Multiple myeloma (MM) cells inhibit certain T-cell functions. We examined the expression of B7-H1 (PD-L1), a B7-related protein that inhibits T-cell responses, in CD138-purified plasma cells isolated from MM patients, monoclonal gammopathy of undetermined significance patients, and healthy donors. We observed that B7-H1 was expressed in most MM plasma cells, but not cells isolated from monoclonal gammopathy of undetermined significance or healthy donors. This expression was increased or induced by IFN-γ and Toll-like receptor (TLR) ligands in isolated MM plasma cells. Blocking the MEK/ERK pathway inhibited IFN-γ–mediated and TLR-mediated expression of B7-H1. Inhibition of the MyD88 and TRAF6 adaptor proteins of the TLR pathway blocked not only B7-H1 expression induced by TLR ligands but also that mediated by IFN-γ. IFN-γ–induced STAT1 activation, via MEK/ERK and MyD88/TRAF6, and inhibition of STAT1 reduced B7-H1 expression. MM plasma cells stimulated with IFN-γ or TLR ligands inhibited cytotoxic T lymphocytes (CTLs) generation and this immunosuppressive effect was inhibited by preincubation with an anti-B7-H1 antibody, the UO126 MEK inhibitor, or by transfection of a dominant-negative mutant of MyD88. Thus, B7-H1 expression by MM cells represents a possible immune escape mechanism that could be targeted therapeutically through inhibition of MyD88/TRAF6 and MEK/ERK/STAT1.
Introduction
In addition to the cytogenic and molecular abnormalities reportedly associated with malignant transformation of plasma cells, interactions between multiple myeloma (MM) cells and the bone marrow micro-environment are crucial for plasma cell survival and proliferation.1,–3 Several factors that mediate MM cell cross-talk with mesenchymal-derived cells, such as vascular endothelial growth factor, an angiogenic factor, have been previously described; however, there is growing evidence that MM cells also interact with immune cells. Plasma cells interact with T cells via the RANK/RANK-L system.4 A recent study demonstrated that the survival and growth of malignant plasma cells was supported by bone marrow dendritic cells via RANK/RANK-L and BAFF-APRIL.5 There is also evidence that malignant plasma cells can be targeted for an immune response. Several tumor antigens are expressed by plasma cells isolated from MM and monoclonal gammopathy of undetermined significance (MGUS) patients, and allogenic bone marrow transplantation can induce a graft-versus-myeloma effect.6,–8 However, MM is also associated with immune dysfunction. Defects in T-cell responses to mitogenic and TCR-mediated stimulation have been reported.9,–11 Interestingly, regulatory T cell (Treg cell) populations are significantly modified in both MGUS and MM patients, suggesting that immune dysfunction is an early event in the malignant transformation process of plasma cells.12,13 However, the factors produced by plasma cells that create these immune defects are poorly defined.
A possible candidate responsible for such T-cell inhibitory mechanisms in MM plasma cells is B7-H1. B7-H1 (also known as PD-L1 or CD274) is a B7 family member and is the ligand for PD-1 (programmed death-1), a member of the CD28 family.14 B7-H1 is broadly distributed in various tissues and cell types and is often expressed after exposure to inflammatory cytokines, especially IFN-γ. B7-H1 interacts with PD-1 and another as yet unknown receptor on T cells and can inhibit T cell activation and cytotoxic T lymphocytes (CTL)-mediated lysis.15,,–18 B7-H1 can also increase T-cell activation.19,–21 Marked expression of B7-H1 has been reported for various human carcinomas and in mouse models expression of B7-H1 enhances tumor growth and allows dormant tumor cells to escape from CTLs.16,22,,,,,,–29 Blocking B7-H1 enhances the effects of cancer vaccines.30,,,–34 Toll-like receptor (TLR) stimulation can also induce B7-H1 expression in mouse tumor cells.35 Thus, B7-H1 overexpression appears as a possible mechanism for tumors to avoid the host's immune response.
Little is known regarding the expression of B7-H1 in B-lineage lymphocytes. We show here that B7-H1 is expressed by malignant plasma cells from most MM patients but not from MGUS patients. This expression is further enhanced by IFN-γ and TLR stimulation via a MEK/ERK-dependent and MyD88/TRAF6-dependent pathway and can inhibit T-cell responses, indicating B7-H1 as a possible immune evasion mechanism in MM.
Patients, materials, and methods
This study was approved by the IRB Tumorothèque du Centre Hospitalier et Universitaire de Lille, Hôpital Calmette, Lille, France.
Patients and cell lines
Bone marrow mononuclear cells from 42 patients with MGUS, 82 patients with MM, and 20 healthy donors were isolated by Ficoll Hypaque sedimentation after the donors had given informed consent, in accordance with the Declaration of Helsinki. Plasma cells were purified using the anti-CD138 plasma cell isolation system (Miltenyi-Biotec, Bergisch Gladbach, Germany) according to the manufacturer's recommendations. Purities of positive and negative fractions were analyzed by flow cytometry using a phycoerythrin-conjugated mouse antihuman CD138 monoclonal antibody (Beckman Coulter, Miami, FL).
RPMI 8226, U266, K562, and HEK-293 cell lines were cultured in RPMI 1640 supplemented with 10% fetal calf serum, 2 mmol l-glutamine, 100 U/mL penicillin, and 100 μg/mL streptomycin at 37°C in 5% CO2.
Flow cytometry and Western blot analyses
Expression of B7-H1 on CD138-selected plasma cells was evaluated by flow cytometry with the specific monoclonal antibody (mAb) phycoerythrin-B7-H1 (Clone MIH1; eBiosciences, San Diego, CA). In certain experiments, B7-H1 expression was also evaluated by Western blot using the N20 goat polyclonal antihuman B7-H1 antibody (Santa Cruz Biotechnology, Santa-Cruz, CA).
T cells were analyzed with an anti-CD3 (clone UCHT1; BD Biosciences), anti-CD4 (clone UCHT1; BD Biosciences), and anti-CD8 (clone RM4–5; BD Biosciences, San Diego, CA) mAbs. To avoid nonspecific binding, cells were pre-incubated with FcR blocking reagent (Miltenyi Biotec, Bergish Gladbach, Germany) for 15 minutes at 4°C before immunolabelling. Cells were analyzed on an EPICS XL3-MCL (Beckman Coulter).
For Western blot analyses, CD138-purified plasma cells were suspended in lysis buffer (20 mM Tris-HCl, pH7.8, 50 mM NaCl, 5 mM EGTA, and 1% v/v Triton X-100) containing freshly added protease and phosphatase inhibitors (1mM phenylmethyhyl sulfonyl fluoride, 1 μM leupeptin, 2 μM aprotinin, 1 mM sodium orthovanadate, and 20 mM glycerophosphate). Lysates were clarified by centrifugation at 4°C and protein concentration determined by Bio-Rad protein assay. Equal quantities of proteins were separated by SDS-PAGE, transferred to PVDF membrane, and sequentially incubated with primary antibodies and HRP-conjugated secondary antibodies, followed by ECL detection. The following antibodies were used: antiphospho ERK (Thr202/Tyr204), antiphospho JNK (Thr183/Tyr185), antiphospho STAT1 (Tyr701), and anti-beta-Actin (all from Cell Signaling Technology), antiphospho JAK2 (Tyr1007/Tyr1008; Biosource, Camarillo, CA), C14 anti-ERK2, N20 antihuman B7-H1, N19 anti-MyD88, C-20 anti-IRF1, and H-274 anti-TRAF6 (Santa Cruz).
Quantitative real-time polymerase chain reaction
Total RNA was extracted from cell lines and MM, MGUS patient, and normal volunteer (ie, primary) plasma cells using conventional techniques. Levels of human MyD88, TLR2, TLR4, and TLR9 mRNA were assessed using a SYBR Green kit (Applied Biosystems, Foster City, CA) and real-time polymerase chain reaction primer sets2 (SuperArray Bioscience Corporation). B7-H1 mRNA expression was measured using the forward primer (5′-AGG AGA TTA GAT CCT GAG GAA AAC C-3′), reverse primer (5′-GGA CTC ACT TGG TAA TTC TGG GA), and TaqMan Probe (5′FAM-CTG GCA CAT CCT C–3′MGB), with the TaqMan Universal Master Mix (Applied Biosystems). Real-time polymerase chain reaction was performed on an ABI PRISM 7700HT sequence detection system. A 2-fold serial dilution of Jurkat cDNA or lung carcinoma cell line A549 cDNA was selected as a calibrator for quantification of MyD88, TLRs, and B7-H1 mRNA. A negative control containing no RNA template was introduced in each run. 18S ribosomal RNA (PDAR 18S; Applied Biosystems) was amplified as an internal control. An equal quantity of cDNA prepared from 10 ng of mRNA was loaded into each well. Results were measured via standard curves and expressed as ratios.
RNA Interference and cDNA transfection
Small interfering RNA (siRNA) oligonucleotides targeting endogenous human MyD88, TRAF6, IRF1 (Santa Cruz, CA), MEK1, STAT1 (Cell Signaling) and their control siRNAs (final concentration 150 μM in a 500 μl volume) were transfected into 2 × 106 RPMI 8226 cells or 0.5-1.0 × 106 CD138-selected primary myeloma cells, using the Amaxa Nucleofector device (Amaxa, Koeln, Germany) with Cell Line-specific Nucleofector kit V and program U15, according to the manufacturer's recommendations. After electroporation, cells were transferred to 2.0 mL of complete medium and cultured for 24 hours before analysis.
For cDNA transfection of RPMI 8226 cells, 2.5 μg of expression plasmids carrying the wild-type human MyD88 cDNA (pUNO-hMyD88), a dominant-negative mutant (pDeNy-hMyD88), or control empty vector, were stably transfected by electroporation and further selected with blasticidin (all from InvivoGen/Cayla, Toulouse, France).
Signal transduction analyses
To study the signal transduction pathway involved in B7-H1 expression, the following cytokines and reagents were used: AG490 JAK2 inhibitor (25 μM), PD98059 MEK1 inhibitor (25 μM), SB203580 p38MAPK inhibitor (3 μM), LY294002 PI-3K inhibitor (25 μM), SN50 NF-kB inhibitor (20 μM), and TLR4 ligand lipopolysaccharide (500 ng/mL LPS, from Escherichia coli strain O111:B4; Calbiochem, San Diego, CA), SP600125 JNK inhibitor (25μM; Biosource, Camarillo, CA), U0126 MEK1/2 inhibitor (20μM; Cell Signaling Technology, Danvers, MA), recombinant human IFN-γ (500 IU/mL; Peprotech, Rocky Hill, NJ), peptidoglycan TLR2 ligand (PGN; 2.5 μg/mL, from Staphylococcus aureus), CpG oligonucleotide TLR9 ligand (type C, ODN M362; 5 μM), and its control CpG DNA (InvivoGen/Cayla, Toulouse, France), phorbol 13-myristate 12-acetate (PMA; 1 ng/mL), actinomycin D (10μg/mL; ActD), and cycloheximide (10 μg/mL; Sigma, Saint Louis, MO). The activity of the transcription factors ATF-2, c-Jun, c-Myc, MEF2, and STAT1 were detected using a TransAM mitogen-activated protein kinase (MAPK) family kit (Active Motif, Carlsbad, CA). NFκB p50/p65 transcription factor activity was detected using an NFκkB p50/p65 transcription factor assay kit (Chemicon, Temecula, CA).
Generation of cytotoxic T cells
T cells from the peripheral blood of a healthy donor were isolated using a Pan T-cell Isolation Kit (Miltenyi Biotec) and cultured in RPMI 1640 medium (Life Technologies, Gaithersburg, MD) supplemented with 10% fetal calf serum, 100 IU penicillin, 100 mg/mL streptomycin, 2 mM l-eukemia, 50 μM β2-mercaptoethanol, and 20 IU/mL IL-2 (PeProtech, Rocky Hill, NJ). The culture medium was changed every 2 days and T cells stimulated with 100 Gy-irradiated RPMI 8226 cells or RPMI 8226 cells transfected with a dominant-negative mutant of MyD88 (RPMI 8226/DN-hMyD88) or control empty vector, at a 1:1 ratio and added twice per week. After 15 days, dead cells were removed and CD8a+ cells purified using a CD8a+ T cell Isolation Kit (Miltenyi Biotec). CTL activity was assessed, using RPMI 8226 cells as targets, with the Cytotox Non-Radioactive 96 kit (Promega). For blocking of B7-H1, stimulator cells or target cells were pre-incubated with B7-H1 (clone MIH1; Clinisciences, Montrouge, France) blocking antibodies at 2 μg/mL.21,36 To induce B7-H1 expression, stimulator cells were pre-incubated in 500 IU/mL IFN-γ. To block IFN-γ B7-H1 expression, stimulator cells were pre-incubated for 1 hour in 20 μM UO126. The specificity of CTL-mediated lysis of RPMI 8226 cells was verified with HEK-293 cells as target. MHC class I-restricted lysis was verified with an anti-HLA-ABC (clone W6/32; eBiosciences) or an isotopic control. The absence of natural killer (NK) cell-mediated cytotoxicity was verified with K562 cells as targets. Effector CD8 T cells were also pre-treated for 2 hours with 100 nM concanamycin A (CMA; Sigma) to inhibit perforin-mediated killing. In certain experiments, CD138-selected primary MM cells were used as stimulator and as CTL targets under the same conditions as those used for RPMI 8226 cells.
Statistical analyses
Statistical analyses were performed with the Sigma Stat 3.11 software (SPSS Sciences, Chicago, IL).
Results
Expression of B7-H1 in plasma cells from MM and MGUS patients and MM cell lines
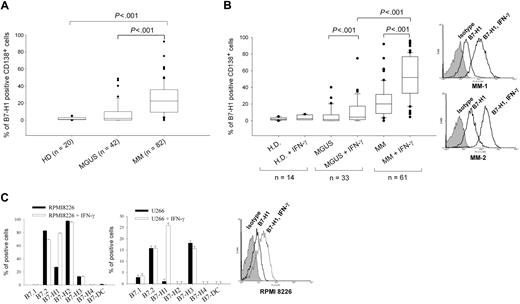
We analyzed B7-H1 expression in CD138-selected cells from 42 MGUS, 82 MM, and 20 healthy donors (patient characteristics detailed in Table 1). B7-H1 expression was not detected in plasma cells from healthy donors (range, 0.1%-2.7%; median, 1; standard deviation [SD], 0.8; Figure 1B). Only 3 MGUS samples of the 42 examined expressed B7-H1 in more than 30% of their plasma cells (range, 0%-48%; median, 2.05; SD, 12.5). However, plasma cells from MM patients expressed significantly higher levels of B7-H1 (range, 0%-92%; median, 23; SD, 18.7) than plasma cells from MGUS patients (P < .001, Mann-Whitney rank-sum test; Figure 1A). No correlation could be found between the level of B7-H1 expression and any patient characteristics examined.
Patient characteristics
| . | MGUS . | MM . |
|---|---|---|
| Total number of patients | 42 | 82 |
| Sex ratio | 1.2 | 2.3 |
| Median age (range) | 61 (32-101) | 64 (36-95) |
| Monoclonal component isotype | ||
| IgG | 33 | 45 |
| IgA | 6 | 16 |
| Light chain | — | 13 |
| Other | 3 | 8 |
| Light chain of the monoclonal component | ||
| Kappa | 28 | 47 |
| Lambda | 14 | 34 |
| Salmon and Durie stage | ||
| I | — | 24 |
| II | — | 10 |
| III | — | 33 |
| Relapsed or refractory | — | 15 |
| . | MGUS . | MM . |
|---|---|---|
| Total number of patients | 42 | 82 |
| Sex ratio | 1.2 | 2.3 |
| Median age (range) | 61 (32-101) | 64 (36-95) |
| Monoclonal component isotype | ||
| IgG | 33 | 45 |
| IgA | 6 | 16 |
| Light chain | — | 13 |
| Other | 3 | 8 |
| Light chain of the monoclonal component | ||
| Kappa | 28 | 47 |
| Lambda | 14 | 34 |
| Salmon and Durie stage | ||
| I | — | 24 |
| II | — | 10 |
| III | — | 33 |
| Relapsed or refractory | — | 15 |
— indicates not applicable.
B7-H1 expression in MM plasma cell lines and in MM and MGUS plasma cells. (A) B7-H1 expression measured by flow cytometry in CD138-selected cells from MM, MGUS, and healthy donors (HDs). (B) Same as (A) but after 24-hour incubation with 500 IU/mL IFN-γ. Two representative histograms of B7-H1 expression of MM cells are also shown. (C) B7-family molecule expression in RPMI 8226 and U266 cells, with or without incubation of 500 IU/mL IFN-γ for 24 hours before flow cytometric analysis. One representative histogram of B7-H1 expression in RPMI 8226 cells is also shown.
B7-H1 expression in MM plasma cell lines and in MM and MGUS plasma cells. (A) B7-H1 expression measured by flow cytometry in CD138-selected cells from MM, MGUS, and healthy donors (HDs). (B) Same as (A) but after 24-hour incubation with 500 IU/mL IFN-γ. Two representative histograms of B7-H1 expression of MM cells are also shown. (C) B7-family molecule expression in RPMI 8226 and U266 cells, with or without incubation of 500 IU/mL IFN-γ for 24 hours before flow cytometric analysis. One representative histogram of B7-H1 expression in RPMI 8226 cells is also shown.
Because B7-H1 is usually an inducible molecule in normal cells, we investigated whether B7-H1 expression could be induced by several cytokines known to have a role in malignant plasma cell survival or to be present in the MM microenvironment. IL-3, IL-4, IL-6, IL-7, granulocyte-macrophage colony-stimulating factor, FLT3-L, SDF-1, and IGF-1 had no effect on B7-H1 expression in plasma cells from MGUS and MM patients (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). However, IFN-γ, which is known to be the main inducer of B7-H1 expression in normal cells, significantly enhanced B7-H1 expression in plasma cells from MM patients (P < .001, paired t test) and MGUS patients (P < .001), but not from healthy donors. Induced B7-H1 protein expression was significantly higher in plasma cells from MM than from MGUS patients (P < .001, Mann-Whitney rank-sum test; Figure 1B). Ninety-three percent of MM patient cells showed IFN-γ–inducible B7-H1 expression. Thus, B7-H1 is overexpressed by MM plasma cells, but not in most MGUS plasma cells, and this expression can be further enhanced by IFN-γ exposure.
We also analyzed the expression of B7 family molecules in 2 MM cell lines. Although expression of B7.2, B7-H1, B7-H2, or B7-H3 was detected in U266 or RPMI 8226 MM cell lines, only B7-H1 expression was enhanced after IFN-γ exposure (Figure 1C).
Induced B7-H1 expression via a MEK/ERK pathway
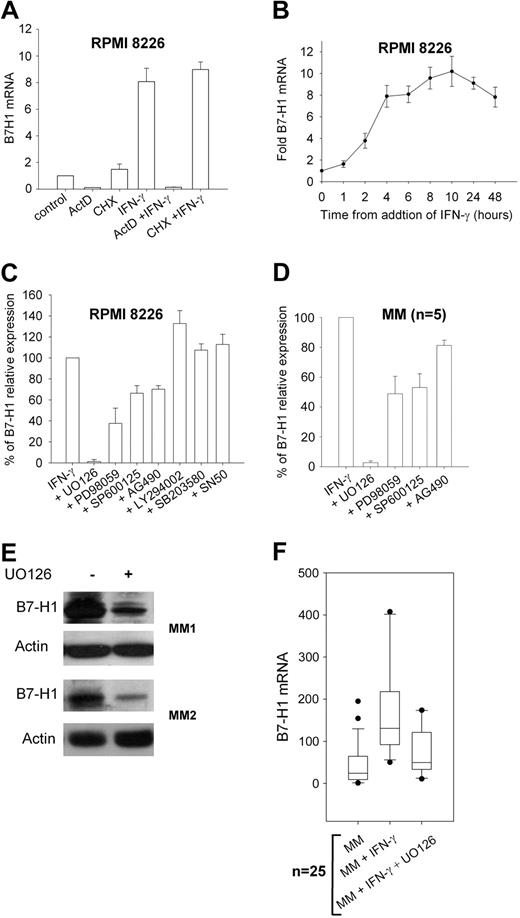
To understand which signaling pathway is involved in IFN-γ–induced B7-H1 expression in MM plasma cells, we first assessed if RNA synthesis was required in this process by blocking RNA synthesis with ActD. The results showed that ActD completely blocked B7-H1 mRNA synthesis (Figure 2A). Blocking protein synthesis with cycloheximide had no effect on B7-H1 mRNA synthesis, indicating that de novo protein synthesis is not required for B7-H1 transcription. Time-course analysis of B7-H1 mRNA levels after the addition of IFN-γ showed that prolonged exposure was needed to reach maximal levels (Figure 2B). We also analyzed B7-H1 mRNA in 10 MGUS samples. Eight were negative for B7-H1 protein expression and showed low but detectable levels of B7-H1 mRNA. IFN-γ upregulated B7-H1 mRNA in only 2 MGUS samples that already expressed B7-H1 protein (data not shown). Thus, the B7-H1 expression induced by IFN-γ in MM plasma cells is mostly attributable to increased B7-H1 mRNA levels. We then incubated the RPMI 8226 MM cell line and plasma cells from MM patients exposed to IFN-γ with several transduction pathway inhibitors (Figure 2C,D). Blocking of p38 MAPK with SB203580, PI3K with LY294002, and NFκB with SN50 did not reduce B7-H1 expression. However, blocking MEK1/2 with UO126 dramatically reduced IFN-γ–induced B7-H1 expression. A slight reduction was also observed after blocking JAK2 with AG490 and JNK with SP600125. We confirmed the results obtained from the flow cytometric analyses with the M1H1 antibody by Western blot analysis using the N20 polyclonal anti-B7-H1 antibody (Figure 2E). UO126 also reduced B7-H1 at the mRNA level in plasma cells from MM patients (Figure 2F).
B7-H1 mRNA expression and signal transduction analysis in MM plasma cells. (A) B7-H1 mRNA levels in RPMI 8226 MM cells measured by real-tie polymerase chain reaction after exposure to 500 IU/mL IFN-γ for 6 hours with or without 10 μg/mL cycloheximide or 10 μg/mL actinomycin D (ActD) for 90 minutes. (B) Time-course analysis of B7-H1 mRNA levels after addition of 500 IU/mL IFN-γ to RPMI 8226 MM cells. (C) Flow cytometric analysis of B7-H1 expression in control RPMI 8226 MM cells (set at 100%) and after 24-hour incubation with IFN-γ with or without 1 hour pre-treatment with signal transduction inhibitors, ie, 20 μM UO126 (MEK1/2), 25 μM PD98059 (MEK1), 25 μM SP600125 (JNK), 25 μM AG490 (JAK2), 25 μM LY294002 (PI3K), 3 μM SB203850 (p38MAPK), and 25 μM SN50 (NFκB). (D) Same as (C) but with 5 MM CD138-selected cell samples. (E) Western blot analysis of B7-H1 levels with N20 polyclonal anti-B7-H1 antibody in 2 MM patient CD138-selected plasma cell samples exposed to 500 IU/mL IFN-γ with or without 1-hour preincubation with 20 μM UO126. (F) B7-H1 mRNA levels in 25 MM CD138-selected cell samples exposed for 24 hours to 500 IU IFN-γ with or without 1-hour pre-treatment with 20 μM UO126.
B7-H1 mRNA expression and signal transduction analysis in MM plasma cells. (A) B7-H1 mRNA levels in RPMI 8226 MM cells measured by real-tie polymerase chain reaction after exposure to 500 IU/mL IFN-γ for 6 hours with or without 10 μg/mL cycloheximide or 10 μg/mL actinomycin D (ActD) for 90 minutes. (B) Time-course analysis of B7-H1 mRNA levels after addition of 500 IU/mL IFN-γ to RPMI 8226 MM cells. (C) Flow cytometric analysis of B7-H1 expression in control RPMI 8226 MM cells (set at 100%) and after 24-hour incubation with IFN-γ with or without 1 hour pre-treatment with signal transduction inhibitors, ie, 20 μM UO126 (MEK1/2), 25 μM PD98059 (MEK1), 25 μM SP600125 (JNK), 25 μM AG490 (JAK2), 25 μM LY294002 (PI3K), 3 μM SB203850 (p38MAPK), and 25 μM SN50 (NFκB). (D) Same as (C) but with 5 MM CD138-selected cell samples. (E) Western blot analysis of B7-H1 levels with N20 polyclonal anti-B7-H1 antibody in 2 MM patient CD138-selected plasma cell samples exposed to 500 IU/mL IFN-γ with or without 1-hour preincubation with 20 μM UO126. (F) B7-H1 mRNA levels in 25 MM CD138-selected cell samples exposed for 24 hours to 500 IU IFN-γ with or without 1-hour pre-treatment with 20 μM UO126.
To confirm that IFN-γ induced B7-H1 expression through a MEK/ERK pathway in malignant plasma cells, we stimulated RPMI 8226 cells or plasma cells from MM patients with IFN-γ and analyzed the phoshorylation of ERK1/2. IFN-γ induced phosphorylation of ERK1/2, which was almost completely blocked by UO126 (Figure 3A,B). IFN-γ also induced phosphorylation of STAT1 at Tyr701. Incubation of RPMI 8226 cells in PMA, a known activator of the MEK/ERK pathway, induced B7-H1 expression and ERK1/2 phosphorylation that were also blocked by UO126 (Figure 3A,C). Finally, silencing MEK1 with a MEK1 siRNA reduced B7-H1 expression both in the RPMI 8226 cell line and in plasma cells from MM patients (Figure 3D).
MEK/ERK-dependent B7-H1 expression in MM cells. (A) Western blot analysis of ERK1/2 and STAT1 phosphorylation in RPMI 8226 MM cells after incubation at indicated times with 500 IU/mL IFN-γ or 1 ng/mL PMA, both with or without 1-hour preincubation with 20 μM UO126. (B) Same as (A) but in a representative MM CD138-selected sample. (C) Flow cytometric analysis of B7-H1 expression in RPMI 8226 cells exposed for 24 hours to 1 ng/mL PMA with or without 1-hour preincubation with 20 μM UO126. (D) Flow cytometric analysis of B7-H1 expression in RPMI 8226 cells or 5 MM CD138-selected cell samples incubated for 24 hours with 500 IU/mL IFN-γ (set at 100%) and transfected with MEK1 or control nonspecific siRNA (NS siRNA).
MEK/ERK-dependent B7-H1 expression in MM cells. (A) Western blot analysis of ERK1/2 and STAT1 phosphorylation in RPMI 8226 MM cells after incubation at indicated times with 500 IU/mL IFN-γ or 1 ng/mL PMA, both with or without 1-hour preincubation with 20 μM UO126. (B) Same as (A) but in a representative MM CD138-selected sample. (C) Flow cytometric analysis of B7-H1 expression in RPMI 8226 cells exposed for 24 hours to 1 ng/mL PMA with or without 1-hour preincubation with 20 μM UO126. (D) Flow cytometric analysis of B7-H1 expression in RPMI 8226 cells or 5 MM CD138-selected cell samples incubated for 24 hours with 500 IU/mL IFN-γ (set at 100%) and transfected with MEK1 or control nonspecific siRNA (NS siRNA).
MyD88-dependent expression of B7-H1
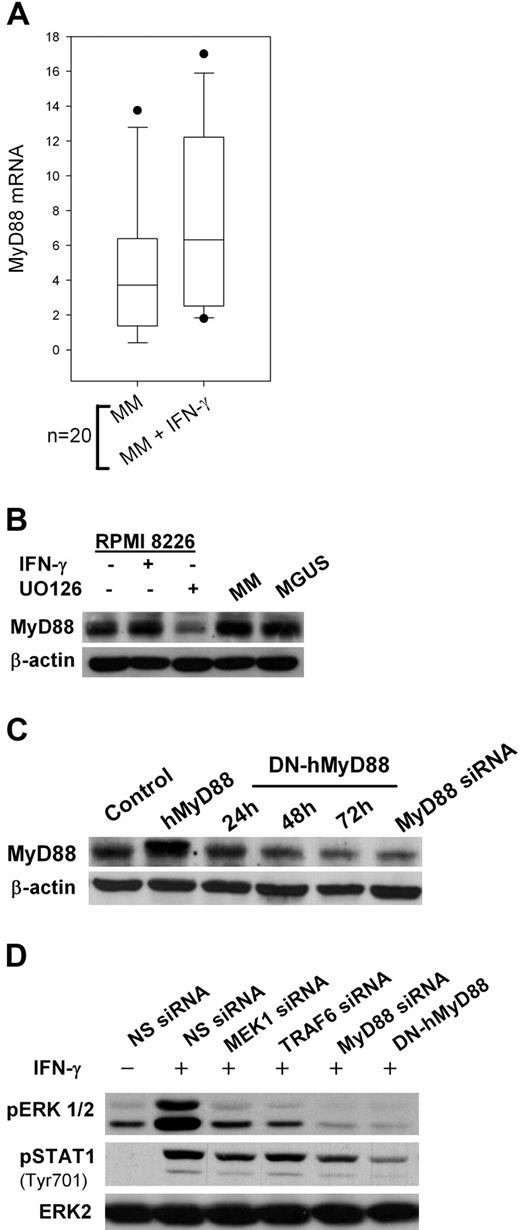
IFN-γ signaling has been extensively investigated through the JAK/STAT pathway.37 However, recent data indicates that MyD88, an adaptor protein essential for TLR signaling, is also involved in IFN-γ signaling.38 We first observed that MyD88 mRNA levels increased after IFN-γ exposure in plasma cells isolated from MM patients (Figure 4A). MyD88 protein was also detected by Western blot in MM and MGUS patients (Figure 4B). No significant difference in MyD88 expression could be detected between CD-138 cells isolated from MM and MGUS patients (data not shown). UO126 reduced IFN-γ–induced MyD88 expression.
MyD88-dependent pathways in MM plasma cells. (A) Real-time polymerase chain reaction analysis of MyD88 RNA levels in 20 primary MM CD138-selected cell samples incubated for 24 hours with 500 IU/mL IFN-γ. (B) Western Blot analysis of MyD88 expression in RPMI 8226 MM cells exposed for 24 hours to 500 IU/mL IFN-γ with or without 1-hour pretreatment with 20 μM UO126 and in CD138-selected samples from MM and MGUS patients. (C) MyD88 expression in RPMI 8226 cells stably transfected with an expression plasmid carrying a human MyD88 cDNA (hMyD88) or a dominant-negative mutant (DN-hMyD88), or with MyD88 siRNA. (D) ERK1/2 and STAT1 phosphorylation in RPMI 8226 cells transfected with MEK1, MyD88, TRAF6 siRNAs, or DN-hMyD88 cDNA and exposed to 500 IU/mL IFN-γ.
MyD88-dependent pathways in MM plasma cells. (A) Real-time polymerase chain reaction analysis of MyD88 RNA levels in 20 primary MM CD138-selected cell samples incubated for 24 hours with 500 IU/mL IFN-γ. (B) Western Blot analysis of MyD88 expression in RPMI 8226 MM cells exposed for 24 hours to 500 IU/mL IFN-γ with or without 1-hour pretreatment with 20 μM UO126 and in CD138-selected samples from MM and MGUS patients. (C) MyD88 expression in RPMI 8226 cells stably transfected with an expression plasmid carrying a human MyD88 cDNA (hMyD88) or a dominant-negative mutant (DN-hMyD88), or with MyD88 siRNA. (D) ERK1/2 and STAT1 phosphorylation in RPMI 8226 cells transfected with MEK1, MyD88, TRAF6 siRNAs, or DN-hMyD88 cDNA and exposed to 500 IU/mL IFN-γ.
We then transfected RPMI 8226 MM cells with siRNAs to silence MyD88 signaling and observed a significant decrease in IFN-γ-induced ERK1/2 phosphorylation (Figure 4C,D). The same effect was observed after transfection with an expression plasmid encoding a dominant-negative mutant of MyD88 (DN-hMyD88). DN-hMyD88 is a truncated form (aa161-296) of MyD88 that contains the C-terminal TIR domain but lacks the death domain.39 This DN-hMyD88 is unable to homodimerize and, therefore, unable to activate the signaling cascade. Silencing TRAF6, an adaptor protein downstream of MyD88, had the same effect. These data suggest there is a self-reinforcing signaling loop between MyD88 and the MEK/ERK pathway in MM cells. Blocking of MEK, MyD88, or TRAF6 also reduced STAT1 phosphorylation at Tyr701 (Figure 4D).
Blocking the MyD88 pathway in MM plasma cells or RPMI 8226 cells with siRNA against MyD88 or TRAF6, or with the dominant-negative mutant of MyD88, blocked IFN-γ–induced B7-H1 expression (Figure 5A,B). Similar to previously published reports, we detected abundant expression of TLR2, TLR4, and TLR9 in 100%, 69%, and 100% of 23 MM plasma cell samples, respectively.40,41 Incubation of these 23 MM plasma cell samples or RPMI 8226 cells with TLR2, TLR4, and TLR9 ligand (PGN, LPS, and ODN, respectively) induced B7-H1 protein and RNA in MM plasma cells, but not in RPMI 8226 cells transfected with the dominant-negative mutant of MyD88 (Figure 5C,D). Five of the 23 MM samples did not show any inducible B7-H1 expression after exposure to LPS, PGN, or ODN. TLR ligands also induced ERK phosphorylation that was blocked by the dominant-negative mutant of MyD88 or UO126 (Figure 5E). Thus, B7-H1 expression induced by IFN-γ in MM plasma cells is mediated though a MyD88- and MEK-dependent pathway that can also be stimulated by TLR ligands.
MyD88-dependent B7-H1 expression in MM cells. (A) Flow cytometric analysis of B7-H1 expression in RPMI 8226 cells and in 5 CD138-selected MM samples after incubation for 24 hours with 500 IU/mL IFN-γ and transfection with NS, MyD88, or TRAF6 siRNAs. (B) Time-course analysis of B7-H1 expression after addition of 500 IU/mL IFN-γ by RPMI 8226 cells transfected with MyD88 or DN-hMyD88 cDNA. (C) B7-H1 expression in RPMI 8226 cells transfected with DN-MyD88 or a control plasmid and incubated for 24 hour with TLR ligands (500 ng/mL LPS, 2.5 μg/mL PGN, 5 μM ODN). (D) B7-H1 expression in 23 CD138-selected MM samples incubated with TLR ligands. (E) ERK1/2 phophorylation in RPMI 8226 or RPMI 8226/DN-hMyD88 cells treated for 30 minutes with TLR ligands with or without 20 μM UO126.
MyD88-dependent B7-H1 expression in MM cells. (A) Flow cytometric analysis of B7-H1 expression in RPMI 8226 cells and in 5 CD138-selected MM samples after incubation for 24 hours with 500 IU/mL IFN-γ and transfection with NS, MyD88, or TRAF6 siRNAs. (B) Time-course analysis of B7-H1 expression after addition of 500 IU/mL IFN-γ by RPMI 8226 cells transfected with MyD88 or DN-hMyD88 cDNA. (C) B7-H1 expression in RPMI 8226 cells transfected with DN-MyD88 or a control plasmid and incubated for 24 hour with TLR ligands (500 ng/mL LPS, 2.5 μg/mL PGN, 5 μM ODN). (D) B7-H1 expression in 23 CD138-selected MM samples incubated with TLR ligands. (E) ERK1/2 phophorylation in RPMI 8226 or RPMI 8226/DN-hMyD88 cells treated for 30 minutes with TLR ligands with or without 20 μM UO126.
MAPK-activated transcription factors and B7-H1
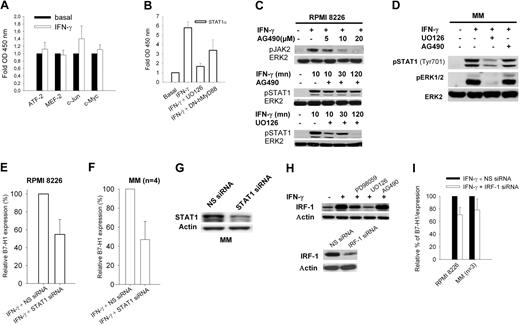
Because our data indicated that the MEK/ERK pathway is a major component of IFN-γ–mediated and TLR-mediated B7-H1 expression, we measured in RPMI 8226 cell nuclear extracts the quantity of phosphorylated ATF2, c-Myc, c-Jun, STAT1α, and MEF2 transcription factors bound on consensus DNA binding sites. All these transcription factors are known downstream effectors of the MAPK pathway. No or insignificant increases in ATF2, c-Myc, c-Jun, and MEF2 were detected after stimulation with IFN-γ (Figure 6A). Using the same method, we also analyzed p50 and p65 NF-kB subunits, but no significant increase was again observed after IFN-γ exposure (data not shown), a point also demonstrated by the absence of an inhibitory effect of the SN50 NF-kB inhibitor on B7-H1 expression (Figure 3A). However, a marked increase in STAT1α transcriptional activity was observed, which was blocked by UO126, and reduced in DN-hMyD88 transfected cells (Figure 6B). Thus, STAT1α appears to be the main transcription factor activated by IFN-γ in MM plasma cell lines, at least partially via MyD88/MEK/ERK-dependent pathways. This was confirmed by detection of STAT1α phosphorylation that was reduced by UO126 (Figure 6C). Inhibition of JAK2 with AG490 had only a slight effect on STAT1 phosphorylation, suggesting that MEK/ERK is the main activating pathway for STAT1 in MM plasma cell lines. Very similar results were observed for plasma cells isolated from MM patients (Figure 6D). Finally, transfection of STAT1 siRNA in RPMI 8226 cells and plasma cells from MM patients reduced IFN-γ–induced B7-H1 expression, suggesting that STAT1α is one of the main effectors of B7-H1 expression in MM plasma cells (Figure 6E-G). A recent study in A549 cells has indicated that IRF-1, which is one of the targets of STAT1α, could mediate IFN-γ–mediated B7-H1 expression.42 Consistent with the STAT1 activity demonstrated in nuclear extracts, we also detected increased IRF1 expression in RPMI 8226 cells exposed to IFN-γ. This expression was reduced by blocking MEK1/2 with UO126 and MEK1 with PD98059, but not JAK2 with AG490 (Figure 6H). Transfection of RPMI 8226 cells or 3 MM plasma cell cultures with IRF-1 siRNA showed only a slight reduction in B7-H1 expression, suggesting that in MM plasma cells IRF-1 is not the main factor responsible for the increased B7-H1 expression induced by IFN-γ (Figure 6I).
STAT1 and IRF-1 activity and B7-H1 expression. (A) TransAM assay of MAPK-regulated transcription factors. (B) TransAM assay of STAT1α transcription factor in nuclear extracts from RPMI 8226 or RPMI 8226/DN-hMyD88 cells incubated for 30 minutes with 500 IU/mL IFN-γ and with or without 1-hour preincubation with 20 μM UO126. (C) JAK2 and STAT1 phosphorylation in RPMI 8226 cells exposed for 30 minutes to 500 IU/mL IFN-γ with or without 1-hour preincubation with 25 μM AG490 or 20 μM UO126. (D) Same as (C) but from a representative CD-138-selected MM sample. (E) Flow cytometric analysis of B7-H1 expression in RPMI 8226 cells transfected with control NS or STAT1 siRNAs. (F) Same as (E) but with 4 CD138-selected MM samples. (G) Western blot analysis of STAT1 expression in a representative CD138-selected MM sample after transfection with control NS or STAT1 siRNAs (H) Western blot analysis of IRF-1 expression after incubation for 24 hours with 500 IU/mL IFN-γ and pre-treated for 1 hour with 20 μM UO126 or 25 μM PD98059. (I) Flow cytometric analysis of B7-H1 expression by RPMI 8226 cells or 3 CD138-selected MM samples incubated for 24 hours with IFN-γ with control NS or IRF-1 siRNA.
STAT1 and IRF-1 activity and B7-H1 expression. (A) TransAM assay of MAPK-regulated transcription factors. (B) TransAM assay of STAT1α transcription factor in nuclear extracts from RPMI 8226 or RPMI 8226/DN-hMyD88 cells incubated for 30 minutes with 500 IU/mL IFN-γ and with or without 1-hour preincubation with 20 μM UO126. (C) JAK2 and STAT1 phosphorylation in RPMI 8226 cells exposed for 30 minutes to 500 IU/mL IFN-γ with or without 1-hour preincubation with 25 μM AG490 or 20 μM UO126. (D) Same as (C) but from a representative CD-138-selected MM sample. (E) Flow cytometric analysis of B7-H1 expression in RPMI 8226 cells transfected with control NS or STAT1 siRNAs. (F) Same as (E) but with 4 CD138-selected MM samples. (G) Western blot analysis of STAT1 expression in a representative CD138-selected MM sample after transfection with control NS or STAT1 siRNAs (H) Western blot analysis of IRF-1 expression after incubation for 24 hours with 500 IU/mL IFN-γ and pre-treated for 1 hour with 20 μM UO126 or 25 μM PD98059. (I) Flow cytometric analysis of B7-H1 expression by RPMI 8226 cells or 3 CD138-selected MM samples incubated for 24 hours with IFN-γ with control NS or IRF-1 siRNA.
T-cell inhibition by IFN-γ–stimulated and TLR-stimulated MM cells
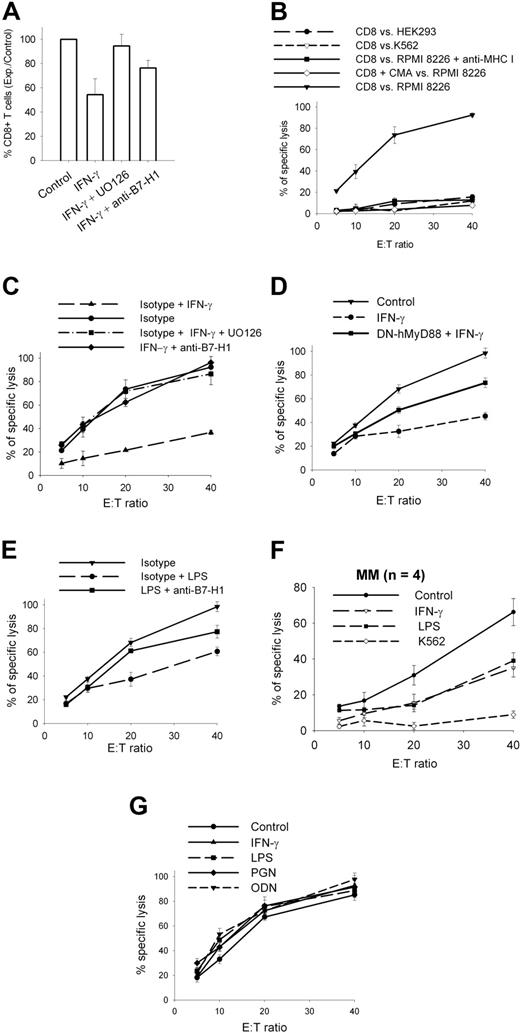
B7-H1 is a known inhibitor of T-cell functions, including CTL-mediated lysis. We cocultured allogenic T-cells with RPMI 8226 MM cells for 2 weeks and measured CD8+ T-cell expansion. Incubation of RPMI 8226 MM cells with IFN-γ significantly reduced CD8+ T-cell expansion (Figure 7A). This effect was partially reversed when RPMI 8226 MM cells were pre-incubated with anti-B7-H1 antibodies or with UO126 (ie, MEK1/2 inhibitor) before being added to the T-cell culture. We then measured CTL-mediated lysis of RPMI 8226 MM cells with CD8+ T cells purified from these different expanded populations (Figure 7B-D). Specific CD8+ T cells expanded with RPMI 8226 MM cells pre-incubated with IFN-γ showed significantly reduced killing of target cells (Figure 7C), and this inhibitory effect of IFN-γ was blocked when UO126 or the anti-B7-H1 antibody was added during pre-incubation. Thus, stimulation of MM plasma cells with IFN-γ reduced, via a MEK/ERK/B7-H1 pathway, CD8 T-cell expansion and CTL activity against MM plasma cells. RPMI 8226/DN-hMyD88 cells showed less efficacy of the IFN-γ inhibitory effect on CTLs generation, confirming that IFN-γ induces a T-cell inhibitory effect in MM plasma cells via MyD88 (Figure 7D). Stimulation of TLR4 by preincubation of RPMI 8226 MM cells with LPS had a similar, but slightly lower, inhibitory effect on CTL generation, which was partially reversed with anti-B7-H1 antibodies (Figure 7E). Similar inhibitory effects of IFN-γ and LPS were also observed with plasma cells from MM patients (Figure 7F). This suggests that the LPS-induced immunosuppressive effect on MM cells is mediated by B7-H1 and, possibly, by other molecules.
T-cell inhibition by MM plasma cells. (A) CD8+ T-cell expansion after a 2-week coculture with irradiated RPMI 8226 MM cells pre-incubated with 500 IU/mL IFN-γ and 20 μM UO126 or anti-B7-H1 or isotype control. (B) CTL activity of CD8-sorted T cells expanded for 2 weeks with irradiated RPMI 8226 cells, against HEK 293, K562, and RPMI 8226 cells. (C) CTL activity against RPMI 8226 cells of CD8-sorted T cells expanded for 2 weeks with irradiated RPMI 8226 cells pre-incubated with 500 IU/mL IFN-γ + /− 20 μM UO126 or anti-B7-H1 or isotype control. (D) Same as (C) but with CD8-sorted T cells expanded with irradiated RPMI 8226/DN-hMyD88 cells. (E) Same as (C) but 500 ng/mL LPS instead used for preincubation of stimulator cells in coculture. (F) CTL activity against CD-138 selected MM cells of CD8-sorted T cells expanded for 2 weeks with irradiated CD138-selected samples pre-incubated for 24 hours with 500 IU/mL IFN-γ or 500 ng/mL LPS used as stimulator cells in coculture. Results are means and SD of 4 samples. (G) Same as (B) but against target cells pre-incubated for 1 hour with TLR ligands (500 ng/mL LPS, 2.5 μg/mL PGN, 5 μM ODN) or 500 IU/mL IFN-γ before CTL assay. Data represent mean and SD of experiments performed twice in quadruplicate.
T-cell inhibition by MM plasma cells. (A) CD8+ T-cell expansion after a 2-week coculture with irradiated RPMI 8226 MM cells pre-incubated with 500 IU/mL IFN-γ and 20 μM UO126 or anti-B7-H1 or isotype control. (B) CTL activity of CD8-sorted T cells expanded for 2 weeks with irradiated RPMI 8226 cells, against HEK 293, K562, and RPMI 8226 cells. (C) CTL activity against RPMI 8226 cells of CD8-sorted T cells expanded for 2 weeks with irradiated RPMI 8226 cells pre-incubated with 500 IU/mL IFN-γ + /− 20 μM UO126 or anti-B7-H1 or isotype control. (D) Same as (C) but with CD8-sorted T cells expanded with irradiated RPMI 8226/DN-hMyD88 cells. (E) Same as (C) but 500 ng/mL LPS instead used for preincubation of stimulator cells in coculture. (F) CTL activity against CD-138 selected MM cells of CD8-sorted T cells expanded for 2 weeks with irradiated CD138-selected samples pre-incubated for 24 hours with 500 IU/mL IFN-γ or 500 ng/mL LPS used as stimulator cells in coculture. Results are means and SD of 4 samples. (G) Same as (B) but against target cells pre-incubated for 1 hour with TLR ligands (500 ng/mL LPS, 2.5 μg/mL PGN, 5 μM ODN) or 500 IU/mL IFN-γ before CTL assay. Data represent mean and SD of experiments performed twice in quadruplicate.
Several reports have shown that B7-H1 can also mediate a direct inhibitory effect on CTL-mediated lysis. We pre-incubated target RPMI 8226 cells with IFN-γ or various TLR ligands and measured CTL lysis with CD8+ T cells, but were unable to detect any inhibitory effect (Figure 7G). Thus, the inhibitory effect on T cells of MM cells stimulated with IFN-γ or TLR ligands probably occurs during the generation of an immune response rather than at the effector phase.
Discussion
Immunosuppressive properties of malignant plasma cells have been previously reported; however, the precise nature of the signals produced by these cells that inhibit T-cell function remain elusive. We observed here that the B7-H1 molecule is expressed by CD-138+ cells isolated from the majority of MM patients but not from MGUS patients (except for a few samples), and not by CD-138+ cells from normal volunteers. These data indicate that B7-H1 expression is a characteristic of malignant plasma cells and suggests that it is acquired during transition from MGUS to MM. Marked B7-H1 expression has been reported for several human and mouse tumors and is associated with a worse prognosis in renal carcinoma.43 Blocking B7-H1 enhances the effect of tumor vaccines.31,,–34 Thus, it can be hypothesized that expression of B7-H1 could also participate in the transition from MGUS to MM by causing inhibition of immunosurveillance. Expression of tumor antigens has been reported in MGUS, suggesting that these cells could be recognized by T cells.7,8 We observed that B7-H1 expression was further enhanced in MM cells by IFN-γ. B7-H1 was the only B7-family molecule induced by IFN-γ in MM cell lines. IFN-γ was the main inducer of B7-H1 expression in malignant CD138-selected cells. Preincubation of MM cells with IFN-γ inhibited CD8+ T-cell expansion and activity of CTLs against MM cells and this effect was blocked by anti-B7-H1 antibodies. Thus, we can hypothesize, that IFN-γ produced by T cells in the MM microenvironment can induce T-cell inhibition by plasma cells through B7-H1. B7-H1 has also been reported to directly inhibit CTL-mediated lysis.16,21,–23 In our experiments with MM cells, induction of B7-H1 expression on target cells just prior to the addition of effector T cells had no effect on CTL-mediated lysis. Thus, expression of B7-H1 by MM cells is likely to inhibit induction of the T-cell response rather than the CTL effector phase. It also suggests that the mechanism of immune escape induced by B7-H1 expression may vary among tumor types.
We observed that only a few MGUS samples showed significant expression of B7-H1 after exposure to IFN-γ. This induced expression was not observed in plasma cells isolated from healthy donors. This may indicate that the B7-H1 response to IFN-γ is a distinct, defining characteristic between MM and MGUS, or that a few MGUS patients can acquire some early immunologic characteristics of MM cells.
Little is known about the mechanisms leading to B7-H1 expression. We inhibited, with specific inhibitors, several components of the JAK/STAT, MAPK, NFκB, and PI3K/AKT pathways known to mediate IFN-γ signaling. Inhibition of MEK1/2 with UO126 or siRNA almost completely blocked INF-γ–induced B7-H1 expression by plasma cells from MM patients. A partial inhibition of B7-H1 expression was observed when JAK2 was blocked with AG490 and JNK with SP600125, but no effect could be observed when the p38MAPK, PI3K, or NFκkB pathways were blocked. UO126 was also able to block the inhibitory effect of IFN-γ–stimulated plasma cells on T cells. Thus, the MEK/ERK pathway seems a major contributor responsible for IFN-γ–induced expression of B7-H1 in malignant plasma cells. Several studies have established that, in addition to the classical JAK/STAT pathway, IFN-γ activates MAPK.37 p38 MAPK can activate STAT1 through phosphorylation of serine 727.44 ERK activates C/EBP-dependent gene transcription through IFN-γ.45 A recent study reported induction of B7-H1 expression in dermal fibroblasts through phosphorylation of ERK and PI3K.46 Our data do not support a role for PI3K/AKT in induced B7-H1 expression, but it seems likely that IFN-γ signaling is mediated through different pathways depending on the cell types involved.
MyD88 adaptor protein is essential for TLR signaling. Stimulation of TLRs triggers the association of MyD88, which in turn recruits IRAK4 (IL-1R associated kinase 4), thereby allowing association with IRAK1 and the adaptor protein TRAF6.47 These associations lead to activation of downstream kinases, allowing activation of MAPK and NFκB. A recent report has described MyD88 as a new component required for IFN-γ signaling: MyD88 associates with IFN-γR1, resulting in p38MAPK activation and stabilization of transcripts encoding TNF-α and IP-10.38 Another report showed that activation of TLR4 on mouse tumor cells can induce B7-H1 expression and TLR expression has recently been described in plasma cells isolated from MM patients. This prompted us to explore the possibility that MyD88 could be a common component involved in B7-H1 expression through IFN-γ and TLR signaling. We first observed that MyD88 was expressed both in MGUS and MM patient CD-138–selected cells and this expression was enhanced by IFN-γ exposure. We observed that ERK phosphorylation induced by IFN-γ was inhibited by transfection of MyD88 siRNA, a dominant-negative mutant of MyD88, or TRAF6 siRNA. Thus, the MyD88 pathway is essential for ERK phosphorylation induced by IFN-γ in MM plasma cells. Then we observed that blocking MyD88 or TRAF6 also inhibited the induction of B7-H1 expression by IFN-γ. Stimulation of MyD88 with TLR ligands also resulted in ERK phosphorylation and B7-H1 expression that was blocked by MyD88 inhibition. Thus, MyD88 is an essential and common component of IFN-γ signaling and TLR-driven expression of B7-H1 in MM plasma cells.
MM plasma cells transfected with a dominant-negative mutant of MyD88 also showed both reduced inhibition of IFN-γ on T-cell expansion and CTL generation, whereas LPS stimulation of MM plasma cells led to T-cell inhibition that was reversible with anti-B7-H1 antibodies. Two recent reports have described TLR expression in malignant plasma cells from MM patients, including TLR1, TLR4, TLR7, and TLR9.40,41 Stimulation of these TLRs resulted in IL-6 production and MM cell proliferation. MM patients are vulnerable to infections and it has been hypothesized that microorganisms might trigger TLR on MM plasma cells and, thereby, promote tumor disease progression. Our data indicate that triggering MyD88 through TLR or IFN-γ in MM plasma cells could contribute to T-cell dysfunction by overexpression of B7-H1. We can hypothesize that T cells recognizing MM-associated tumor antigens would produce IFN-γ that then induces B7-H1 overexpression on malignant plasma cells, and this then blocks T-cell activation. Repeated infections in patients would also contribute to B7-H1 expression through PAMP released by microorganisms and, thereby, further promote tumor cell escape from T cells.
A previous study regarding B7-H1 expression in macrophages and Th1 cells indicated that B7-H1 expression induced by IFN-γ is dependent on STAT1.48 Our data indicate that IFN-γ–induced B7-H1 expression in MM plasma cells is mediated through MyD88/TRAF6 and MEK/ERK. Classical TLR signaling through MyD88 can activate MAPK and NFκB. We investigated which transcription factors were activated by measuring in nuclear extracts the quantity of transcription factors bound on specific DNA sequences. We only detected an increase in STAT1 binding after IFN-γ stimulation, which was inhibited by a dominant negative mutant of MyD88 and UO126. Blocking STAT1 with siRNA reduced B7-H1 expression. Thus, in malignant plasma cells, STAT1 is involved in B7-H1 expression induced by IFN-γ. These results are similar to those obtained by Loke et al48 with mouse STAT1−/− macrophages stimulated with LPS and IFN-γ. However, our results constitute the first demonstration that IFN-γ signaling and B7-H1 expression is connected in malignant plasma cells through a MyD88/MEK/ERK-dependent and, at least partially, STAT1-dependent pathway. IRF-1, which is one of the main targets of IFN-γ signaling, was upregulated in plasma cells exposed to IFN-γ but reduced when UO126 and PD98059 were added, thus confirming a major role for the MEK/ERK pathway in IFN-γ signaling in malignant plasma cells. A recent study in A549 cells demonstrated that IRF-1 binds to a specific site in the B7-H1 putative promoter and contributes to the constitutive expression of B7-H1 in these cells.42 In this study, B7-H1 expression was inhibited by AG490, suggesting stimulation via a direct JAK/STAT/IRF-1 pathway. We observed only a slight reduction of B7-H1 expression in plasma cells transfected with IRF-1 siRNA and the JAK2 inhibitor AG490 had only a modest effect. Thus, B7-H1 expression is likely to be modulated through different pathways among different tumor cell types. This does not rule out a role for a direct JAK2/STAT1/IRF-1 pathway in malignant plasma cells, but does indicate that the MyD88/MEK/ERK/STAT1 pathway is a main inducer of B7-H1 in these cells.
In summary, we have shown that B7-H1 is expressed on malignant plasma cells, that it is involved in inhibition of T-cell responses by these malignant cells, and is upregulated by IFN-γ and TLR ligands through a common pathway involving MEK/ERK and MyD88. These findings may lead to new therapeutic developments; for instance, blocking the MEK/ERK pathway in MM patients with specific inhibitors at selected times just before T-cell–based immunotherapy. Further development of MEK inhibitors, usable in vivo, would be especially helpful to further investigate this potential targeted therapy against immune escape mechanisms by malignant cells.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Ligue Nationale Contre le Cancer (Comité du septentrion). J.Z. is a recipient of a grant from the Fondation pour la Recherche Médicale and from the Institut de Recherche sur le Cancer de Lille.
Authorship
Contribution: B.Q. designed the study; J.L., A.H., D.H., and A.S. performed the research and analyzed the data; D.W., V.C., and K.K. provided patient samples and critical suggestions; and J.L. and B.Q. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bruno Quesnel, Service des Maladies du Sang, Centre Hospitalier et Universitaire de Lille, Rue Polonovski, 59037, Lille, France; e-mail: brunoquesnel@hotmail.com.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal