like structures on Matrigel (Figure 1E-F). Therefore, we determined that the adherent cells were likely to be EPCs and further characterized these cells by the following experiments.
Separation of EPCs according to the ALDH activity
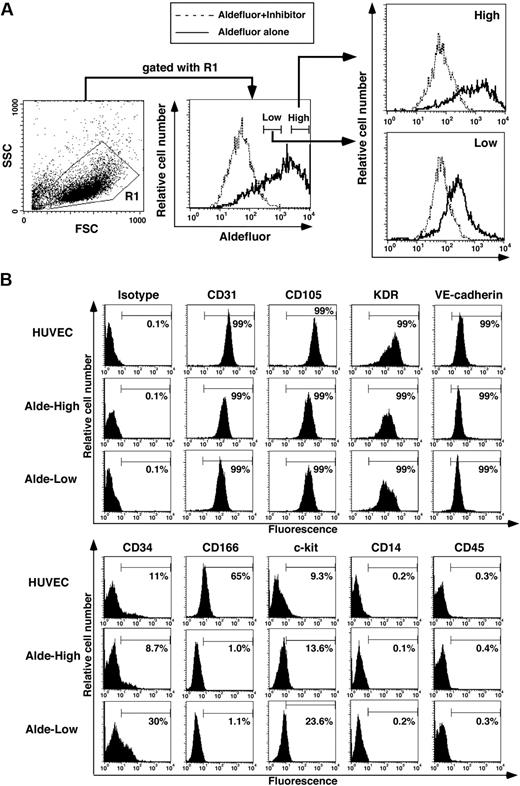
In hematopoietic cells originating from UCB, lineage-negative cells with high ALDH activity express primitive hematopoietic cell surface markers more frequently and possess a greater ability to repopulate compared to those with low ALDH activity.19 We therefore examined whether the expression level of ALDH can be used as a marker for segregating EPCs with different functional properties. As shown in Figure 2A, we divided UCB-derived EPCs into 2 fractions according to their expression level of ALDH. Alde-High and Alde-Low cells were found at a frequency of 24.6% ± 9.4% and 37.3% ± 1.5%, respectively (n = 3). Both populations expressed EC-specific cell surface markers (CD31, CD105, VE-cadherin, and KDR) (Figure 2B). In addition, hematopoietic cell surface markers (CD45 and CD14) were not expressed in either fraction. We also examined markers for immature cells that are commonly expressed in ECs and hematopoietic cells, such as CD34, CD166, and c-kit. While the expressions of c-kit and CD166 were similarly observed in Alde-High and Alde-Low EPCs, we found that the frequency of CD34+ cells was slightly higher in Alde-Low EPCs than in Alde-High EPCs (Figure 2B).
Isolation of Alde-High and Alde-Low EPCs. (A) EPCs were separated on the basis of ALDH activity into Alde-High and Alde-Low cells. The dotted lines represent samples containing cells with Aldefluor reagent and its inhibitor, whereas the solid lines represent samples containing cells with Aldefluor reagent alone. (B) HUVECs, Alde-High EPCs, and Alde-Low EPCs were analyzed by FACS for the expressions of CD31, CD105, KDR, VE-cadherin, CD34, CD166, c-kit, CD45, and CD14.
Isolation of Alde-High and Alde-Low EPCs. (A) EPCs were separated on the basis of ALDH activity into Alde-High and Alde-Low cells. The dotted lines represent samples containing cells with Aldefluor reagent and its inhibitor, whereas the solid lines represent samples containing cells with Aldefluor reagent alone. (B) HUVECs, Alde-High EPCs, and Alde-Low EPCs were analyzed by FACS for the expressions of CD31, CD105, KDR, VE-cadherin, CD34, CD166, c-kit, CD45, and CD14.
To examine whether the expression of CD34 is specific to a certain subpopulation of EPCs, EPCs were subdivided by fluorescence-activated cell sorting (FACS) on the basis of CD34 expression and cultured under the same conditions as for Alde-High and Alde-Low EPCs (data not shown). Strikingly, CD34 expression was lost in the CD34+ cell fraction after a few passages, while no CD34− cells turned positive for CD34 during culturing. These results suggest that CD34 may not be an ideal marker for estimating the functional role of EPCs, although CD34 is expressed specifically in immature EPCs and Alde-Low EPCs may contain more immature cells compared with Alde-High EPCs.
Analyses of EPCs under hypoxic conditions
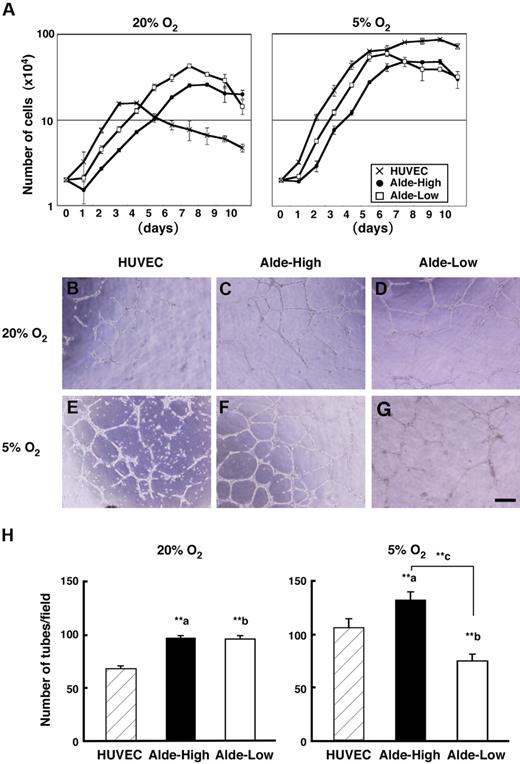
Since Ingram et al suggested a hierarchical model of UCB-derived EPCs based on their proliferative potential,12 we next examined the correlation between the ALDH expression level and the proliferative activity of EPCs. A previous study demonstrated that the severity of ischemia correlates with the migration of EPCs to the ischemic region and the proliferative activity of EPCs, indicating that hypoxia acts as the major stimulus of EPC function.27 We therefore examined cell proliferation by scoring the cell number daily under conditions of normoxia and hypoxia (Figure 3A). Both Alde-High and Alde-Low EPCs reached a plateau on day 7 under normoxic conditions (20% O2). Of interest, Alde-Low EPCs reached a plateau 2 days earlier than Alde-High EPCs under hypoxic conditions (Alde-Low, day 5; Alde-High, day 7; 5% O2) and both cell types grew more rapidly than during normoxia. The doubling time of EPCs was significantly shorter in Alde-Low compared to Alde-High cells in both normoxia (Alde-Low, 29.3 ± 0.4 hours and Alde-High, 40.1 ± 2.7 hours, P < .01) and hypoxia (Alde-Low, 22.5 ± 0.5 hours and Alde-High, 26.0 ±1.6 hours, P < .05). We also examined the proliferative activity of HUVECs and made a comparison. The doubling time of HUVECs was 21.4 ± 3.8 hours in normoxia and 23.9 ± 0.8 hours in hypoxia. Although the doubling time of HUVECs was faster than those of EPCs, HUVECs reached a plateau within short period and confluent cell number was much smaller compared to those of EPCs in normoxia. Under hypoxic condition, HUVECs proliferated at comparable level to both EPCs. These data clearly indicate that Alde-Low EPCs are highly proliferative compared to Alde-High EPCs and HUVECs under normoxic condition.
Characterization of Alde-High and Alde-Low EPCs under hypoxic conditions. (A) HUVECs and EPCs were incubated in 20% (left) or 5% (right) oxygen and harvested every 24 hours until the cultures reached confluency. The average cell number in triplicate dishes was determined (mean ± SD) by counting. Note that HUVECs (crisscross) reached a plateau at lower number of cells, and the growth rate of Alde-Low cells (white squares) was faster than that of Alde-High cells (black circles) under normoxic and hypoxic conditions. (B-G) EC tube formation on Matrigel. HUVECs (B,E), Alde-High EPCs (C,F), and Alde-Low EPCs (D,G) were plated on Matrigel and cultured under normoxic (20% O2) or hypoxic (5% O2) conditions for 8 to 12 hours. (H) The number of tubes formed on Matrigel was scored in each field using microscopy and the mean ± SD was obtained from triplicate wells. a indicates HUVECs versus Alde-High EPCs; b, HUVECs versus Alde-Low EPCs; and c, Alde-High versus Alde-Low EPCs; **P < .01.
Characterization of Alde-High and Alde-Low EPCs under hypoxic conditions. (A) HUVECs and EPCs were incubated in 20% (left) or 5% (right) oxygen and harvested every 24 hours until the cultures reached confluency. The average cell number in triplicate dishes was determined (mean ± SD) by counting. Note that HUVECs (crisscross) reached a plateau at lower number of cells, and the growth rate of Alde-Low cells (white squares) was faster than that of Alde-High cells (black circles) under normoxic and hypoxic conditions. (B-G) EC tube formation on Matrigel. HUVECs (B,E), Alde-High EPCs (C,F), and Alde-Low EPCs (D,G) were plated on Matrigel and cultured under normoxic (20% O2) or hypoxic (5% O2) conditions for 8 to 12 hours. (H) The number of tubes formed on Matrigel was scored in each field using microscopy and the mean ± SD was obtained from triplicate wells. a indicates HUVECs versus Alde-High EPCs; b, HUVECs versus Alde-Low EPCs; and c, Alde-High versus Alde-Low EPCs; **P < .01.
In contrast to the clear difference in cell growth, we did not observe any significant difference in the extent of tube formation between Alde-High and Alde-Low EPCs under normoxic conditions (Figure 3B-3G). Surprisingly however, Alde-High and Alde-Low EPCs did show striking differences in tube formation under hypoxic conditions. Alde-Low EPCs were spindle shaped and had elongated cytoplasmic poles under hypoxia, but failed to form capillary tubes (Figure 3G). In contrast, Alde-High EPCs could form more capillary networks under hypoxic conditions (Figure 3F). The number of tubes formed significantly decreased in Alde-Low EPCs during hypoxia (normoxia, 96.2 ± 3.3 per field and hypoxia, 73.7 ± 6.9 per field, P < .01 [n = 3]), while an increased number of tubes emerged in Alde-High EPCs (normoxia, 97.5 ± 2 per field and hypoxia, 125.2 ± 8.5 per field, P < .01 [n = 3]) (Figure 3F). The number of tubes increased in HUVECs during hypoxia (normoxia, 68.7 ± 2.3 per field and hypoxia, 105.7 ± 8.5 per field, P < .01 [n = 3]) (Figure 3H). While the tube formation of HUVECs was less than those of EPCs in normoxia, they increased at comparable number in hypoxia to that of Alde-High EPCs.
Analyses of gene expression profiles in EPCs under conditions of hypoxia
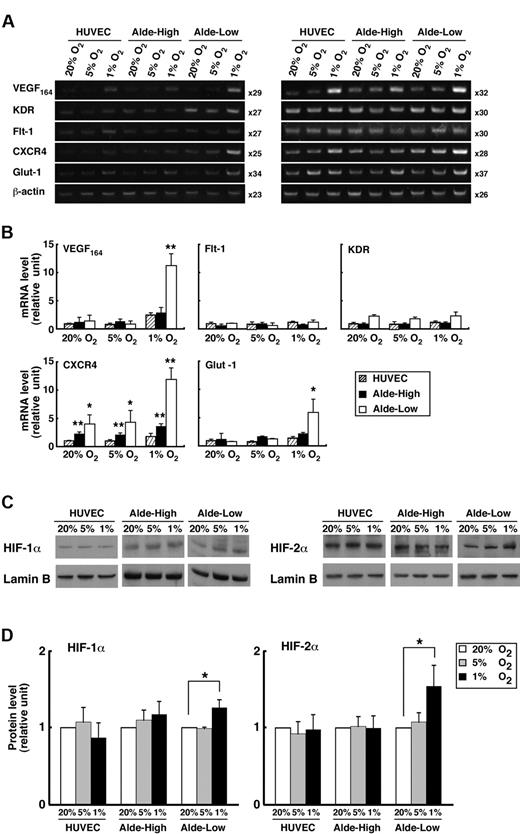
We examined the expression of genes related to the proliferation and migration of ECs after exposing EPCs to hypoxia (1% or 5% O2) for 8 hours (Figure 4A-B). During normoxia, gene expression did not significantly differ between the Alde-High and Alde-Low cell populations. In contrast, during hypoxia, VEGF, CXCR4, and GLUT-1 were appreciably up-regulated in Alde-Low EPCs compared to Alde-High EPCs, although the expressions of VEGF receptors, Flt-1, and KDR were comparable between Alde-High and Alde-Low EPCs. While CXCR4 was significantly expressed in Alde-High and Alde-Low EPCs compared to HUVECs in both normoxia and hypoxia, remarkable up-regulation was observed in Alde-Low EPCs under 1% oxygen concentration. These data suggest that Alde-Low EPCs are more active in their response to hypoxia and that the 2 types of EPCs may act differently in the neovascularization of ischemic tissue.
Comparison of mRNA expression in Alde-High and Alde-Low EPCs. (A) The mRNA expression levels of each factor in HUVECs, Alde-High EPCs, and Alde-Low EPCs were examined by RT-PCR under normoxic (20% O2) or hypoxic (1% O2) conditions. Semiquantitative RT-PCR analysis was performed in 2 independent cycles. (B) The mRNA expression levels of the factors in HUVECs, Alde-High EPCs, and Alde-Low EPCs were measured by densitometry and expressed as histograms (striped bar indicates HUVECs; black bar, Alde-High EPCs; and white bar, Alde-Low EPCs). The expression levels seen in HUVECs under normoxic conditions were normalized to a value of 1 as the standard for each factor. *P < .05; **P < .01. (C-D) Western blot analyses were performed to examine HIF protein levels in HUVECs, Alde-High EPCs, and Alde-Low EPCs under hypoxic condition. Left panel represents the HIF-1α expression in ECs, and right panel represents the HIF-2α expression. Quantification of the protein levels was analyzed by a densitometer. The values shown are the averages of 3 independent experiments (mean ± SD). Data are expressed as fold induction compared to the values taken from HUVECs cultured under normoxic condition (20% O2). White bar indicates 20% O2; gray bar, 5% O2; black bar, 1% O2. *P < .05.
Comparison of mRNA expression in Alde-High and Alde-Low EPCs. (A) The mRNA expression levels of each factor in HUVECs, Alde-High EPCs, and Alde-Low EPCs were examined by RT-PCR under normoxic (20% O2) or hypoxic (1% O2) conditions. Semiquantitative RT-PCR analysis was performed in 2 independent cycles. (B) The mRNA expression levels of the factors in HUVECs, Alde-High EPCs, and Alde-Low EPCs were measured by densitometry and expressed as histograms (striped bar indicates HUVECs; black bar, Alde-High EPCs; and white bar, Alde-Low EPCs). The expression levels seen in HUVECs under normoxic conditions were normalized to a value of 1 as the standard for each factor. *P < .05; **P < .01. (C-D) Western blot analyses were performed to examine HIF protein levels in HUVECs, Alde-High EPCs, and Alde-Low EPCs under hypoxic condition. Left panel represents the HIF-1α expression in ECs, and right panel represents the HIF-2α expression. Quantification of the protein levels was analyzed by a densitometer. The values shown are the averages of 3 independent experiments (mean ± SD). Data are expressed as fold induction compared to the values taken from HUVECs cultured under normoxic condition (20% O2). White bar indicates 20% O2; gray bar, 5% O2; black bar, 1% O2. *P < .05.
Since the expressions of VEGF, CXCR4, and GLUT-1 are regulated by the HIF transcription factors that are stabilized under hypoxic condition, we hypothesized that the HIF protein levels are increased in Alde-Low, but not in Alde-High, EPCs during hypoxia. We therefore examined the expressions of HIFs in EPCs by Western blot analysis (Figure 4C-D). Indeed, the expressions of both HIF-1α and HIF-2α were significantly increased in response to hypoxia (1% O2) in Alde-Low EPCs (HIF-1α: 1.26 ± 0.10-fold increase, P < .05 [n = 3]; HIF-2α: 1.54 ± 0.27-fold increase, P < .05 [n = 3]). In contrast, the induction of HIFs under conditions of hypoxia was not observed in Alde-High EPC and HUVECs. Consistent with the previous report using HeLa cells, 5% oxygen concentration was not sufficient to increase accumulation of HIF proteins in ECs used in this experiments.29 These results indicate that the up-regulation of HIF protein levels under conditions of hypoxia causes the induction of target genes in Alde-Low EPCs, while the response to hypoxia is not significant in Alde-High EPCs and HUVECs.
Role of EPCs in neovascularization in vivo
Our data suggest that Alde-Low EPCs are highly proliferative and highly responsive to hypoxia compared to Alde-High EPCs and HUVECs. To assess how these types of ECs are involved in neovascularization in vivo, we studied the effects of hypoxia on angiogenic and repair processes following surgery of the dorsal skin in immunosuppressant mice. In this model, an oxygen tension gradient occurs in the ischemic tissue, with the caudal portion of the flap having one fifth the oxygen tension of the upper region adjacent to the head of the flap, as previously reported.27
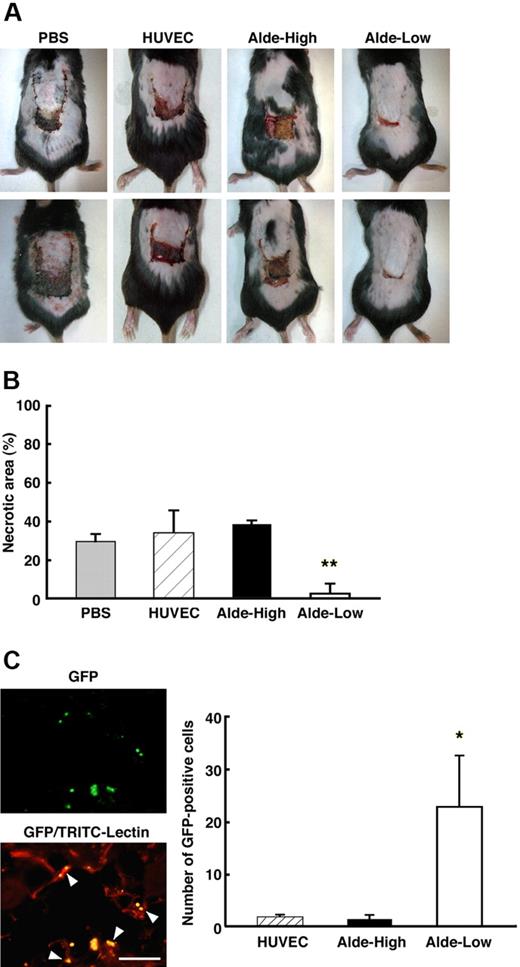
After the dorsal skin surgery, ECs were injected into the mice and the therapeutic effect was assessed for HUVECs, Alde-High EPCs, and Alde-Low EPCs (Figure 5). Seven days subsequent to the surgery, the flap did not fully cover the surgical region in the absence of an EC injection, and the edge of the flap, where the oxygen tension was the lowest, became necrotic (Figure 5A left column). The injection of HUVECs or Alde-High EPCs also failed to rescue the ischemic region and the surface area of necrosis was similar to that of the PBS injection (Figure 5A middle 2 columns). Remarkably, the necrotic region was barely observable when Alde-Low EPCs were injected into the mice (Figure 5A right column). The areas of necrosis as percentages of the total flap areas after surgery were as follows: PBS, 30.0% ± 3.7%; HUVECs, 34.6% ± 11.4%; Alde-High EPCs, 38.9% ± 2.0%; and Alde-Low EPCs, 2.9% ± 5.1%; P < .01 (n = 3) (Figure 5B). Since the experiments were done by using mice with a mixed genetic background, the injection of cyclosporin-A was necessary to suppress immunorejection. To exclude the direct effects of cyclosporin-A on functions of EPCs, the same experiment has been done by using BALB/c nu/nu mice without the cyclosporin-A treatment. Consistent with the results shown in Figure 5A-B, the injection of Alde-Low EPCs resulted in a near-complete repair of ischemic area, whereas that of Alde-High EPCs or HUVECs failed to rescue the region from necrosis (Figure S1, available on the Blood website; see the Supplemental Figure link at the top of the online article).
Alde-Low EPCs were curative of ischemic tissue. (A) Skin incisions were created on the dorsal surfaces of mice, and PBS alone, HUVECs, Alde-High EPCs, or Alde-Low EPCs were injected (top and bottom photographs/each condition). The effectiveness of ECs to recover tissue from ischemia was examined on day 7 following surgery. (B) The necrotic regions in the 4 types of mice (n = 3) were measured. Note that the area of necrosis was smallest in the mice injected with Alde-Low EPCs. Gray bar indicates PBS; striped bar, HUVECs; black bars Alde-High EPCs; and white bar, Alde-Low EPCs. **P < .01. (C) Neovascularization was examined in the region of the incision after the injection of Alde-Low EPCs. Newly formed vessels were analyzed for the detection of GFP-positive Alde-Low EPCs (GFP; top) and whole vessel formation by TRIC-conjugated lectin (GFP-TRIC-Lectin; bottom). The colocalization of GFP-positive EPCs and TRIC-positive fine capillaries can be observed (GFP-TRIC-Lectin, arrowheads). The numbers of GFP-positive HUVECs, Alde-High EPCs, and Alde-Low EPCs that had migrated to the dorsal skin were scored on the seventh day after surgery (left). *P < .05. Bar indicates 100 μm.
Alde-Low EPCs were curative of ischemic tissue. (A) Skin incisions were created on the dorsal surfaces of mice, and PBS alone, HUVECs, Alde-High EPCs, or Alde-Low EPCs were injected (top and bottom photographs/each condition). The effectiveness of ECs to recover tissue from ischemia was examined on day 7 following surgery. (B) The necrotic regions in the 4 types of mice (n = 3) were measured. Note that the area of necrosis was smallest in the mice injected with Alde-Low EPCs. Gray bar indicates PBS; striped bar, HUVECs; black bars Alde-High EPCs; and white bar, Alde-Low EPCs. **P < .01. (C) Neovascularization was examined in the region of the incision after the injection of Alde-Low EPCs. Newly formed vessels were analyzed for the detection of GFP-positive Alde-Low EPCs (GFP; top) and whole vessel formation by TRIC-conjugated lectin (GFP-TRIC-Lectin; bottom). The colocalization of GFP-positive EPCs and TRIC-positive fine capillaries can be observed (GFP-TRIC-Lectin, arrowheads). The numbers of GFP-positive HUVECs, Alde-High EPCs, and Alde-Low EPCs that had migrated to the dorsal skin were scored on the seventh day after surgery (left). *P < .05. Bar indicates 100 μm.
The extent of neovascularization in the ischemic tissue after the injection of ECs was analyzed by tracking the EPCs or HUVECs with coexpressed GFP and fluorescent microscopy (Figure 5C). A greater number of GFP-positive cells was observed in mice injected with Alde-Low EPCs compared to those injected with Alde-High EPCs or HUVECs (Figure 5C). We also found that some GFP-positive Alde-Low EPCs had incorporated into capillary blood vessels in the skin (Figure 5C). These results clearly demonstrate that Alde-Low EPCs are more functional in the improvement of neovascularization and tissue repair in the mouse skin flap model.
Migration assay in ischemic tissue
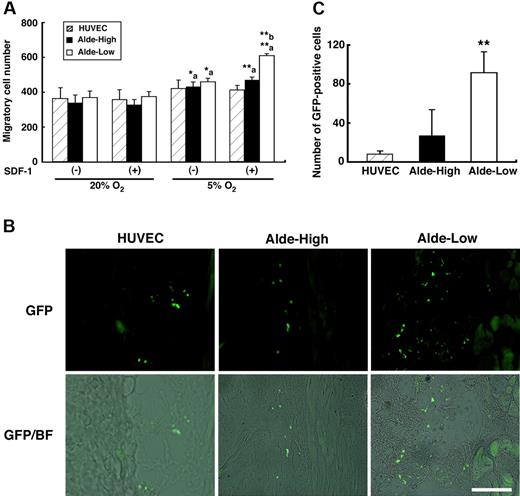
The homing of EPCs following intravascular administration is an important process for their incorporation into ischemic tissue. We observed that the expression of CXCR4, one of the homing receptors, is up-regulated in Alde-Low EPCs under hypoxic conditions (Figure 4A-B), suggesting that Alde-Low EPCs may possess a greater ability for migration toward ischemic tissue than Alde-High EPCs. In order to assess the ability for migration, we cultured and HUVECs, Alde-High EPCs, and Alde-Low EPCs by using transwells in the absence and presence of SDF-1 (Figure 6A). We found that the ability for migration was significantly increased in hypoxia (5% O2) compared to that in normoxia in both Alde-High and Alde-Low EPCs, but not in HUVECs. Notably, when SDF-1 was added in lower chambers under hypoxic conditions, the migration of Alde-Low EPCs was significantly greater than that of Alde-High EPCs (Alde-High: 471.3 ± 15.3 cells per well vs Alde-Low: 610 ± 10.8 cells per well, P < .01 [n = 3]).
Alde-Low EPCs are highly migratory. (A) Migration assay by using transwell culture system. The asterisk ‘a’ represent the significant increase of EPC migration under hypoxic conditions (5% O2) compared to those under normoxic conditions (20% O2). The asterisk ‘b’ represents the greater migratory ability of Alde-Low EPCs compared that of Alde-High EPCs under hypoxia in the presence of SDF-1. Striped bar represents HUVECs, black bar represents Alde-High EPCs, and white bar represents Alde-Low EPCs. *P < .05; **P < .01. (B) Skin incisions were made on the dorsal surfaces of mice and HUVECs (left), Alde-High EPCs (middle), or Alde-Low EPCs (right) were injected. The ability of ECs to migrate was examined within 24 hours after the surgery. Bar indicates 100 μm. (C) The numbers of migrated GFP-positive cells in the skin were scored using fluorescent microscopy. Three mice injected with either HUVECs, Alde-High EPCs, or Alde-Low EPCs were used for estimating the extent of EC migration and the mean ± SD was obtained. The striped bar represents HUVECs, the black bar represents Alde-High EPCs, and the white bar represents Alde-Low EPCs. *P < .05.
Alde-Low EPCs are highly migratory. (A) Migration assay by using transwell culture system. The asterisk ‘a’ represent the significant increase of EPC migration under hypoxic conditions (5% O2) compared to those under normoxic conditions (20% O2). The asterisk ‘b’ represents the greater migratory ability of Alde-Low EPCs compared that of Alde-High EPCs under hypoxia in the presence of SDF-1. Striped bar represents HUVECs, black bar represents Alde-High EPCs, and white bar represents Alde-Low EPCs. *P < .05; **P < .01. (B) Skin incisions were made on the dorsal surfaces of mice and HUVECs (left), Alde-High EPCs (middle), or Alde-Low EPCs (right) were injected. The ability of ECs to migrate was examined within 24 hours after the surgery. Bar indicates 100 μm. (C) The numbers of migrated GFP-positive cells in the skin were scored using fluorescent microscopy. Three mice injected with either HUVECs, Alde-High EPCs, or Alde-Low EPCs were used for estimating the extent of EC migration and the mean ± SD was obtained. The striped bar represents HUVECs, the black bar represents Alde-High EPCs, and the white bar represents Alde-Low EPCs. *P < .05.
We then examined the ability for migration in vivo by injecting HUVECs, Alde-High EPCs, and Alde-Low EPCs into the mice treated with the FLAP surgery (Figure 6B-C). Consistent with the results from transwell cultures, the number of GFP-positive EPCs was significantly higher in Alde-Low EPCs compared to Alde-High EPCs and HUVECs in the dorsal skin 24 hours after injection (HUVECs, 7.7 ± 3.6; Alde-High, 27.2 ± 26.3 cells per field; Alde-Low, 91.6 ± 21.5 cells per field; Alde-High vs Alde-Low, P < .05 [n = 3]). These data specify that Alde-Low EPCs can recruit to ischemic tissue more effectively than Alde-High EPCs and hence a larger number of EPCs can contribute to neovascularization in ischemic tissue.
Discussion
Neovascularization in adults is recognized in the process of angiogenesis involving the recruitment of pre-existing ECs in ischemic tissues. Recent studies suggest that BM-derived EPCs also play an essential role in the process of vasculogenesis. EPCs derived from PB, UCB, and BM have been shown to be involved in neovascularization during vascular injury, ischemia, and tumor growth.30,31 So far, the clinical applications for EPC transfusion are limited because of the small number of available cells.7 UCB has an advantage over PB and BM as a source of EPCs, because of its accessibility and higher EPC content.32 The aim of this study was to characterize UCB-derived EPCs and investigate their potential use in therapeutic vasculogenesis. We found that UCB-derived EPCs can be separated into 2 distinct phenotypes on the basis of ALDH activity. Furthermore, EPCs with low ALDH activity possess a higher proliferative potential and ability to migrate than those with high ALDH activity. Finally, we showed that EPCs with low ALDH activity display a greater ability to regenerate ischemic tissue in vivo.
Currently, the concepts of EPCs have become increasingly complex and confusing. The discovery of a complete hierarchy of EPCs within the vessel wall could provide an alternative explanation for the widespread distribution of EPCs.12,33 So far, the state of maturation of ECs is defined by the expression of endothelial markers, including VEGF receptor-2 (VEGFR-2, KDR), vascular endothelial-cadherin (VE-cadherin), von Willebrand factor (VWF), CD31, CD34, and CD146. However, none of these surface markers could be used as a potential marker for isolating functionally active EPCs. For instance, CD133 is recognized as a cell surface marker associated with progenitor cells in several cell lineages, including hematopoietic, endothelial, and neural tissues.34,35 While the expression of CD133 is used as a marker for EPCs derived from BM,32 a recent study demonstrated that CD133 is barely expressed in EPCs derived from UCB.12 CD34 expression is commonly used as a marker for isolating circulating endothelial cells (CECs).32 Although the surface antigens CD45 and CD14 are generally used as markers for hematopoietic cells, the expression of these markers was observed in “early EPCs” after short-term in vitro culturing.10,36,37 However, a recent study has revealed that CD45− and CD14+ “colony forming unit–endothelial cells (CFU-ECs)” differentiate into macrophages and fail to participate in the process of vasculogenesis in vivo.11 Collectively, these studies show that the expression of surface antigens is not identical among EPCs from different sources and may not be specific to ECs. Therefore, it may be difficult to find a simple and solid procedure for the isolation of functional EPCs founded on the expression of cell surface markers.
In this regard, we propose that ALDH activity instead may serve as an excellent marker for isolating EPCs from UCB for clinical cell therapy for the following reasons. In contrast to our observation that the expression of CD34 declines during the culturing of DiI-Ac-LDL–positive/CD45−/CD31+ cells, ALDH activity was retained in Alde-Low and Alde-High EPCs from UCB even after several passages (data not shown). Thus, ALDH activity may prove to be a solid and reproducible marker for purifying a specific subfraction of EPCs for clinical cell therapy. In addition, our staining procedure can be easily adopted without excessive cellular manipulation. Finally, since Aldefluor does not intercalate into DNA,38 selection by ALDH activity appears to be a relatively safe process compared to that utilizing DNA-bound fluorochromes. However, because of its low frequency in UCB, Alde-Low cells need to be cultured in vitro to obtain sufficient number of cells for transplantation. This could be a cause of hesitation for clinical use, unless this system has a significant advantage in transplantation efficiency compared to the existing procedures. Additional in vivo transplantation experiments comparing various procedures should be examined in our future studies.
Ingram et al illustrated that EPCs can be separated into a highly proliferative population, as well as other populations, by exploiting single cell assays.12 They also demonstrated that the cell surface markers expressed on UCB-derived EPCs did not differ significantly from those of HUVECs, suggesting that the proliferative activity of EPCs is a key factor in evaluating the maturation of EPCs.33 Indeed, we did not observe a major difference in the expression of cell surface markers between Alde-High and Alde-Low EPCs. On the other hand, compared to Alde-High EPCs, Alde-Low EPCs had a greater growth potential in both normoxia and hypoxia. These data suggest that Alde-High EPCs are more mature EPCs than Alde-low EPCs, and that ALDH activity is a unique marker for evaluating the state of maturation of EPCs. Further studies are required to determine the exact status of maturation of Alde-High and Alde-Low EPCs.
It is interesting that Alde-Low EPCs failed to form fine capillary networks in vitro, while injection of these cells significantly enhanced neovascularization in vivo. Our data of the expression of HIF target genes may, at least in part, explain the discrepancy between the in vitro and in vivo studies. It is of note that the expression of VEGF164 mRNA is significantly greater in Alde-Low cells compared to Alde-high and control cells under hypoxia (Figure 4B). Because the expression of KDR, a receptor for VEGF, showed only a small increase in Alde-Low cells compared to Alde-high cells (Figure 4B), high level of VEGF in Alde-Low cells may not be able to induce tube formation in an autocrine manner in vitro. In contrast, VEGF from Alde-Low EPCs could enhance proliferation of endothelial cells in pre-existing vessels in ischemic tissues and thereby induce neovascularization in vivo. The other point is that the expression of CXCR4 was significantly increased in Alde-Low EPCs compared to Alde-High and control cells under hypoxia (Figure 4B). SDF-1, a ligand for CXCR4, has been proven to stimulate the recruitment of EPCs to the site of ischemia.39 In addition, overexpression of SDF-1 increases the homing and incorporation of EPCs into ischemic tissue.39,40 In fact, we have demonstrated that Alde-Low EPCs have greater migratory activity in vitro compared to Alde-high EPCs (Figure 6A). Collectively, the homing process may be a critical determinant of neovascularization in ischemic tissue in our mouse flap model, while this may not be an advantage to form vascular tubes in the Matrigel.
A recent report demonstrated that ALDH activity is a promising marker for isolating HSCs with a high capability of repopulation. Remarkably, the study showed that hematopoietic lineage-negative cells with high ALDH activity (ALDHhiLin−) displayed a more efficient repopulating function in vivo.19 Furthermore, the ALDH activity correlated well with the expression of primitive HSC phenotypes such as CD34+CD38− and CD34+CD133+.19,20 Therefore, the study proposed that cell isolation based on ALDH activity in combination with lineage deprivation can be carried out as the established procedure according to the cell surface markers.13,19,20 In contrast to the case of HSCs, no suitable surface marker has been proposed to separate immature and mature EPCs that can be used in combination with ALDH activity. Moreover, because the peak of Aldefluor intensity is close between Alde-High and Alde-Low cells, it is difficult to separate the 2 populations clearly only with the ALDH activity. We consider that the used of additional marker(s) may improve the precision of isolating Alde-Low EPCs, which are currently under investigation.
In summary, the isolation of EPCs from UCB using low ALDH activity has been unearthed by our study as an efficient, safe, and reproducible method for obtaining highly proliferative and migratory EPCs. This method of isolation of such EPCs should prove promising in therapeutic neovascularization for regenerating ischemic tissue. Further assessment is requisite for full comprehension of the functional properties of EPCs following transfusion.
Acknowledgments
We thank Tania O'Connor for critical reading of the paper.
This work was supported by a grant from SORST, the Japan Science and Technology Agency.
Authorship
Contribution: M.N. designed the research and analyzed the data; T.Y. performed the research; H.H., K.O., K.K., T.K., and H.Y. contributed a new approach for the isolation of EPCs; M.S. provided reagents; M.N. and K.O. wrote the paper; and O.O. designed the research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Osamu Ohneda, Department of Regenerative Medicine, University of Tsukuba, 1–1-1 Tennoudai, Tsukuba 305-8575, Japan; e-mail: oohneda@md.tsukuba.ac.jp

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal