CD137 is a member of the TNFR-family with costimulatory function. Here we show that it also has many favorable characteristics as a surrogate marker for antigen-specific activation of human CD8+ T cells. Although undetectable on unstimulated CD8+ T cells, it is uniformly up-regulated 24 hours after stimulation on virtually all responding cells regardless of differentiation stage or profile of cytokine secretion, which circumvents limitations of current surrogate markers for defining the repertoire of responding cells based on only individual functions. Antibody-labeled responding CD137+ cells can be easily and efficiently isolated by flow sorting or magnetic beads to substantially enrich antigen-specific T cells. To test this approach for epitope discovery, we examined in vitro priming of naive T cells from healthy donors to Wilms tumor antigen 1 (WT1), a protein overexpressed in various malignancies. Two overlapping pentadecamers were identified as immunogenic, and further analysis defined WT1(286–293) as the minimal amino acid sequence and HLA-Cw07 as the HLA restriction element. In conclusion, this approach appears to be an efficient and sensitive in vitro technique to rapidly identify and isolate antigen-specific CD8+ T cells present at low frequencies and displaying heterogeneous functional profiles, and does not require prior knowledge of the specific epitopes recognized or the HLA-restricting elements.
Introduction
Methods to assess antigen-specific CD8 T cells are essential for understanding cellular immunity under normal and pathologic conditions. Peptide/MHC (pMHC) multimers1 accurately detect antigen-specific T cells, but the knowledge of the immunogenic peptide, its restricting HLA allele, and the availability of the reagent are limiting and rarely sufficient to characterize the breadth of responding cells. Evaluating the repertoire of responses has required identifying—and often subsequently isolating—antigen-specific T cells using assays that focus on specific functions such as production of a particular cytokine or degranulation in response to antigen.2,3 However, such functional assays often reflect only a subset of specifically activated cells, as the in vivo CD8+ T-cell response is composed of functionally diverse cells that cannot be identified using a single cytokine or the detection of degranulation.4,5 For example, different T-cell subpopulations, such as naive T cells, central memory T cells, or T cells primed under Th1- or Th2-like conditions, show very heterogeneous cytokine profiles, and can therefore be tracked only functionally using multicytokine flow cytometry.6 A surface molecule that is uniformly up-regulated in response to antigen would therefore facilitate the assessment of the complete antigen-specific response. Favorable characteristics of such a surrogate marker would include specific surface expression after activation over a transient but sufficiently prolonged time period to allow reliable detection, and absent expression if unstimulated and during resting phases. Such an expression pattern would facilitate not only the identification but also the selection of viable, activated T cells using fluorescence-activated cell sorting or magnetic beads bound to the specific antibody.
CD137 (4–1BB) was originally identified as a molecule expressed on activated mouse and human CD8+ and CD4+ T cells.7,–9 It is a member of the TNFR family and mediates costimulatory and antiapoptotic functions, promoting T-cell proliferation and T-cell survival.10,11 CD137 has been reported to be up-regulated—depending on the T-cell stimulus—from 12 hours to up to 5 days after stimulation.9,10,12 Antibody-mediated blockade of the CD137/CD137L interaction has been shown to increase survival of murine allografts,13 and depletion of human CD137+ donor-derived T cells after stimulation with allogeneic recipient cells in vitro has recently been described as an approach to reduce alloreactivity.14 Here we demonstrate that CD137 has many characteristics required for efficient positive selection of specifically activated CD8+ T cells with a heterogeneous functional profile, including memory and naive responses to viral-associated (cytomegalovirus [CMV] and hepatitis C virus [HCV] related) and tumor-associated self-antigens such as MART1/Melan-A15 and WT1, a transcription factor overexpressed in many malignancies and a potential target for immunotherapy.16,17 Furthermore, this approach for positive selection of responding cells appears useful for identifying and validating T-cell responses to unknown antigens or epitopes, as evidenced by our studies characterizing a novel epitope in WT1.
Materials and methods
Peripheral blood mononuclear cells (PBMCs) from healthy donors were harvested by leukopheresis after informed consent in accordance with the Declaration of Helsinki. Institutional review board (IRB) approval for these studies was granted by the IRB of the Fred Hutchinson Cancer Research Center. T-cell cultures were maintained in RPMI 1640 medium supplemented with 12.5 mM HEPES, 4 mM l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin (Invitrogen, Carlsbad, CA), 50μM β-mercaptoethanol (Sigma, St Louis, MO), and 10% human serum. Melanoma cell lines A375 (gift from S. Rosenberg, NCI, Bethesda, MD) and Mel526 (gift from M. Lotze, University of Pittsburgh, PA) were maintained in RPMI 1640 with 25 mM HEPES, 4 mM l-glutamine, 50 U/mL penicillin, 50 mg/mL streptomycin, 10 mM sodium pyruvate, 1 mM nonessential amino acids, and 10% FBS (HyClone, Logan, UT). Both lines express the HLA-A2 allele, but only Mel526 expresses the MART-1 Ag. The T2 cell line is a TAP-deficient T-cell/B-cell hybrid expressing the HLA-A2 allele.
Peptides
The HLA-A*0201–restricted peptides representing defined epitopes from Melan-A(26-35L) (ELAGIGILTV), WT1(126-134) (RMFPNAPYL), HIV-1/gag/p17(77-85) (SLYNTVATL), CMV/pp65(495-503) (NLVPMVATV), and CMV/pp65(417-426) (TPRVTGGGAM) were synthesized by Synpep (Dublin, CA). HCV/NS3(1406-1415) (KLVALGINAV) was synthesized by Proimmune (Oxford, United Kingdom). A peptide library spanning WT1, consisting of 15-mers overlapping by 11, was purchased from JPT (Berlin, Germany).
Induction of T-cell lines
T-cell lines were generated as previously described with minor modifications.18 Briefly dendritic cells (DCs) were derived from adherent monocytes by culture for 5 days in Cellgenix DC Medium (Cellgenix, Freiburg, Germany) supplemented with 1% human serum, 800 IU/mL GM-CSF (Berlex, Seattle, WA), and 1000 IU/mL IL-4 (R&D Systems, Minneapolis, MN). On day 3, 1.5 mL fresh medium, supplemented with 1600 IU/mL GM-CSF and 1000 IU/mL IL4, was added to each well. On day 5, cells were harvested and matured for 24 hours in fresh DC medium supplemented with a cytokine cocktail of 800 IU/mL GM-CSF, 1000 IU/mL IL-4, 10 ng/mL LPS (derived from E coli 055:B5; Sigma), and 50 IU/mL IFNγ (Intermune, Brisbane, CA). During the maturation phase, DCs were pulsed with 10 μg/mL peptide. For experiments using the peptide library, each individual peptide was at a concentration of 1.8 μg/mL.
Naive CD8+ T cells were obtained by depleting CD45RO+ cells from PBMCs using anti-CD45RO microbeads (Miltenyi Biotec, Auburn, CA), followed by positive selection using anti-CD8 microbeads. This approach resulted in more than 95% pure CD45RO−CD8+ populations.
Mature, peptide-pulsed DCs were washed and incubated with CD8+ cells at a ratio of 1:2-1:10 in complete T-cell medium supplemented with 100 μM 1-methyltryptophan (Sigma) and each was distributed in 96-well v-bottom plates or 24-well-plates (2 × 105 to 5 × 105 T cells/well, respectively). On day 4, IL-7 and IL-15 (final concentration (f.c.) 5 ng/mL; R&D Systems) were added with medium. Cell lines were supplemented every 2 to 3 days with medium and cytokines.
For restimulation, autologous PBMCs were pulsed with peptide (10 μg/mL) for 2 hours and irradiated (30 Gy). Each T-cell line was harvested, resuspended in medium without cytokines, and mixed with 1 × 106 PBMCs in a 6-well plate. Twenty-four hours later, 2 mL medium was added containing cytokines (f.c. 50 IU/mL IL-2, 5 ng/mL IL-7, and 5 ng/mL IL-15). T-cell cloning was performed as described recently.18
Immunophenotyping and pMHC-multimer staining
Antibodies used for immunophenotyping include anti–CD8-FITC or -PE, anti–CD69-PE, anti–CD25-PE or -FITC, anti–CD137-PE or -APC, anti–CD134-PE, anti–HLA-DR-PE, and anti–CD38-PE (all from BD Pharmingen, San Diego, CA). pMHC-multimer staining was performed using APC-labeled pMHC multimers (Proimmune) and incubating the cells for 20 minutes at room temperature (RT), followed by 20-minute incubation at RT with anti-CD8 antibody. Dead cells were excluded using 7-AAD (BD Pharmingen).
Intracellular cytokine staining and CD107a mobilization shift assay
The assay was performed as described.3,19 Briefly, 2 × 105 T cells were stimulated for 5 hours in 96-well plates using 4 × 105 peptide-pulsed T2 cells or DCs. CD107a-FITC (BD) and 10 μg/mL brefeldin A (Sigma) were added at the beginning of the stimulation period. After 5 hours, cells were stained for CD107a and costained for CD8. Cells were fixed, permeabilized, and stained with antibodies against IFNγ, TNFα, or IL-2 (BD), using FIX/PERM and PERM/Wash solution (BD).
CD137 enrichment assay, IFNγ-secretion assay, and chromium release assay
T-cell lines were generated from naive T cells as described in “Introduction.” Twenty-four hours after restimulation, T cells were washed and stained for 20 minutes on ice with anti–CD137-APC (5 μL/100 μL) (BD). After washing, the cells were incubated with anti–APC-microbeads (Miltenyi Biotec) and selected using MS or LS columns.
The IFNγ-secretion assay (Miltenyi Biotec) was performed as described by the manufacturer. The peptide stimulus was the same as for CD137 enrichment, with a stimulation period of 4 hours to detect peak IFNγ responses. The cells were allowed to secrete IFNγ for 45 minutes, and then cooled in the appropriate volume of cold buffer. All further procedures were performed on ice.
Chromium release assays were performed as described elsewhere.18
Statistics
Pearson correlation (2-tailed) for data derived from 2 methods was calculated using GraphPadPrism software (GraphPad, San Diego, CA). For each analysis, the P value and the coefficient of determination (r2) are indicated.
Results
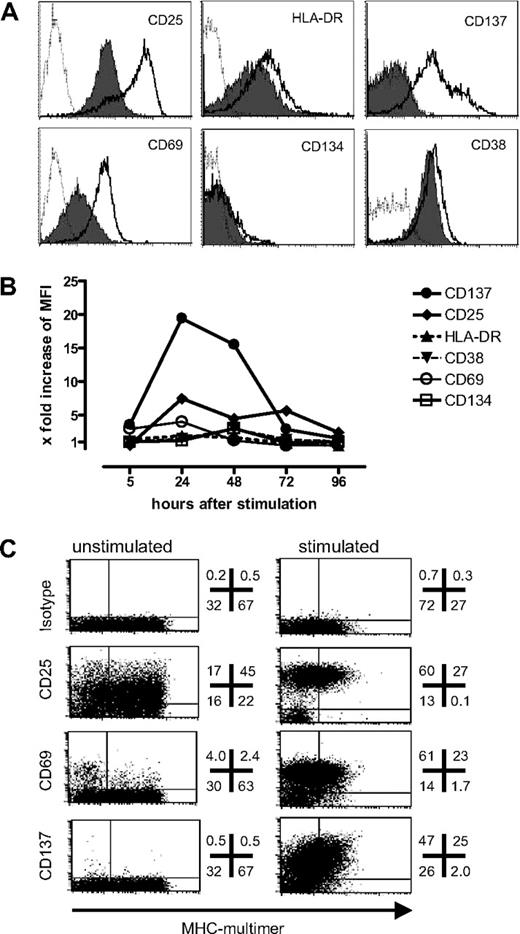
To identify surface markers that could serve as surrogate markers for functional activation, a T-cell clone specific for the heteroclitic HLA-A*0201–restricted epitope of MelanA/MART1(26–35L) that had entered a resting phase at the end of a 14-day expansion cycle was stimulated with peptide-pulsed T2 cells as APCs. Twenty-four hours after stimulation, CD69, CD25, CD137, CD38, and HLA-DR were all up-regulated (Figure 1A). Among these, CD69, CD25, and CD137 exhibited a large enough shift in fluorescence intensity to permit separation of activated cells from unstimulated cells. CD137 expression was increased after 5 hours, peaked by 24 hours, and then gradually declined during the period from 48 to 72 hours (Figure 1B). Among the other markers, only CD25 showed similar kinetics to CD137, whereas expression of CD69, CD38, and HLA-DR was more variable, usually peaking after 6 hours but then quickly returning to baseline levels. CD134 was still negative at 24 hours, but increased slightly by 48 hours. Among the 3 markers that were up-regulated sufficiently for specific detection—CD25, CD137, and CD69—only CD137 was not expressed on unstimulated, resting T cells, which is essential if the marker is to be used for a bead-based selection method. Similar results were obtained for 5 different T-cell clones with various specificities (data not shown).
Expression level and kinetics of activation markers after antigen-specific activation. To evaluate a panel of activation markers, a T-cell clone specific for the HLA-A*0201–restricted epitope of Melan-A(26-35L) was stimulated with peptide-pulsed T2 cells, and assessed for (A) antigen-specific up-regulation of denoted markers 24 hours after stimulation (thick lines) compared with control peptide (filled) or isotype-matched antibodies (dotted line); and (B) expression kinetics of the panel of markers in the same experiment (x-fold increase: MFI of the specifically stimulated sample/MFI of the sample stimulated with the irrelevant control peptide). (C) A polyclonal Melan-A–specific T-cell line generated by 2 in vitro stimulations was assessed for baseline expression and specific up-regulation 24 hours after stimulation of the 3 most informative activation markers (numbers indicate the percentage of viable CD8+ T cells in each quadrant).
Expression level and kinetics of activation markers after antigen-specific activation. To evaluate a panel of activation markers, a T-cell clone specific for the HLA-A*0201–restricted epitope of Melan-A(26-35L) was stimulated with peptide-pulsed T2 cells, and assessed for (A) antigen-specific up-regulation of denoted markers 24 hours after stimulation (thick lines) compared with control peptide (filled) or isotype-matched antibodies (dotted line); and (B) expression kinetics of the panel of markers in the same experiment (x-fold increase: MFI of the specifically stimulated sample/MFI of the sample stimulated with the irrelevant control peptide). (C) A polyclonal Melan-A–specific T-cell line generated by 2 in vitro stimulations was assessed for baseline expression and specific up-regulation 24 hours after stimulation of the 3 most informative activation markers (numbers indicate the percentage of viable CD8+ T cells in each quadrant).
As CD25, CD137, and CD69 were most informative, their expression was next analyzed in a Melan-A(26–35L)–specific T-cell line to assess baseline expression and up-regulation in a polyclonal population, generated by 2 rounds of stimulation. Twenty-four hours after the third stimulation the cells were stained using PE-labeled mAbs for the respective markers and costained with anti-CD8-antibody and the pMHC multimer. As expected, TCR down-regulation in response to antigen hampered pMHC-multimer staining, with fewer multimer+ cells and lower intensity staining detected at 24 hours compared with before stimulation (Figure 1C), making this unreliable for tracking antigen-specific cells after activation. All 3 markers were strongly up-regulated on a large fraction of cells. However, similar to what had been observed with clones, baseline expression was minimal for CD137, whereas low or intermediate expression for CD69 and CD25, respectively, was observed in unstimulated cells (Figure 1C). In a parallel set of samples, triple staining for CD25, CD69, and CD137 was performed. CD137+ cells were nearly uniformly triple positive (96%), whereas gating on CD69+ or CD25+ cells defined more heterogeneous populations with 18% and 17%, respectively, negative for CD137, which may represent nonspecifically stimulated cells in culture expressing the observed higher baseline levels of CD69 and CD25 (data not shown). Of note antigen-independent up-regulation of CD137 in response to IL-15, as has been reported with high doses (100 ng/mL),20 was not observed in the culture systems used for this study, as a lower more physiologic dose (5 ng/mL) of IL-15 was routinely used. Thus, CD137 appeared to have the most favorable expression profile among the markers tested for identifying specifically activated CD8+ T cells.
CD137 expression correlates with functional activation of CD8 T-cell clones and lines
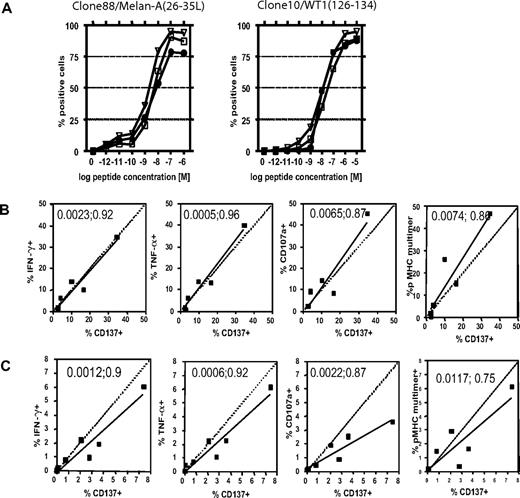
As CD137 up-regulation is a function of T-cell activation, the threshold for inducing CD137 expression was compared with IFNγ and TNFα production after stimulation with titrated amounts of the cognate antigen. Two T-cell clones, clone 88 specific for Melan-A(26-35L) and clone 10 specific for WT1(126–134), were stimulated with T2-cells pulsed with titrated amounts of peptide. For optimal results, these functional assays require different incubation times—therefore samples were set-up in parallel and cytokine production assessed after 5 hours and CD137 expression determined after 24 hours of incubation. The cells showed comparable activation thresholds for cytokine production and CD137 expression, with similar peptide concentrations necessary to attain half-maximal activation (Clone 88 with an EC50 [M] for CD137 = 2.5 × 10−9, IFNγ = 3.7 × 10−9, and TNFα = 1.5 × 10−9; Clone 10 with an EC50 [M] for CD137 = 9.5 × 10−9, IFNγ = 2.0 × 10−8, and TNFα = 9.2 × 10−9) (Figure 2A). The correlation between cytokine production and CD137 expression was highly significant (Clone 88 with [P/r2] for CD137 vs IFNγ = P < .001/0.99 and CD137 vs TNFα = P < .001/0.98; Clone 10 with [P/r2] for CD137 vs IFNγ = P < .001/0.97 and CD137 vs TNFα = P < .001/0.98).
CD137 up-regulation as a surrogate marker for specifically activated CD8+ T cells in vitro and ex vivo. The activation threshold for several markers of response was compared in responding T-cell clones (A), T-cell lines (B), or memory T cells directly ex vivo (C). (A) Two HLA-A*0201–restricted T-cell clones, specific for either Melan-A(26-35L) or WT1(126-134), were stimulated with titrating amounts of peptides. IFNγ (squares) and TNFα (triangles) production was assessed after 5 hours of stimulation in the presence of brefeldin A, and CD137 expression (circles) was determined 24 hours after stimulation (note: these reflect optimal time points for detecting each of these events). (B) Six separate T-cell lines specific for the NS3(1406-1415)-epitope of HCV were generated, and assessed for cytokine production and degranulation after 5 hours of stimulation, and CD137 expression after 24 hours and pMHC-multimer staining of the unstimulated sample. Pearson correlation (2-tailed) was calculated, and the P value and the coefficient of determination (r2) appear in each plot. (C) PBMCs from 7 CMV+/HLA-A*0201+ donors were thawed, rested overnight, and analyzed for pMHC multimer staining, cytokine production, and degranulation after 5 hours of stimulation and CD137 expression at 24 hours. P value; r2 appear in each plot.
CD137 up-regulation as a surrogate marker for specifically activated CD8+ T cells in vitro and ex vivo. The activation threshold for several markers of response was compared in responding T-cell clones (A), T-cell lines (B), or memory T cells directly ex vivo (C). (A) Two HLA-A*0201–restricted T-cell clones, specific for either Melan-A(26-35L) or WT1(126-134), were stimulated with titrating amounts of peptides. IFNγ (squares) and TNFα (triangles) production was assessed after 5 hours of stimulation in the presence of brefeldin A, and CD137 expression (circles) was determined 24 hours after stimulation (note: these reflect optimal time points for detecting each of these events). (B) Six separate T-cell lines specific for the NS3(1406-1415)-epitope of HCV were generated, and assessed for cytokine production and degranulation after 5 hours of stimulation, and CD137 expression after 24 hours and pMHC-multimer staining of the unstimulated sample. Pearson correlation (2-tailed) was calculated, and the P value and the coefficient of determination (r2) appear in each plot. (C) PBMCs from 7 CMV+/HLA-A*0201+ donors were thawed, rested overnight, and analyzed for pMHC multimer staining, cytokine production, and degranulation after 5 hours of stimulation and CD137 expression at 24 hours. P value; r2 appear in each plot.
The HLA-A*0201–restricted heteroclitic Melan-A/MART1(26-35L) epitope is unique in that a fairly high precursor frequency of naive antigen-specific T cells in healthy humans can often be found.15 Therefore, to examine a prototypic in vitro response with a more typical precursor frequency, responses to an A*0201-restricted epitope derived from the nonstructural protein 3 of the hepatitis C virus (NS3(1406–1415))21 were studied in cell lines derived from healthy, HCV seronegative humans. To compare CD137 expression with other standard assays used for identification of activated T cells in this setting of lower frequency responses, cytokine flow cytometry (CFC) and the CD107a-mobilization shift assays to assess degranulation3 were performed in parallel. Excellent correlations of CD137 expression were found with CFC data (CD137 vs IFNγ [P;r2] = .002;0.92 and CD137 vs TNFα [P;r2] = .001;0.96), as well as with the CD107a mobilization shift assay (CD137 vs CD107a [P;r2] = .007;0.87) and pMHC multimer staining (CD137 vs pMHC multimer [P;r2] = .007;0.86) (Figure 2B).
We next examined if CD137 could serve as a surrogate marker for antigen-specific activation to assess the response of memory CD8+ T cells in PBMCs ex vivo. PBMCs from 7 HCMV-seropositive, HLA-A*0201–positive donors were thawed, rested overnight, and then stimulated to compare functional responses to the immunodominant epitope of pp65(495–503). Again, CD137 expression correlated with cytokine production ([P;r2] for IFNγ = .001;0.9 and TNFα = .001;0.92), degranulation ([P;r2] for CD107a = .002;0.87) and pMHC multimer staining ([P;r2] .012;0.75) (Figure 2C), demonstrating that CD137 can be used as a surrogate marker to detect direct activation of responding cells in PBMCs. Of note, proliferation of stimulated antigen-specific cells had not commenced before the time of CD137 staining, as determined by CFSE labeling (data not shown), affirming that the results reflect the number of antigen-specific cells capable of responding in PBMCs detected by each assay.
Viable CD137+ T cells can be easily isolated and are antigen-specific
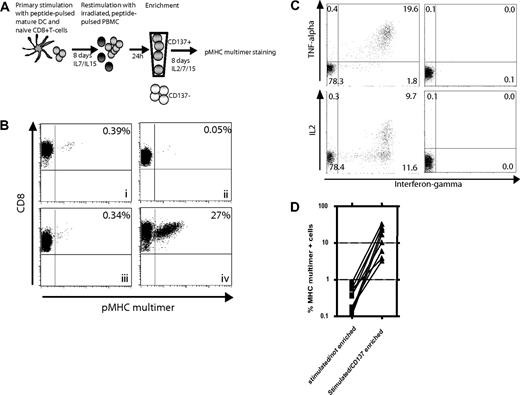
Without knowledge of the recognized epitope and an available tetramer, the isolation and expansion of T cells specific for a selected target or antigen for either analysis or use in adoptive therapy can be difficult. In initial experiments, we were unsuccessful in generating robust manipulable responses to a panel of candidate peptides derived from the weakly immunogenic WT1 antigen, using the IFNγ-secretion assay to isolate cells (data not shown). Therefore, as an alternative strategy, we examined selection of cells that demonstrated up-regulation of CD137 after antigen stimulation for enrichment of responding T cells from T cell cultures containing an initially low frequency of antigen-specific T cells. Before assessing this technique for epitope discovery of unknown antigens, as described later for WT1, we wanted to validate the approach using a model foreign antigen known to induce responses. A polyclonal T cell response was generated against the HCV/NS3(1406-1415) epitope by stimulation of naive CD8+ T cells from an HCV-seronegative donor with peptide-pulsed DCs, and cultured for 1 week in medium containing IL-7 and IL-15 as previously described.18 CD45RO-CD8+ cells were routinely used as the naive starting population, but a similar T-cell response against this epitope could also be induced using CD62L+CD45RO−CD8+ T cells or CD45RA+CCR7+CD8+ T cells. In contrast, HCV/NS3(1406-1415)-specific T cells could not be generated from CD45RO+ T cells (data not shown). The primed cells were restimulated and enriched for CD137+ cells 24 hours later using magnetic beads, and placed back in cytokine-containing medium for 8 days to allow expansion and re-expression of the TCR before being analyzed using pMHC multimers (Figure 3A). The frequency of HCV/NS3(1406-1415)-specific T cells in fresh PBMCs from seronegative, healthy donors, as assessed by pMHC-multimer staining, is below the limits of detection (not shown), but a small percentage (0.39%) of pMHC-multimer+ cells was detectable 8 days after primary stimulation (Figure 3Bi). After a second stimulation, no significant enrichment of this small population of reactive cells was detectable (Figure 3Biii), which is not uncommon for low-frequency responses presumably due to cell death of some of the specific T cells and cytokine-mediated expansion of nonspecific bystander T cells. However more reactive cells were present than if the line remained in culture and was not restimulated with antigen (Figure 3Bii; 0.34% vs 0.05%). In contrast, if enriched after restimulation and expanded for 1 week, 27% of the cells were pMHC-multimer+ T cells (Figure 3Biv), representing a 79-fold enrichment compared with cells not enriched and a 9-fold absolute expansion in antigen-specific cell numbers (Table 1). Adding the antibody to the cells, without enrichment, did not have a measurable stimulatory effect (data not shown). To demonstrate that the enriched T cells were functional, intracellular cytokine staining in response to peptide was performed. More than 20% of the CD8 T cells (79% of the MHC multimer+ cells) produced IFNγ and most of them also produced TNFα. Almost 10% produced IL-2, representing 36% of the MHC multimer+ population (Figure 3C). The enrichment procedure was repeated for 9 individual T-cell lines and enrichment resulted in achieving frequencies of 3% to 33% (mean, 16.15%) (Figure 3D). The average enrichment, calculated from the pooled data of these experiments, was 74-fold over the stimulated but unenriched control. To assess reproducibility of the enrichment procedure, one T-cell line was generated as described above, restimulated and then split in 5 aliquots processed for enrichment in parallel. The mean percentage of pMHC multimer+ cells 1 week after enrichment was 8.6% with a standard error of 0.92% (data not shown). The procedure was also applicable for different peptide antigens (Melan-A(26-35L), HIV-1 gag p17(77-85)) and for T-cells lines after various2,–4 rounds of stimulation (data not shown).
Use of CD137 expression for enrichment of antigen-specific T cells. (A) Experimental outline: Naive CD45RO−CD8+ T cells were stimulated with peptide-pulsed DCs and cultured for 1 week with IL-7 and IL-15 added on day 4. On day 8, the cells were restimulated with irradiated, autologous, peptide-pulsed PBMCs. Twenty-four hours later, CD137+ cells were isolated with magnetic beads and cultured for an additional 8 days in medium containing IL-2, IL-7, and IL-15 to allow both expansion and re-expression of the TCR. The cells were then analyzed for specificity using pMHC multimers. (B) Enrichment of an HCV/NS3(1406-1415)-specific T-cell line by selection of CD137+ cells. Eight days after primary stimulation of naive T cells, an aliquot was stained with an NS3(1406-1415)/MHC multimer to determine the frequency of antigen-specific cells before restimulation and/or enrichment (i). These cells were then split and either cultured in IL-2–, IL-7–, and IL-15–containing medium without any restimulation (ii), restimulated and cultured but not enriched (iii), or restimulated and enriched for CD137+ cells 24 hours later and then cultured for an additional 8 days (iv), and then stained with anti-CD8 antibody and the pMHC multimer. (C) Parallel to the pMHC-multimer staining, an aliquot of the enriched T-cell population was also restimulated with peptide-pulsed T2 cells and cytokine production was assessed after 5 hours (left). T cells incubated with unloaded T2 cells served as control (right). (D) Nine different HCV/NS3-specific T-cell lines were generated and enriched 24 hours after the second stimulation as above, and the number of pMHC multimer+ T cells assessed 8 days later and compared with lines not enriched (2-tailed, paired t test: P = .002).
Use of CD137 expression for enrichment of antigen-specific T cells. (A) Experimental outline: Naive CD45RO−CD8+ T cells were stimulated with peptide-pulsed DCs and cultured for 1 week with IL-7 and IL-15 added on day 4. On day 8, the cells were restimulated with irradiated, autologous, peptide-pulsed PBMCs. Twenty-four hours later, CD137+ cells were isolated with magnetic beads and cultured for an additional 8 days in medium containing IL-2, IL-7, and IL-15 to allow both expansion and re-expression of the TCR. The cells were then analyzed for specificity using pMHC multimers. (B) Enrichment of an HCV/NS3(1406-1415)-specific T-cell line by selection of CD137+ cells. Eight days after primary stimulation of naive T cells, an aliquot was stained with an NS3(1406-1415)/MHC multimer to determine the frequency of antigen-specific cells before restimulation and/or enrichment (i). These cells were then split and either cultured in IL-2–, IL-7–, and IL-15–containing medium without any restimulation (ii), restimulated and cultured but not enriched (iii), or restimulated and enriched for CD137+ cells 24 hours later and then cultured for an additional 8 days (iv), and then stained with anti-CD8 antibody and the pMHC multimer. (C) Parallel to the pMHC-multimer staining, an aliquot of the enriched T-cell population was also restimulated with peptide-pulsed T2 cells and cytokine production was assessed after 5 hours (left). T cells incubated with unloaded T2 cells served as control (right). (D) Nine different HCV/NS3-specific T-cell lines were generated and enriched 24 hours after the second stimulation as above, and the number of pMHC multimer+ T cells assessed 8 days later and compared with lines not enriched (2-tailed, paired t test: P = .002).
CD8+ T cells, selected based on their expression of CD137, expand after in vitro culture
| Exp. no. . | Epitope . | % multimer+ before selection . | Total no. of selected cells, ×105 . | % multimer+ cells 1 wk after selection . | Absolute no. of cells after 1 wk, ×106 . | Absolute no. of antigen-specific cells,* ×106 . | Expansion factor, x-fold . |
|---|---|---|---|---|---|---|---|
| 1 | HCV(1406-1415) | 0.39 | 1.1 | 27 | 3.8 | 1.0 | 9.3 |
| 2 | HCV(1406-1415) | 1.6 | 6.0 | 61 | 8.1 | 4.9 | 8.2 |
| 3 | WT1(126-134) | 2.4 | 2.2 | 49 | 2.3 | 1.1 | 5.1 |
| 4 | MelanA(26-35L) | 25 | 4.5 | 72 | 4.5 | 3.2 | 7.2 |
| 5 | MelanA(26-35L) | 8 | 1.4 | 87 | 2.2 | 1.9 | 13.7 |
| Exp. no. . | Epitope . | % multimer+ before selection . | Total no. of selected cells, ×105 . | % multimer+ cells 1 wk after selection . | Absolute no. of cells after 1 wk, ×106 . | Absolute no. of antigen-specific cells,* ×106 . | Expansion factor, x-fold . |
|---|---|---|---|---|---|---|---|
| 1 | HCV(1406-1415) | 0.39 | 1.1 | 27 | 3.8 | 1.0 | 9.3 |
| 2 | HCV(1406-1415) | 1.6 | 6.0 | 61 | 8.1 | 4.9 | 8.2 |
| 3 | WT1(126-134) | 2.4 | 2.2 | 49 | 2.3 | 1.1 | 5.1 |
| 4 | MelanA(26-35L) | 25 | 4.5 | 72 | 4.5 | 3.2 | 7.2 |
| 5 | MelanA(26-35L) | 8 | 1.4 | 87 | 2.2 | 1.9 | 13.7 |
The absolute number of antigen-specific cells was calculated by (the absolute number of cells × percentage of MHC multimer+ cells)/100.
Due to the exceedingly low precursor frequency of antigen-specific T cells in the naive repertoire, multiple rounds of restimulation are often required until antigen-specific T cells can be successfully and efficiently expanded on a clonal level. Because enrichment for CD137+ cells in bulk T-cell populations was feasible after only 2 stimulations (Figure 3B), we asked whether the approach would also allow for clonal expansion of T cells after only 2 rounds of in vitro stimulation. Purified naive CD8+ T cells were stimulated with the HCV/NS3 epitope and, 24 hours after the second stimulation, T cells were cloned from either the unenriched or from the CD137-enriched group. At 30 days from the first stimulation of the T cells (20 days after cloning), the growing clones were screened using pMHC multimers. In the CD137+ group, 250 of 480 plated wells contained growing cells. Sixty-seven percent of these wells contained clones that were HCV/NS3(1406-1415) specific (overall specific cloning efficiency, 35%). In contrast, only 75 of 480 wells from the unenriched control group showed cell growth, with only 15% of these wells containing clones that were pMHC multimer+ (overall specific cloning efficiency, 2.3%) (Table 2).
Cloning of HCV-specific T cells after the second stimulation with or without CD137 enrichment*
| . | Not enriched . | CD137 enriched . |
|---|---|---|
| Total no. of wells | 480 | 480 |
| Percentage of specific T cells prior to cloning | 0.11 | 0.11 |
| Initial cell no./well | 4 | 4 |
| Total no. of wells with positive cell growth (%) | ∼ 75 (16) | ∼ 250 (52) |
| No. of wells tested by pMHC-multimer | 55 | 64 |
| % of multimer+ clones/CD8+ clone | 15 | 67 |
| % of multimer+ cells in parallel bulk culture | 12 | 62 |
| Overall specific cloning efficiency, %* | 2.3 | 35 |
| . | Not enriched . | CD137 enriched . |
|---|---|---|
| Total no. of wells | 480 | 480 |
| Percentage of specific T cells prior to cloning | 0.11 | 0.11 |
| Initial cell no./well | 4 | 4 |
| Total no. of wells with positive cell growth (%) | ∼ 75 (16) | ∼ 250 (52) |
| No. of wells tested by pMHC-multimer | 55 | 64 |
| % of multimer+ clones/CD8+ clone | 15 | 67 |
| % of multimer+ cells in parallel bulk culture | 12 | 62 |
| Overall specific cloning efficiency, %* | 2.3 | 35 |
Cloning was performed 24 hours after the second stimulation in medium containing irradiated allogenous B-LCLs and PBMCs as feeder cells and IL-2 and IL-15. Plates were evaluated by pMHC-multimer staining 20 days after cloning.
Specific cloning efficiency was determined as the percentage of wells with specific cell growth in relation to the total number of wells.
CD137 is a more uniform marker of antigen-specific activation than monitoring production of only a single cytokine
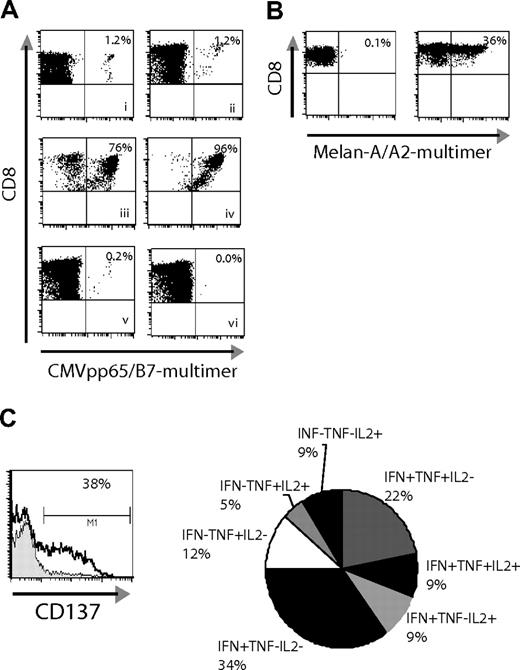
Methods based on cytokine secretion after activation are often used to enrich antigen-specific CD8+ cells, and we compared the utility of the IFNγ-secretion method with the CD137-enrichment method for selecting responding T cells derived from memory or naive populations. Both assays were compared for direct ex vivo enrichment from a starting population of CMV-specific memory T cells, which are known to secrete high levels of IFNγ.22 PBMCs from a CMV+ patient were stimulated with the HLA-B7–restricted epitope CMVpp65(417-426) and responding cells enriched based on capture of IFNγ-secreting cells at 4 hours or enrichment for CD137+ cells at 24 hours after stimulation. Flow cytometric controls showed that both procedures resulted in comparable and adequate enrichment (data not shown). One week after the enrichment step, the groups were compared by pMHC multimer staining. Both assays resulted in excellent enrichment (IFNγ = 76% and CD137 = 96%) (Figure 4Aiii-iv). To determine how effectively the 2 methods define the complete antigen-specific population, the negative fractions were examined for remaining antigen-specific T cells. To ensure complete removal of cells that can be identified by either method, the negative fractions (flowthrough) from the above enrichment procedures were passed over a second column designed to deplete any remaining cells attached to beads, and the depleted fraction cultured and analyzed one week later. After depletion of IFNγ+ cells, a small percentage (0.2%) of pMHC multimer binding CMV+ T cells was detectable (Figure 4Av), suggesting that some cells had either not been labeled sufficiently by the antibody for selection or were not secreting IFNγ at the time of enrichment. In contrast, no pMHC-multimer+ cells were detected 1 week after depletion of CD137+ cells, suggesting that by 24 hours after stimulation CD137 is uniformly up-regulated on all antigen-specific T cells (Figure 5Avi). Similar results were obtained when analyzing the negative fractions (flowthrough) after stimulation of T-cell lines against different specificities without use of an additional depletion column (data not shown).
CD137 enrichment is effective with memory and naive T-cell populations. (A) PBMCs from a CMV+/HLA-B7+ donor were used to compare the CD137 enrichment method versus the IFNγ-secretion assay (all plots, except the ex vivo staining, show groups after an additional 8 days of culture): (i) CMVpp65(417-426)-pMHC multimer+ cells ex vivo; (ii) stimulation with peptide, no enrichment; (iii) stimulation with peptide and enrichment with IFNγ-secretion assay; (iv) stimulation with peptide and CD137 enrichment; (v) IFN− group (flowthrough/depleted); (vi) CD137− cells (flowthrough/depleted). (B) PBMCs from a healthy donor were stimulated with Melan-A(26-35L) peptide directly ex vivo for 24 hours and then either cultured for an additional week in medium containing IL-7 and IL-15 (left), or enriched for CD137+ cells and cultured for one week (right). The enriched sample was then restimulated a second time (day 8 of culture) and assessed for (C) up-regulation of CD137 at 24 hours after peptide-specific stimulation (left, black line) or stimulation with irrelevant control peptide (filled). In parallel, the pattern of cytokine responses was determined by intracellular cytokine staining (right, 5-hour stimulation period, % responsive CD8+ cells noted).
CD137 enrichment is effective with memory and naive T-cell populations. (A) PBMCs from a CMV+/HLA-B7+ donor were used to compare the CD137 enrichment method versus the IFNγ-secretion assay (all plots, except the ex vivo staining, show groups after an additional 8 days of culture): (i) CMVpp65(417-426)-pMHC multimer+ cells ex vivo; (ii) stimulation with peptide, no enrichment; (iii) stimulation with peptide and enrichment with IFNγ-secretion assay; (iv) stimulation with peptide and CD137 enrichment; (v) IFN− group (flowthrough/depleted); (vi) CD137− cells (flowthrough/depleted). (B) PBMCs from a healthy donor were stimulated with Melan-A(26-35L) peptide directly ex vivo for 24 hours and then either cultured for an additional week in medium containing IL-7 and IL-15 (left), or enriched for CD137+ cells and cultured for one week (right). The enriched sample was then restimulated a second time (day 8 of culture) and assessed for (C) up-regulation of CD137 at 24 hours after peptide-specific stimulation (left, black line) or stimulation with irrelevant control peptide (filled). In parallel, the pattern of cytokine responses was determined by intracellular cytokine staining (right, 5-hour stimulation period, % responsive CD8+ cells noted).
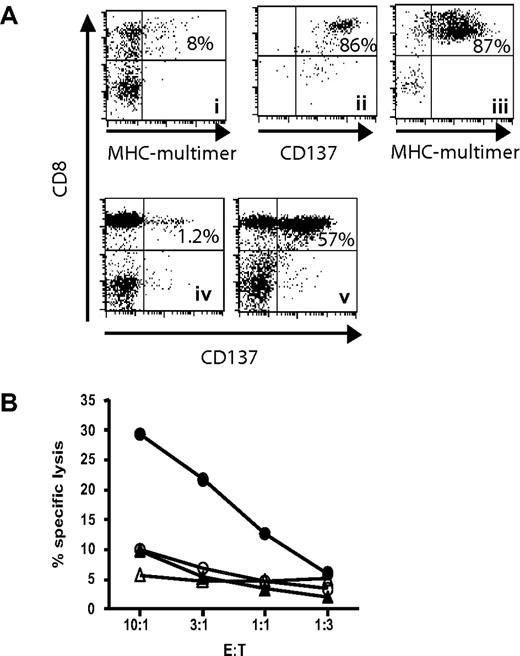
CD137 enrichment includes tumor-lytic T cells. (A) A Melan-A–specific T-cell line was generated by 2 rounds of stimulation (i). The cells were restimulated with peptide-pulsed autologous PBMCs, and 24 hours later CD137+ cells were positively selected using antibody/bead complexes (ii). Selected T cells were cultured for additional 8 days until pMHC multimer staining was performed (iii). This enriched line was then coincubated for 24 hours (ratio 1:1) with the HLA-A2+ Melan-A− melanoma cell line A325 (iv) or with the HLA-A2+ Melan-A+ melanoma cell line Mel526 (v), before staining for CD137. (B) The same enriched line (filled symbols) and the CD137− flowthrough (open symbols) were also tested in a 4-hour chromium release assay using the melanoma cell lines as targets (circles indicate targets; Melan-A+, Mel526 melanoma cells; and triangles, Melan A− A325 melanoma cells).
CD137 enrichment includes tumor-lytic T cells. (A) A Melan-A–specific T-cell line was generated by 2 rounds of stimulation (i). The cells were restimulated with peptide-pulsed autologous PBMCs, and 24 hours later CD137+ cells were positively selected using antibody/bead complexes (ii). Selected T cells were cultured for additional 8 days until pMHC multimer staining was performed (iii). This enriched line was then coincubated for 24 hours (ratio 1:1) with the HLA-A2+ Melan-A− melanoma cell line A325 (iv) or with the HLA-A2+ Melan-A+ melanoma cell line Mel526 (v), before staining for CD137. (B) The same enriched line (filled symbols) and the CD137− flowthrough (open symbols) were also tested in a 4-hour chromium release assay using the melanoma cell lines as targets (circles indicate targets; Melan-A+, Mel526 melanoma cells; and triangles, Melan A− A325 melanoma cells).
Unlike the CD8 memory response to CMV in which IFNγ is the dominant activation-induced cytokine,22 naive T cells are known to secret low quantities of IFNγ, a function that is acquired later after differentiation into Tc1-like effector cells. It is therefore difficult to isolate naive CD8+ T cells using the IFNγ-secretion assay as has been described before.23 Indeed, after stimulating naive T cells with the Melan-A peptide, we were unable to enrich for antigen-specific cells using the IFNγ-secretion method (data not shown). However when PBMCs were stimulated directly ex vivo with the Melan-A peptide and CD137+ cells isolated 24 hours later and cultured for 1 week, 36% of the cells were pMHC multimer+ (Figure 4B right panel). Stimulation of PBMCs with peptide without enrichment did not result in the outgrowth in 1 week of a detectable Melan-A–specific population from this donor (Figure 4B left panel), whose natural frequency of naive Melan-A–specific cells in PBMCs was below the detection limit with pMHC multimers (data not shown). Thus, in contrast to IFNγ production, CD137 can be used for early isolation of responding CD8+ T cells from primary responses.
The functional response of these cells that had been enriched for CD137+ cells 24 hours after primary sensitization was assessed after 1 week in culture by restimulation with peptide-pulsed PBMCs. The percentage of CD137+ cells detected 24 hours after restimulation correlated well with the percentage of pMHC-multimer+ cells present before restimulation (Figure 4C left panel and 4B right panel). However, consistent with the origin of these cells from a naive repertoire and stimulation under conditions lacking strong Th1/Tc1-polarizing signals, the responding cells expressed a heterogeneous cytokine profile, with 26% of the responding cells making either IL-2, TNFα, or both, but not IFNγ (Figure 4C right panel). Thus, selection based on production of a single cytokine alone, such as IFNγ, would have resulted in an underestimation of this responding population.
Enrichment of CD137+ T cells upon antigen stimulation includes T cells with lytic capacity against antigen+ tumor cells
We next wanted to know if the isolated and enriched T-cell population includes T cells that were able to recognize endogenously processed and presented antigen by tumor cells. A Melan-A–specific T-cell line was generated by 2 rounds of in vitro stimulation, resulting in 8% pMHC-multimer+ T cells (Figure 5Ai). This line was restimulated with peptide-pulsed PBMCs and CD137+ cells were selected 24 hours later (Figure 5Aii). When these cells were further expanded for one week, 87% of the T cells were Melan-A multimer+ (Figure 5Aiii). This line was then tested for recognition of the HLA-A2+, Melan-A+ melanoma cell line Mel526, or the HLA-A2+ Melan-A− melanoma cell line A325. Twenty-four hours after coincubation, 57% of the T cells up-regulated CD137 in response to Mel526 (Figure 5Aiv), whereas no relevant up-regulation was observed when incubated with the Melan-A− tumor line A325 (Figure 5Av). Most importantly, specific lysis of the Melan-A+ tumor targets was observed only in the enriched T-cell line, whereas cells saved from the flowthrough after enrichment did not show lytic capacity (Figure 5B). This demonstrates, that the CD137+ population contains affinity T cells capable of recognizing endogenously processed and presented antigen.
The CD137 assay as a tool for adoptive immunotherapy and epitope discovery
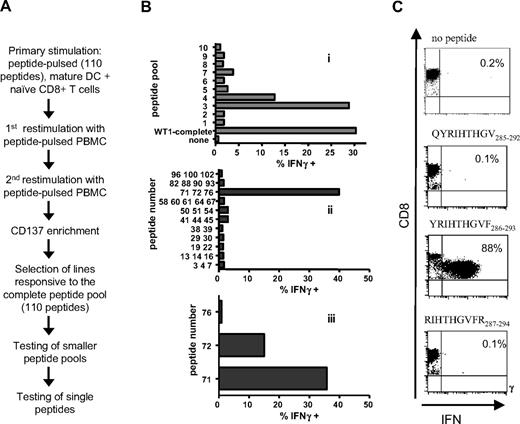
In the context of malignancy, a selection procedure that does not require the exact knowledge of the epitope or the HLA-restriction element could be used to identify novel antigens and broaden the spectrum of patients eligible for immunotherapy trials.24 WT1, a pro-oncogenic protein overexpressed in hematologic and oncologic malignancies,16 is being evaluated as a target protein for immunotherapy.17 As only few epitopes are defined so far,25,–27 we examined if novel WT1-specific epitopes could be identified by selecting for CD137+ T cells responding to stimulation with a peptide library, consisting of 15-mers spanning the complete WT1 protein with each overlapping by 11 amino acids. Naive CD8+ T cells from healthy donors were stimulated with autologous DCs pulsed with the peptide library and restimulated twice. Twenty-four hours after the third stimulation, CD137+ T cells were enriched using magnetic beads as described. One week later, enriched lines were tested for recognition of the peptide pool, with positive responses then resolved by subsequent sequential stimulations of aliquots of the responding cells deconvoluting the peptide pool (Figure 6A). Initial experiments using either no enrichment method or the IFNγ-secretion assay failed to enrich or detect any positive responses to the peptide pool (data not shown). By contrast, in 3 of 3 experiments using naive CD8 T cells from 2 different donors enriched for CD137+ cells, 5 immunogenic epitopes were identified (data not shown). One of these epitopes has been characterized in more detail by deconvoluting the peptide pool representing WT1, and found to be contained in 2 overlapping pentadecamers, denoted as 71 and 72 (Figure 6B). To identify the HLA restriction element, 23 Epstein-Barr virus (EBV)–transformed, partially HLA-matched B-cell lines were pulsed with either peptide 71 or peptide 72, and used as APCs. HLA-Cw0701 was identified as the restriction element, with HLA-Cw0702 also presenting the epitope, as 15 of 16 B-cell lines expressing HLA-Cw07 presented the peptide as measured by intracellular IFNγ staining, whereas 7/7 HLA-Cw07–negative B-cell lines did not present the peptide (Table 3). In a T-cell line, generated from a second donor, who shared only the Cw07-allele with the original donor, the minimal essential amino acid sequence was then determined. The WT1(286-294) (YRIHTHGVF) 9-mer induced a strong IFNγ response, whereas loss of tyrosine in position 1 or the phenylalanine in position 9 described as an anchor in the binding motif for HLA-Cw0728 abrogated recognition (Figure 6C; Table 4).
Presentation of WT1(286-293) by peptide-pulsed B-LCLs with partially matching HLA type
| No. . | Matching allele . | Nonmatching alleles . |
|---|---|---|
| Original donor: A1/A24/B8/B40/Cw03/Cw07* | ||
| B-cell-lines not presenting the epitope (IFNγ+ T cells <5%) | ||
| 1 | A1 | A30/B44/B57/Cw5/Cw6 |
| 2 | A1 | A11/B53/B57/Cw4/Cw6 |
| 3 | A24 | A32/B35/B44/Cw4/Cw4 |
| 4 | A24/Cw3 | A68/B35/B44/Cw4 |
| 5 | A24/Cw3 | A3/B15/B35/Cw4 |
| 6 | — | A30/A32/B27/B50/Cw1/Cw4 |
| 7 | A24/B40 | A32/B35/Cw2/Cw4 |
| 8 | B40/Cw7/Cw7*† | A203/A20/B38 |
| B-cell-lines presenting the epitope (IFNγ+ T cells >5%) | ||
| 9 | A1/B8/Cw7/Cw7* | A2/B18 |
| 10 | A1/B8/Cw7/Cw7 | A29/A44 |
| 11 | A1/B8/Cw7/Cw7 | A2/B7 |
| 12 | A1/B8/Cw7/Cw7 | A3/B49 |
| 13 | A1/B8/Cw7/Cw7* | A3/B49 |
| 14‡ | A1/B8/Cw3/Cw7 | A2/B55 |
| 15 | A24/B40/Cw1/ Cw7* | A32/B35 |
| 16 | B40/Cw3/Cw7† | A3/A3/B39 |
| 17 | B40/Cw7/Cw7 | A203/A20/B38 |
| 18 | Cw7/Cw7*† | A2/A3/B7/B18 |
| 19 | Cw72 | A3/A3/B7/B41/Cw17 |
| 20 | Cw7* | A2/A3/B18/B44/Cw12 |
| 21 | Cw7† | A3/A29/B7/B44/Cw16 |
| 22‡ | Cw7/Cw7 | A2/A2/B7/B7 |
| 23‡ | Cw7* | A2/A23/B27/B49/C1 |
| No. . | Matching allele . | Nonmatching alleles . |
|---|---|---|
| Original donor: A1/A24/B8/B40/Cw03/Cw07* | ||
| B-cell-lines not presenting the epitope (IFNγ+ T cells <5%) | ||
| 1 | A1 | A30/B44/B57/Cw5/Cw6 |
| 2 | A1 | A11/B53/B57/Cw4/Cw6 |
| 3 | A24 | A32/B35/B44/Cw4/Cw4 |
| 4 | A24/Cw3 | A68/B35/B44/Cw4 |
| 5 | A24/Cw3 | A3/B15/B35/Cw4 |
| 6 | — | A30/A32/B27/B50/Cw1/Cw4 |
| 7 | A24/B40 | A32/B35/Cw2/Cw4 |
| 8 | B40/Cw7/Cw7*† | A203/A20/B38 |
| B-cell-lines presenting the epitope (IFNγ+ T cells >5%) | ||
| 9 | A1/B8/Cw7/Cw7* | A2/B18 |
| 10 | A1/B8/Cw7/Cw7 | A29/A44 |
| 11 | A1/B8/Cw7/Cw7 | A2/B7 |
| 12 | A1/B8/Cw7/Cw7 | A3/B49 |
| 13 | A1/B8/Cw7/Cw7* | A3/B49 |
| 14‡ | A1/B8/Cw3/Cw7 | A2/B55 |
| 15 | A24/B40/Cw1/ Cw7* | A32/B35 |
| 16 | B40/Cw3/Cw7† | A3/A3/B39 |
| 17 | B40/Cw7/Cw7 | A203/A20/B38 |
| 18 | Cw7/Cw7*† | A2/A3/B7/B18 |
| 19 | Cw72 | A3/A3/B7/B41/Cw17 |
| 20 | Cw7* | A2/A3/B18/B44/Cw12 |
| 21 | Cw7† | A3/A29/B7/B44/Cw16 |
| 22‡ | Cw7/Cw7 | A2/A2/B7/B7 |
| 23‡ | Cw7* | A2/A23/B27/B49/C1 |
T cells specific for WT-1(286-293) were tested for recogniton of peptide-pulsed B-LCLs with partially matching HLA-type and subsequent INFγ production (CFC).
HLA-Cw0701.
HLA-Cw0702.
Presentation only at 25 μg/mL peptide.
Implementing CD137 enrichment for epitope discovery. (A) Experimental outline: After 3 rounds of stimulation with a pool of 110 overlapping 15-mers, CD137+ cells were enriched and further expanded. The immunogenic peptides were subsequently identified by step-wise testing of smaller peptide pools. (Bi) An enriched T-cell line was tested for IFNγ production in response to autologous EBV-transformed B lymphoblastoid cell lines (B-LCLs) pulsed with pools of peptides (10 or 11 peptides/pool). (ii) Peptides derived from pools 3, 4 (clearly positive), and 7 (potentially positive) were tested in smaller pools containing 2 to 5 peptides, in which the peptides were rearranged by selecting partially overlapping peptide sequences. (iii) Identification of peptides 71 and 72 as the stimulating peptides. (C) Fine mapping of the minimal essential amino acid sequence was performed using a T-cell line generated from a second HLA-Cw07+ donor after 3 stimulations with the immunogenic peptide. A panel of peptides was synthesized and tested for recognition of the minimal essential amino acid (see also Table 4) using peptide-pulsed, autologous B-LCLs as stimulators.
Implementing CD137 enrichment for epitope discovery. (A) Experimental outline: After 3 rounds of stimulation with a pool of 110 overlapping 15-mers, CD137+ cells were enriched and further expanded. The immunogenic peptides were subsequently identified by step-wise testing of smaller peptide pools. (Bi) An enriched T-cell line was tested for IFNγ production in response to autologous EBV-transformed B lymphoblastoid cell lines (B-LCLs) pulsed with pools of peptides (10 or 11 peptides/pool). (ii) Peptides derived from pools 3, 4 (clearly positive), and 7 (potentially positive) were tested in smaller pools containing 2 to 5 peptides, in which the peptides were rearranged by selecting partially overlapping peptide sequences. (iii) Identification of peptides 71 and 72 as the stimulating peptides. (C) Fine mapping of the minimal essential amino acid sequence was performed using a T-cell line generated from a second HLA-Cw07+ donor after 3 stimulations with the immunogenic peptide. A panel of peptides was synthesized and tested for recognition of the minimal essential amino acid (see also Table 4) using peptide-pulsed, autologous B-LCLs as stimulators.
Fine mapping of WT1(286-293) as the minimal essential amino acid sequence using peptide-pulsed, autologous B-LCLs as APCs
| Peptide position . | Sequence . | Recognition (5% threshold) . |
|---|---|---|
| 277-291 | TTPILCGAQYRIHTH | − |
| 281-295 | LCGAQYRIHTHGVFR | + |
| 285-299 | QYRIHTHGVFRGIQD | + |
| 286-300 | YRIHTHGVFRGIQDV | + |
| 289-303 | HTHGVFRGIQDVRRV | − |
| 284-294 | AQYRIHTHGVFR | + |
| 284-293 | AQYRIHTHGVF | + |
| 284-292 | AQYRIHTHGV | − |
| 285-294 | QYRIHTHGVFR | + |
| 285-293 | QYRIHTHGVF | + |
| 285-292 | QYRIHTHGV | − |
| 286-294 | YRIHTHGVFR | + |
| 286-293 | YRIHTHGVF | + |
| 287-294 | RIHTHGVFR | − |
| Peptide position . | Sequence . | Recognition (5% threshold) . |
|---|---|---|
| 277-291 | TTPILCGAQYRIHTH | − |
| 281-295 | LCGAQYRIHTHGVFR | + |
| 285-299 | QYRIHTHGVFRGIQD | + |
| 286-300 | YRIHTHGVFRGIQDV | + |
| 289-303 | HTHGVFRGIQDVRRV | − |
| 284-294 | AQYRIHTHGVFR | + |
| 284-293 | AQYRIHTHGVF | + |
| 284-292 | AQYRIHTHGV | − |
| 285-294 | QYRIHTHGVFR | + |
| 285-293 | QYRIHTHGVF | + |
| 285-292 | QYRIHTHGV | − |
| 286-294 | YRIHTHGVFR | + |
| 286-293 | YRIHTHGVF | + |
| 287-294 | RIHTHGVFR | − |
T cells generated by stimulation with the 15-mer for WT-1(281-295) were tested by CFC for recogniton of autologous B-LCLs pulsed with the indicated peptides (10 μg/mL).
Discussion
The biologic role of CD137 to provide a survival signal to activated T cells has been demonstrated in vivo and in vitro.9,29 Here we describe a novel use of CD137 based on the kinetics of expression after antigen recognition to identify and expand antigen-specific CD8+ cells. This new application has several advantages over alternative techniques for evaluating CD8+ T-cell responses: (1) it defines a broad and, based on pMHC multimer binding, nearly complete repertoire of the antigen-specific CD8+ T cells in a heterogeneous population, which cannot be achieved by analyzing the production of a single cytokine alone; (2) it allows the detection, isolation, and subsequently the rapid expansion of antigen-specific CD8+ cells even if present initially at a very low precursor frequency; (3) it can be performed independent of knowledge of the immunogenic epitopes and MHC-restriction elements; (4) it correlates well with already established assays such as CFC; and (5) it requires a simple staining procedure of a surface antigen that has a relatively long window of up-regulated expression after stimulation.
Exploiting the characteristics of CD137 expression provides a broader picture of the CD8+ T-cell population responding to antigen than the analysis of a single cytokine alone, as demonstrated using naive T cells. Based on these data and preliminary data using polarizing priming conditions, such as priming in the presence of exogenous IL-4 (not shown), CD137 appears to serve as a uniform marker for different T-cell subsets such as central memory cells or CD8+ T cells primed under Th2/Tc2 conditions. Screening for responsive CD137+ T-cell populations, followed by analysis of production of different cytokines should enhance the detection, monitoring, and selection of diverse responding CD8+ T-cell subpopulations, and could facilitate vaccine development requiring quantitative and qualitative characterization of the CD8+ T-cell response.6,30
Our own preliminary data, as well as the work done by Wehler et al studying depletion of alloreactive T cells,14 indicate that CD137 is also specifically up-regulated on CD4+ T cells. However further work is required to define specificity and kinetics of CD137 up-regulation on CD4+ cells and its comparison with selection methods based on expression of CD154.31,32
The ability to detect and expand ex vivo high-avidity CD8+ T cells specific for candidate tumor-associated antigens is essential not only for pursuing adoptive T-cell immunotherapy but also for validating the immunogenicity of candidate antigens. We have recently demonstrated the feasibility of generating in vitro T-cell clones from a naive repertoire for the known HLA-A*0201–restricted WT1(126–134) epitope.18 By using CD137 expression as a means to enrich responding cells, we now extend these findings and demonstrate that specific CD8+ T cells can be generated from a naive T-cell repertoire against previously undefined epitopes. By permitting early enrichment after stimulation of naive cells, the likelihood of detecting and expanding clones is increased and the culture time and number of restimulations necessary are reduced. As each round of restimulation drives the cells further toward terminal differentiation or “exhaustion,”33 methods to clone reactive cells after fewer divisions can have a direct impact on the functional quality of the resulting T cells. In fact, preliminary data suggest a fraction of CD8+ T-cell clones generated after only 2 rounds of stimulation have a functional and phenotypic profile resembling early/intermediate effector memory cells34 with maintained CD28 expression and IL-2-production (data not shown).
The identification of novel T-cell epitopes in relatively weakly immunogenic proteins often requires screening of a vast number of potential epitopes for each candidate protein. By using APCs pulsed with a pool of peptides encompassing the whole WT1 protein and enriching and testing the responding CD137+ cells, it has proved possible to generate and characterize novel T-cell responses against this weakly immunogenic self-antigen. The use of such a peptide library for the analysis and expansion of memory T cells against CMV35,–37 - or HIV38 -derived proteins has been described recently, and offers the advantage that it does not require an a priori selection of candidate peptides. For example, in silico analysis, predicting peptides from within the protein with favorable binding characteristics to a selected HLA-molecule, has often been used to narrow the number of candidate peptides,39 but prediction of binding scores, especially for less common HLA alleles, is sometimes unreliable or not available. In fact, the newly identified epitope WT1(286-294) models at only 8.2% of the maximal possible score, ranking as the 25th best peptide for HLA-Cw0702 from the complete amino acid sequence of WT1 as computed using the BIMAS algorithm40 (the other commonly used algorithm, SYFPEITHI,41 does not provide calculations for this allele) and would likely have been missed if peptides had been selected on the basis of in silico prediction as demonstrated for other antigens.42
Adoptive immunotherapy with antigen-specific CD8+ T cells has been shown to be effective in the treatment of human tumors,43 but is limited by the time and difficulty of isolating and expanding functional cells.24 Implementing novel technologies that facilitate obtaining a larger repertoire of specific T cells, accomplishing this in a faster more cost-efficient way, and generating cells that can exhibit broader functional profiles will facilitate broader and more effective application of this strategy. The CD137-enrichment method should prove useful for helping to achieve these goals.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the Leukemia & Lymphoma Society (LLS 7040–03) and the National Institute of Health (P01 CA18029, R37 CA33084). M.W. and J.K. are fellows of the Deutsche Krebshilfe (Germany).
The authors gratefully acknowledge expert technical help from Natalie Duerkopp and Audrey Mollerup.
National Institutes of Health
Authorship
Contribution: M.W. designed, performed, and analyzed experiments; J.K., H.N., W.Y.H., and P.D.G. designed and analyzed experiments; T.J.M. and M.B. provided vital reagents; and M.W. and P.D.G. wrote the paper. All authors edited and approved the written paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthias Wolfl, Pediatric Stem Cell Transplant Program, University Children's Hospital Wuerzburg, Josef-Schneider-Strasse 2, Wuerzburg D-97080, Germany; e-mail: woelfl_m@klinik.uni-wuerzburg.de or mwolfl@arcor.de; Philip D. Greenberg, University of Washington, BB1325 Health Sciences Building, Box 356527, Seattle, WA 98195; e-mail: pgreen@u.washington.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal