Extensive chronic graft-versus-host disease (ecGVHD) is characterized by fibrosis similar to that of patients with systemic sclerosis (scleroderma). Since stimulatory autoantibodies against the platelet-derived growth factor (PDGF) receptor (PDGFR) have been found in patients with scleroderma and are responsible for the activation of skin fibroblasts, we tested the hypothesis that these autoantibodies are also present in patients affected by ecGVHD. Serum from 39 patients subjected to allogeneic stem cell transplantation for hematologic malignancies (22 with ecGVHD and 17 without cGVHD) and 20 healthy controls was assayed for the presence of stimulatory autoantibodies to the PDGFR by incubating purified IgG with mouse-embryo fibroblasts lacking PDGFR α or β chains or with the same cells expressing PDGFR α. Stimulatory antibodies to the PDGFR were found selectively in all patients with ecGVHD but in none of the patients without cGVHD. Higher levels were detected in patients with generalized skin involvement and/or lung fibrosis. Antibodies recognized native PDGFR, induced tyrosine phosphorylation, accumulation of reactive oxygen species (ROS), and stimulated type 1 collagen gene expression through the Ha-Ras-ERK1/2-ROS signaling pathway. The biologic activity of these autoantibodies suggests a role in the development of fibrosis and argues for a common pathogenetic trait in ecGVDH and scleroderma phenotypes.
Introduction
Chronic graft-versus-host disease (cGVHD) is the most frequent long-term complication of allogeneic hematopoietic stem cell transplantation (HCT), occurring in 20% to 70% of subjects surviving more than 100 days. It represents the major cause of late death in this group of patients, and in survivors it is often associated with severe impairment of quality of life.1
Chronic GVHD most commonly affects the skin, liver, eyes, and mouth, though other sites may also be involved. A clinical classification of cGVHD distinguishes patients with limited disease, in which only the skin or liver is involved, and patients with extensive cGVHD (ecGVHD) characterized by generalized or localized skin manifestations and/or hepatic dysfunction including involvement of other organs.2 Skin lesions in ecGVHD are reminiscent of systemic sclerosis (scleroderma; SSc). Patients developing extensive cGVHD with scleroderma-like skin manifestations also have other clinical signs similar to those of patients with scleroderma. Fibrosis of the skin and of several visceral organs, such as lung, heart and esophagus has been described in both diseases,3 including activation of immune system and cytokine abnormalities.4,–6 The hypothesis that fetal microchimerisms play a role in the pathogenesis of scleroderma7 further supports the notion of the similarity between scleroderma and cGVHD.
We have recently demonstrated that patients with scleroderma are characterized by serum stimulatory autoantibodies targeting the receptor of platelet-derived growth factor (PDGFR).8 These antibodies trigger an intracellular loop, involving Ha-Ras-extracellular-signal-regulated kinases 1 and 2 (ERK 1/2)–reactive oxygen species (Ha-Ras-ERK 1/2–ROS), which leads to increased collagen gene expression and myofibroblast phenotype conversion of normal human primary fibroblasts.8–9 These findings strongly argue for a pathogenetic role of anti-PDGFR autoantibodies in the fibrosis of scleroderma patients.
Keeping these data in mind, we have evaluated patients with extensive cGVHD for the presence of stimulatory autoantibodies directed against the PDGFR.
Patients and methods
Patients
Thirty-nine white patients (18 men and 21 women), median age 43 years (range, 17 to 70), who had received allogeneic stem cell transplantation for malignant hematologic disorders, were studied. Twenty-two had extensive chronic GVHD, whereas 17 had not developed any sign of GVHD during the period of observation after stem cell transplantation. Diagnosis of ecGVHD was made according to established criteria.10 Skin disease was generalized in 9 of 22 patients and associated with lung fibrosis in 4 additional patients. Nine patients had minimal skin disease with either lung fibrosis (5 patients), sicca syndrome (3 patients), or hepatic and intestinal involvement (1 patient). The median time interval between transplantation and the enrollment in the study was 23 months (range, 16 to 36) in the group of patients with ecGVHD, and 42 months (range, 9 to 51) in patients without GVHD. The stem cell source was peripheral blood progenitor cells in 14 patients (12 in the ecGVHD group and 2 in those without GVHD) and bone marrow in 24 patients (10 in the ecGVHD group and 14 in those without GVHD). Cord blood was used in 1 patient who did not develop GVHD. At the time of the investigation none of the 17 patients without GVHD were receiving immunosuppressive treatment, whereas all 22 patients with ecGVHD were receiving immunosuppressive drugs.
Clinical characteristics of the 39 patients who received transplants
| . | . | Patients with ecGVHD, 22 . | Patients without cGVHD, 17 . |
|---|---|---|---|
| Recipient, age | 43 | 47 | 53 |
| Median, range | 17–70 | 22–70 | 17–68 |
| Recipient, gender | |||
| Male/female | 18/21 | 12/10 | 6/11 |
| Donor, age | 35 | 39.5 | 45.5 |
| Median, range | 11–71 | 24–69 | 11–71 |
| Donor, gender | |||
| Male/female | 21/18 | 12/10 | 9/8 |
| Diagnosis | |||
| ANLL/ALL/ | 11/3 | 6/1 | 5/2 |
| NHL/HL/MM | 5/1/3 | 1/0/3 | 4/1/0 |
| CML/CLL/MDS | 10/2/2 | 8/1/1 | 2/1/1 |
| Other | 2 | 1 | 1 |
| Status before transplantation | |||
| CR/PR | 13/12 | 6/7 | 7/5 |
| SD/PD | 3/11 | 1/8 | 2/3 |
| HLA-id. sibling/MUD/CB/ | 26/9/1 | 16/5/0 | 10/4/1 |
| HLA mismatch/HLA haplo. | 2/1 | 0/1 | 2/0 |
| Stem cell source | |||
| PBSC/BM/CB | 14/24/1 | 12/10/0 | 2/14/1 |
| Status | |||
| Alive/dead | 29/10 | 13/9 | 16/1 |
| Relapse | |||
| Yes/no | 11/28 | 8/14 | 3/14 |
| . | . | Patients with ecGVHD, 22 . | Patients without cGVHD, 17 . |
|---|---|---|---|
| Recipient, age | 43 | 47 | 53 |
| Median, range | 17–70 | 22–70 | 17–68 |
| Recipient, gender | |||
| Male/female | 18/21 | 12/10 | 6/11 |
| Donor, age | 35 | 39.5 | 45.5 |
| Median, range | 11–71 | 24–69 | 11–71 |
| Donor, gender | |||
| Male/female | 21/18 | 12/10 | 9/8 |
| Diagnosis | |||
| ANLL/ALL/ | 11/3 | 6/1 | 5/2 |
| NHL/HL/MM | 5/1/3 | 1/0/3 | 4/1/0 |
| CML/CLL/MDS | 10/2/2 | 8/1/1 | 2/1/1 |
| Other | 2 | 1 | 1 |
| Status before transplantation | |||
| CR/PR | 13/12 | 6/7 | 7/5 |
| SD/PD | 3/11 | 1/8 | 2/3 |
| HLA-id. sibling/MUD/CB/ | 26/9/1 | 16/5/0 | 10/4/1 |
| HLA mismatch/HLA haplo. | 2/1 | 0/1 | 2/0 |
| Stem cell source | |||
| PBSC/BM/CB | 14/24/1 | 12/10/0 | 2/14/1 |
| Status | |||
| Alive/dead | 29/10 | 13/9 | 16/1 |
| Relapse | |||
| Yes/no | 11/28 | 8/14 | 3/14 |
ecGVHD indicates extensive chronic graft-versus-host disease; ANLL, acute nonlymphoblastic leukemia; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma; HL, Hodgkin lymphoma; MM, multiple myeloma; CML, chronic myelogenous leukemia; CLL, chronic lymphocytic leukemia; MDS, myelodysplastic syndrome; CR, complete remission; PR, partial remission; SD, stable disease; PD, progressive disease; HLA-id. sibling, marrow from identical siblings; MUD, matched-unrelated donor; CB, cord blood; HLA mismatch, mismatched marrow from parents/siblings; HLA haplo, marrow from haploidentical parents/siblings; PBSC, peripheral blood stem cell; and BM, bone marrow.
Treatment of the patients with and without GVHD
| . | . | Patients with estensive cGVHD, 22 . | Patients without cGVHD, 17 . |
|---|---|---|---|
| ecGVHD prophylaxis | |||
| CsA + MTX | 37 | 22 | 15 |
| CsA + MMF | 1 | 0 | 1 |
| CsA + MTX + ATG | 1 | 0 | 1 |
| ecGVHD therapy | |||
| prednisone | 2 | ||
| prednisone + azathioprine | 1 | ||
| prednisone+ rituximab | 2 | ||
| CsA + prednisone | 3 | ||
| CsA + prednisone+ azathioprine | 8 | ||
| miscellaneous including ECP | 6 | ||
| ecGVHD targets | |||
| skin (generalized scleroderma) | 9 | ||
| skin-liver-sicca syndrome | 2 | ||
| skin-lung-sicca syndrome | 1 | ||
| sicca syndrome | 1 | ||
| skin-sicca syndrome | 4 | ||
| skin-lung | 5 |
| . | . | Patients with estensive cGVHD, 22 . | Patients without cGVHD, 17 . |
|---|---|---|---|
| ecGVHD prophylaxis | |||
| CsA + MTX | 37 | 22 | 15 |
| CsA + MMF | 1 | 0 | 1 |
| CsA + MTX + ATG | 1 | 0 | 1 |
| ecGVHD therapy | |||
| prednisone | 2 | ||
| prednisone + azathioprine | 1 | ||
| prednisone+ rituximab | 2 | ||
| CsA + prednisone | 3 | ||
| CsA + prednisone+ azathioprine | 8 | ||
| miscellaneous including ECP | 6 | ||
| ecGVHD targets | |||
| skin (generalized scleroderma) | 9 | ||
| skin-liver-sicca syndrome | 2 | ||
| skin-lung-sicca syndrome | 1 | ||
| sicca syndrome | 1 | ||
| skin-sicca syndrome | 4 | ||
| skin-lung | 5 |
MTX indicates methotrexate; MMF, mycophenolate mofetil; CsA, cyclosporin A; ATG, antithymocyte globulin; and ECP, photopheresis
Control groups included 20 age-, sex-, and race-matched healthy volunteers.
The study was approved by the institutional ethics committee (Università Politecnica delle Marche, Ancona, Italy).
After oral and written informed consent in accordance with the Declaration of Helsinki, a blood sample was taken from patients and controls and spun in a refrigerated centrifuge after clot formation. The supernatants were collected and stored at −20°C until assayed, usually within 4 weeks.
Bioassay for anti-PDGFR autoantibodies
As previously described, a functional bioassay was used to detect anti-PDGFR autoantibodies.8 Briefly, mouse-embryo fibroblasts expressing PDGFR α subunit (Fα cells) were exposed in vitro to immunopurified IgG. Control cells were mouse-embryo fibroblasts devoid of PDGFR (F−/− cells). Cells were plated in duplicate at a density of 20 000 cells in 1.83-cm2 wells, cultured for 24 hours at 37°C in 0.2 percent fetal calf serum, and incubated in the presence of 1 mL of normal or patient immunopurified IgG (200 μg/mL) for 15 minutes at 37°C before reactive oxygen species (ROS) production determination.
Fluorimetric determination of intracellular ROS generated by adherent fibroblasts was carried out after loading the cells with 2′,7′-dichlorofluorescein diacetate (DCFH-DA 10 μM, Molecular Probes, PoortGebouw, The Netherlands) as described.8 Each IgG sample was tested in duplicate, and the average was recorded. The results were expressed as Stimulation Index (SI), which corresponds to (S-C)/(P-C), where S is the DCF fluorescence intensity of the test IgG, C is DCF fluorescence intensity of a negative control obtained by cell cultured without IgG, and P of a positive control obtained incubating the cells with PDGF (15 ng/mL for 15 minutes). The intraplate variation was less than 3%. The samples were recorded as positive if the SI was greater than 95th percentile of controls.
In selected experiments ROS generation was evaluated in cells exposed to the PDGF receptor tyrosine kinase inhibitor (AG 1296; 2 μM for 1–2 hours) and the epidermal growth factor (EGF) receptor tyrosine kinase inhibitor (AG 1478; 2 μM for 2 hours) (Calbiochem, San Diego, CA).
IgG isolation
IgG fractions were purified by affinity chromatography on protein A/G-sepharose column (Pierce, Rockford, IL) from serum of healthy subjects and patients with and without ecGVHD.
Fibroblast cultures
Human skin fibroblasts obtained from punch biopsies taken from the forearms of healthy volunteers, and the involved skin of patients who fulfilled the criteria for the diagnosis of ecGVHD10 were cultured as described.8 Fibroblasts between the fourth and the ninth subpassage were used for all experiments.
Cell lysis and immunoblotting
Cell culture plates were lysed with 0.3 mL of cold radioimmunoprecipitation assay (RIPA) buffer (1× phosphate-buffered saline (PBS), 1% Nonidet P-40, 0.5% sodium deoxycholate, 0.1% sodium dodecyl sulfate (SDS), 2 mM sodium orthovanadate, 2 μg/mL aprotinin, 1 mM phenylmethylsulfonyl fluoride (PMSF) and processed as described.8,9
Real-time PCR for collagen
Levels of α1 (I) and α2 (I) collagen RNA transcripts were quantitated by real-time polymerase chain reaction (PCR). Normal human fibroblasts were treated with normal and ecGVHD IgG (200 μg/mL for 15 minutes), and total RNA from the samples was isolated using RNeasy (QIAGEN, Hilden, Germany). cDNA sequences derived from the National Center for Biotechnology Information database (NCBI, http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?CMD = search&DB = nucleotide; accession numbers GAPDH: BC013310; COL α1(I): BC036531, COL α2(I): BC054498) were used to design the forward and reverse primers to the sequence using Beacon Designer software (PREMIER Biosoft International, Palo Alto, CA). The primers were as follows:
GAPDH FW: 5′-CCCTTCATTGACCTCAACTACATG-3′;
GAPDH REV: 5′-TGGGATTTCCATTGATGACAAGC-3′;
COL α1 (I) FW: 5′-AGGGCCAAGACGAAGACATC-3′;
COL α1 (I) REV: 5′-AGATCACGTCATCGCACAACA-3′;
COL α2 (I) FW: 5′-AGGTCAAACAGGAGCCCGTGGG-3′;
COL α2 (I) REV: 5′-GCACCTGGGAAGCCTGGAGGG-3′.
cDNA was reverse transcribed from 2 μg of total RNA of each sample, using M-MLV RT kit (Gibco Invitrogen, Paisley, United Kingdom). cDNAs were mixed with SYBR-Green PCR IQ Super mix (Bio-Rad, Hercules, CA) and primers, and real-time PCR was performed. GAPDH gene was used as internal control. For each single well amplification reaction, a threshold cycle (CT) was observed in the exponential phase of amplification, and the quantitation of relative expression levels was achieved using standard curves for both the target and endogenous controls. All assays were performed in triplicate.
Statistical analysis
Stimulation Index in the study groups is expressed as a median value and a range. Comparisons between the levels of anti-PDGFR autoantibodies of scleroderma and control groups were performed with the nonparametric Mann-Whitney test. All reported P values are 2-sided. Data were analyzed with the use of SAS software (SAS Institute, Cary, NC).
Results
Immunoglobulins from patients with extensive cGVHD induce ROS in fibroblasts
To detect antibodies stimulating PDGFR in patients with ecGVHD and eliminate the effects of PDGF or other cytokines present in serum, we purified total IgG from patients and controls and determined their biologic activity by measuring the induction of ROS levels in a mouse-embryo cell line expressing recombinant α PDGFR subunit (Fα cells). As negative control, the immunoglobulins were tested on the same cell line lacking PDGFR genes and therefore unable to express any PDGFRs (F−/− cells).
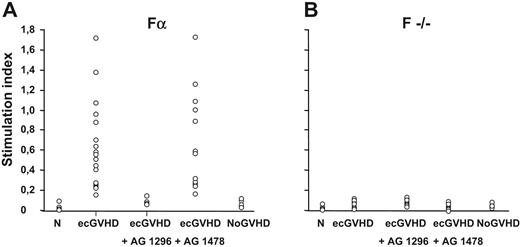
Figure 1 shows that the levels of ROS induced by IgG from patients with ecGVHD (median value, 0.47; range, 0.15 to 1.71) were significantly higher (P > .001) than ROS generated by normal IgG (median value, 0; range, 0 to 0.02) or IgG from patients who received transplants without cGVHD (median value, 0.01; range, 0 to 0.15) (P > .001). Using the 99th percentile as the upper limit of normal values, antibodies stimulating ROS levels were found in all patients with ecGVHD and in none of the controls.
Antibodies against PDGF receptor in patients with extensive cGVHD. Levels of reactive oxygen species in mouse-embryo fibroblasts Fα (panel A) and F−/− cells (panel B) incubated with IgG (200 μg/mL for 15 minutes per 20 000 cells) from healthy subjects (N; n = 20), extensive chronic GVHD patients (ecGVHD; n = 22), and from patients who had not developed GVHD after transplantation (NoGVHD; n = 17). Fα cells were also pre-incubated with the selective inhibitors of PDGFR tyrosine kinase (AG 1296, 2 μM for 2 hours) and of the epidermal growth factor (EGF) receptor tyrosine kinase (AG 1478; 2 μM for 2 hours).
Antibodies against PDGF receptor in patients with extensive cGVHD. Levels of reactive oxygen species in mouse-embryo fibroblasts Fα (panel A) and F−/− cells (panel B) incubated with IgG (200 μg/mL for 15 minutes per 20 000 cells) from healthy subjects (N; n = 20), extensive chronic GVHD patients (ecGVHD; n = 22), and from patients who had not developed GVHD after transplantation (NoGVHD; n = 17). Fα cells were also pre-incubated with the selective inhibitors of PDGFR tyrosine kinase (AG 1296, 2 μM for 2 hours) and of the epidermal growth factor (EGF) receptor tyrosine kinase (AG 1478; 2 μM for 2 hours).
IgG from patients with extensive cGVHD specifically recognize PDGFR
To determine the receptor specificity of the IgG of ecGVDH patients, we pretreated the cells with selective PDGFR or EGFR tyrosine kinase inhibitors, AG 1296 or AG 1478, respectively. PDGFR, but not EGFR, inhibitor prevented ROS induction by IgG from ecGVHD patients (Figure 1A). Also, the ecGVHD IgG did not stimulate ROS in F−/− cells that do not express PDGFR (Figure 1B). Finally, ecGVHD IgG, but not IgG from the patients without GVHD, immunoprecipitated PDGFR α and β subunits from Fα cells (Figure 2A). PDGFR-interacting antibodies in ecGVHD IgG were completely removed by pre-absorption with Fα cells but not with F−/− cells. The depleted supernatant lost the ability to stimulate ROS production (data not shown). These data indicate that Fα cells were able to remove selectively PDGFR-interacting antibodies from ecGVHD IgG (data not shown).
Immunoglobulins from patients with ecGVHD immunoprecipitate and phosphorylate PDGF receptor. (A) Fα and F−/− lysates were immunoprecipitated with IgG purified from extensive cGVHD patients (ecGVHD) and patients without GVHD (No GVHD). The filters were developed with specific antibodies against the PDGFR α and β subunits (WB). Total lysates indicate immunoblots of total proteins. Representative results from 1 of 3 experiments are shown. (B) Normal human fibroblasts were stimulated with different concentrations of IgG from patients with ecGVHD (15 minutes), PDGF (15 ng/mL for 15 minutes), or grown in 0.2% fetal calf serum. Cell lysates were immunoprecipitated with a polyclonal antibody against PDGFR (subunit α), and the immunoblots were developed with a specific antibody against phosphorylated tyrosine. FCS indicates fetal calf serum.
Immunoglobulins from patients with ecGVHD immunoprecipitate and phosphorylate PDGF receptor. (A) Fα and F−/− lysates were immunoprecipitated with IgG purified from extensive cGVHD patients (ecGVHD) and patients without GVHD (No GVHD). The filters were developed with specific antibodies against the PDGFR α and β subunits (WB). Total lysates indicate immunoblots of total proteins. Representative results from 1 of 3 experiments are shown. (B) Normal human fibroblasts were stimulated with different concentrations of IgG from patients with ecGVHD (15 minutes), PDGF (15 ng/mL for 15 minutes), or grown in 0.2% fetal calf serum. Cell lysates were immunoprecipitated with a polyclonal antibody against PDGFR (subunit α), and the immunoblots were developed with a specific antibody against phosphorylated tyrosine. FCS indicates fetal calf serum.
To determine unambiguously the stimulatory nature of antibody-receptor interaction, we challenged normal fibroblasts with increasing concentrations of IgG, derived from patients with ecGVHD, for 15 minutes and tested tyrosine phosphorylation of the PDGFR. IgG derived from ecGVHD patients induced tyrosine phosphorylation of the PDGFR in a dose-dependent manner (Figure 2B). We also investigated a possible relationship between ROS-inducing activity of immunoglobulins and the clinical features of ecGVHD phenotype. Pooling the data together, we found that significantly higher levels of the antibodies, in terms of high biologic activity, were present in 13 patients with generalized skin disease, compared with the levels found in 9 patients with localized skin disease and organ involvement (P > .03).
Biologic activity of PDGFR stimulatory antibodies present in the serum of patients with chronic GVHD
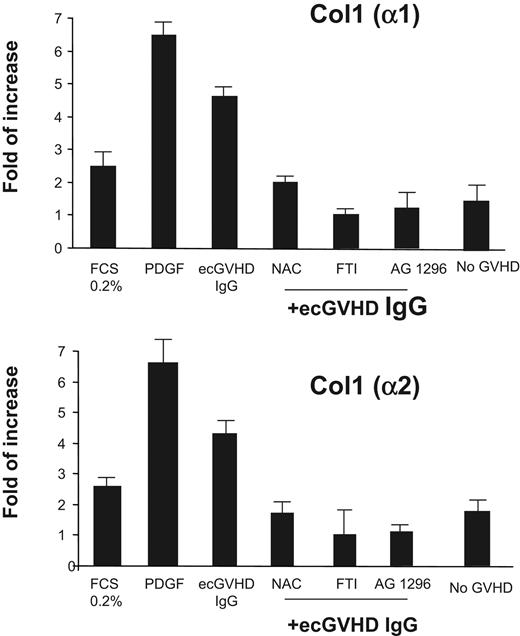
To determine the biologic effects induced by ecGVHD IgG, we assayed the expression of type I collagen genes in normal human fibroblasts exposed to ecGVHD IgG. Type I collagen α1 and α2 chain mRNAs were induced by ecGVHD IgG but not by IgG from patients without GVHD as shown by real-time PCR (Figure 3). Also, PDGF receptor tyrosine kinase inhibitor (AG 1296 2 μM for 1 hour before treatment) inhibited type I collagen gene expression (Figure 3). ecGVHD IgG induced–expression of type I collagen was inhibited by N-acetyl cysteine (20 mM for 1 hour before treatment), a potent ROS scavenger. Pretreatment of the cells with FTI-277 (20 μM for 2 hours), a farnesyl protein transferase inhibitor, prevented ecGVHD IgG stimulation of collagen expression. These data indicate that both membrane-anchored Ha-Ras and ROS signals, similarly to scleroderma, are essential for PDGFR stimulatory activity of ecGVHD IgG.9
Stimulatory antibodies to PDGFR from patients with ecGVHD induce type I collagen. Type I collagen gene expression by normal human fibroblasts after incubation with 0.2% fetal calf serum for 48 hours, PDGF (15 ng/mL for 15 minutes), extensive cGVHD IgG (ecGVHD; 200 μg/mL for 15 minutes), IgG from patients without GVHD (No GVHD IgG; 200 μg/mL for 15 minutes) in the presence and absence of AG1296 (2 μM for 1 hour before treatment), N-acetylcysteine (NAC; 20 mM for 1 hour before treatment), and farnesyl protein transferase inhibitor FTI-277 (20 μM for 2 hours before treatment). Real-time quantitative (RT)-PCR of the transcripts of genes encoding the α1 and α2 chain of type I collagen was performed as described in “Material and methods.”
Stimulatory antibodies to PDGFR from patients with ecGVHD induce type I collagen. Type I collagen gene expression by normal human fibroblasts after incubation with 0.2% fetal calf serum for 48 hours, PDGF (15 ng/mL for 15 minutes), extensive cGVHD IgG (ecGVHD; 200 μg/mL for 15 minutes), IgG from patients without GVHD (No GVHD IgG; 200 μg/mL for 15 minutes) in the presence and absence of AG1296 (2 μM for 1 hour before treatment), N-acetylcysteine (NAC; 20 mM for 1 hour before treatment), and farnesyl protein transferase inhibitor FTI-277 (20 μM for 2 hours before treatment). Real-time quantitative (RT)-PCR of the transcripts of genes encoding the α1 and α2 chain of type I collagen was performed as described in “Material and methods.”
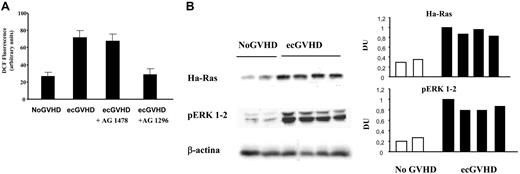
To demonstrate the key role of the Ha-Ras-ERK 1/2-ROS circuitry in the activation of fibroblasts, we isolated fibroblasts from patients with extensive cGVHD and assayed the levels of Ha-Ras, P-ERK 1/2 and reactive oxygen species. We have previously reported8,9 that Ha-Ras protein levels are stabilized by ROS-PDGFR activation. Figure 4 shows that fibroblasts derived from eGVHD patients, cultured in low serum, contained high levels of Ha-Ras, ROS, and P-ERK1/2. The PDGFR-specific inhibitor reduced significantly these markers to normal levels (Figure 4).
Ha-Ras-ERK 1/2-ROS signaling in fibroblasts from patients with cGVHD. (A) Levels of reactive oxygen species, evaluated as DCF fluorescence intensity (arbitrary units) in 3 fibroblast cell lines from patients with and without GVHD (ecGVHD and No GVHD, respectively). Fibroblasts from patients with ecGVHD also were pre-incubated with selective inhibitors of EGFR and PDGFR (AG 1478 and AG 1296, respectively; 2 μM for 2 hours). (B) Left part. The data shown were obtained with 2 and 4 fibroblast cell lines from patients with and without GVHD (ecGVHC and No GVHD, respectively) cultured in 0.2% FCS for 48 hours before being harvested. Immunoblots were developed with specific antibodies against Ha-Ras on cell lysates immunoprecipitated with a monoclonal anti–pan-Ras antibody. Phosphorylated forms of ERK 1/2 were detected by immunoblotting with phosphospecific antibodies. The right part of the panel shows the densitometric analysis of the blots of the left part.
Ha-Ras-ERK 1/2-ROS signaling in fibroblasts from patients with cGVHD. (A) Levels of reactive oxygen species, evaluated as DCF fluorescence intensity (arbitrary units) in 3 fibroblast cell lines from patients with and without GVHD (ecGVHD and No GVHD, respectively). Fibroblasts from patients with ecGVHD also were pre-incubated with selective inhibitors of EGFR and PDGFR (AG 1478 and AG 1296, respectively; 2 μM for 2 hours). (B) Left part. The data shown were obtained with 2 and 4 fibroblast cell lines from patients with and without GVHD (ecGVHC and No GVHD, respectively) cultured in 0.2% FCS for 48 hours before being harvested. Immunoblots were developed with specific antibodies against Ha-Ras on cell lysates immunoprecipitated with a monoclonal anti–pan-Ras antibody. Phosphorylated forms of ERK 1/2 were detected by immunoblotting with phosphospecific antibodies. The right part of the panel shows the densitometric analysis of the blots of the left part.
These data indicate that in cells derived from ecGVHD patients, PDGFR signaling is constitutively stimulated. Constitutive signaling by ROS-Ha-Ras-ERK1/2 by PDGFR is linked to overproduction of collagen and ultimately to fibrosis.
Discussion
The present study shows the presence of antibodies directed against the receptor of PDGF in patients with extensive chronic GHVD. Their stimulatory activity is proved by the ability to stimulate (1) tyrosine phosphorilation of PDGFR, (2) expression of type I collagen α1 and α2 gene, and (3) Ha-Ras-ERK 1/2–ROS signaling pathway.8
The presence of stimulatory antibodies in autoimmune diseases is rare. Insofar, only in scleroderma and Graves disease agonistic antibodies to PDGFR and TSHR, respectively, have been reported.8,11 In fact, the bulk of antibodies found in autoimmune diseases is generally represented by antibodies targeted toward several intracellular or extracellular components. These antibodies are able to bind their targets but do not stimulate selective intracellular signaling pathway. The findings reported here add a new level of complexity to the scleroderma and ecGVDH phenotypes and suggest a common mechanism underlying the pathogenesis of these disorders. The similarity between the signals leading to fibrosis in scleroderma and extensive cGVHD has been previously suggested; for a review, see Artlett et al.12 For instance, several investigators consider ecGVHD a model of scleroderma.13,–15 It is possible that in scleroderma and in extensive chronic GVHD the same mechanism may impair self tolerance toward PDGF receptor.7,12
Autoantibodies against several antigens have been reported both in animal models of cGVDH and in clinical studies,4,–6,16 although it remains controversial at best whether they play an actual role in pathogenesis. The autoantibodies described here explain the fibrotic lesions in ecGVHD because (1) they stimulate type I collagen gene expression, and (2) their levels correlate with the severity of fibrosis. Furthermore, there is evidence that cGVHD is a T-helper 2 disease, involving a sustained CD4 + T-cell help for B cells, probably caused by derangement of negative selection process of autoreactive T lymphocytes.17
Finally, our data confirm the pathogenetic relevance of the Ha-Ras-ERK 1/2-ROS cascade in triggering and maintaining the activation of fibroblasts as already described.8,9 Targeting this circuitry and the inhibition of any of the molecules involved in the cascade (Ras-Erk1/2-ROS) are crucial for the development of a successful treatment of fibrosis in ecGVHD.18,19
Although our data indicate a mechanistic explanation of the phenotype in ecGVHD fibroblasts, there are many unsolved questions to be answered. First, the precise epitope on PDGFR recognized by the autoantibodies: this is relevant for a more sensitive and less cumbersome diagnostic test, as well as for development of targeted therapy. Second, the presence of the antibodies we have described suggests a pathogenetic link between scleroderma and ecGVDH but does not provide information on the initial cause of both diseases. The mechanism by which PDGFR becomes a self antigen is not known. Autoimmunity may be induced by epitopes exposed as a result of a prolonged injury or viral infection or incompatibility between donor and recipient in terms of PDGF receptor genes.
The data reported in this study are to be considered preliminary, awaiting validation from larger clinical investigations, which also should establish the positive and negative predictive value of anti-PDGF receptor autoantibodies for the development of GVHD.
The practical implication deriving from our findings is a possible targeted therapy based on inhibition of PDGFR-ROS and Ha-Ras signaling. We speculate that blocking PDGFR activation with imatinib mesylate, a tyrosine kinase inhibitor, might be of clinical benefit. However, our data indicate that inhibition of PDGFR in the presence of excess of ROS does not completely suppress the Ha-Ras-ERK 1/2-ROS cascade.9 For example, H2O2 generated in fibroblasts may diffuse and activate adjacent cells, irrespective of PDGFR activation.
We believe that targeting multiple steps of the Ha-Ras-ERK-ROS circuitry is the only way to overcome the longterm biologic action of the autoantibodies to PDGFR, and it will ultimately be clinically rewarding.18,19
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by Società Italiana di Reumatologia (SIR), AILS (Associazione Italiana per la Lotta alla Sclerodermia), Ministero Italiano per l'Università e la Ricerca Scientifica (MIUR 2003), AIRC (Associazione Italiana Ricerca sul Cancro), and a generous grant from Fondazione Cariverona.
We are indebted to Dr Andrius Kazlauskas (Schepens Eye Research Institute, Boston, MA) for providing F−/− and Fα cells and for reviewing the manuscript.
Authorship
Contribution: S.S. and A.O. collaborated in the design of the study, performed the cellular studies, and contributed to writing the paper. A.G. and E.V.A. designed the study, carried out a comprehensive analysis of the data, and wrote the paper. M.L. and G.M. performed the analysis of collagen gene expression real-time polymerase chain reaction. P.L. and A.B. collected the patient clinical and serological data; and N.C., A.P., and S.T. performed the immunoprecipitation, immunohistologic, and immunofluorescence experiments.
S.S. and A.O. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Armando Gabrielli, Dipartimento di Scienze Mediche e Chirurgiche–Sezione di Clinica Medica, Università Politecnica delle Marche, Polo Didattico, Via Tronto, 10, 60020 Ancona, Italy; e-mail: a.gabrielli@univpm.it.