To the editor:
HLA-identical siblings are prime donors for allogenic hematopoietic stem cell transplantation (HSCT). Despite matching for MHC antigens, the risk for graft-versus-host disease (GVHD) remains high.1 Fetomaternal microchimerism has been associated with better survival of maternal compared with paternal grafts2,3 in non–T-cell–depleted, haploidentical HSCT. Fetomaternal4,–6 and transmaternal sibling microchimerism7 have been associated with autoimmune diseases. In analogy, we hypothesized that pretransplantation encounters of recipient cells with their later donors through fetomaternal trafficking should have an impact on outcome in HLA-identical sibling HSCT.
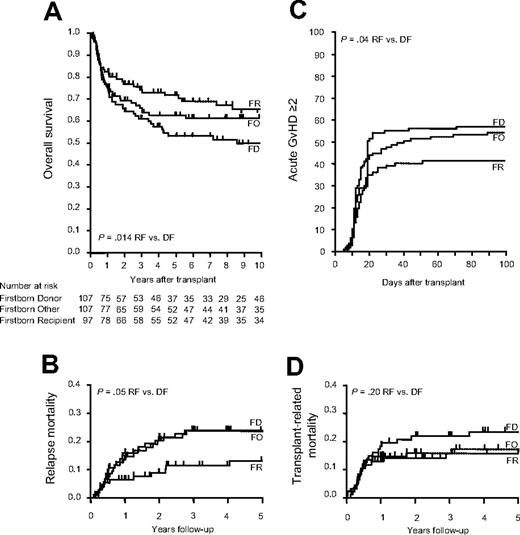
This retrospective single-center cohort study analyzed overall survival (OS), transplantation-related mortality (TRM), relapse mortality (RM), and incidence and severity of acute and chronic GVHD after HLA-identical sibling HSCT. We defined 3 groups based on birth sequence: firstborn donor (FD), firstborn recipient (FR), and others (FO). A total of 311 consecutive patients with complete information on sibling sequence that received transplants from 1980 to 2004 were included. Recipient and donor age and sex, diagnosis, stage of the disease, transplantation date and conditioning regimen, stem-cell source (bone marrow versus peripheral blood stem cells), graft manipulation (T-cell depletion), acute and chronic GVHD and dates of relapse and last clinical visit or death were recorded. A total of 97 patients were FRs, and 107 recipients received a graft from an FD. A total of 107 patients were neither firstborn nor received a graft from a firstborn sibling (FO), and served as a control group. FR patients were by definition older than FD patients, and FO patients had more siblings. No other significant differences for pretransplantation variables were observed (data not shown). A total of 187 (60.1%) patients were alive with a median follow-up of 8.9 years. OS at 10 years was better in FR than FD patients (49.6% vs 63.7%, P = .014; Figure 1A), and cumulative incidence of death from relapse was lower in FR (Figure 1B). Cumulative incidence of grade 2 or higher acute GVHD was 41.2% in FR versus 57.0% in FD (P = .035; Figure 1C). FO patients showed intermediate results. TRM and chronic GVHD were not different among groups (Figure 1D and data not shown). Multivariate analyses strongly confirmed the findings of the univariate tests and excluded recipient/donor age or sibling number as reasons for the observed results (Table 1).
Transplant outcomes depending on sibling sequence. Kaplan-Meier estimate and log-rank comparison of OS (A). Cumulative incidence of RM (B), incidence of acute GVHD (C), and TRM (D). Comparisons were done using the Gray test.
Transplant outcomes depending on sibling sequence. Kaplan-Meier estimate and log-rank comparison of OS (A). Cumulative incidence of RM (B), incidence of acute GVHD (C), and TRM (D). Comparisons were done using the Gray test.
Multivariate analysis for OS and RH
| . | Relative risk . | 95% confidence interval . | P . |
|---|---|---|---|
| OS | |||
| FD | 1.00 | — | — |
| FR | 0.58 | 0.38-0.92 | .02 |
| FO | 0.79 | 0.53-1.21 | .07 |
| Early disease | 1.00 | — | — |
| Advanced disease | 1.75 | 1.19-2.58 | .005 |
| Year of transplantation (risk reduction per year) | 0.97 | 0.94-0.99 | .048 |
| RM | |||
| FD | 1.00 | — | — |
| FR | 0.19 | 0.08-0.46 | < .001 |
| FO | 0.65 | 0.34-1.23 | .19 |
| Early disease | 1.00 | — | — |
| Advanced disease | 2.72 | 1.57-4.70 | < .001 |
| Year of transplantation (risk reduction per year) | 0.97 | 0.94-0.99 | .048 |
| . | Relative risk . | 95% confidence interval . | P . |
|---|---|---|---|
| OS | |||
| FD | 1.00 | — | — |
| FR | 0.58 | 0.38-0.92 | .02 |
| FO | 0.79 | 0.53-1.21 | .07 |
| Early disease | 1.00 | — | — |
| Advanced disease | 1.75 | 1.19-2.58 | .005 |
| Year of transplantation (risk reduction per year) | 0.97 | 0.94-0.99 | .048 |
| RM | |||
| FD | 1.00 | — | — |
| FR | 0.19 | 0.08-0.46 | < .001 |
| FO | 0.65 | 0.34-1.23 | .19 |
| Early disease | 1.00 | — | — |
| Advanced disease | 2.72 | 1.57-4.70 | < .001 |
| Year of transplantation (risk reduction per year) | 0.97 | 0.94-0.99 | .048 |
Multivariate analysis for survival and relapse mortality by Cox proportional hazard models. Variables included in the final models were tested by using a time-dependent covariate method to determine whether the proportional hazards assumption was met. Only significant (P < .05) results are shown.
We describe a significant impact of sibling primacy on the incidence of acute GVHD, relapse incidence, and overall survival in HLA-identical HSCT: patients who were born as a first child in a family had the best survival, with a significant reduction in acute GVHD and relapse mortality. Possible mechanisms include fetomaternal and transmaternal sibling cell trafficking and tolerization of the donor. If confirmed, birth order could be integrated into the donor-selection algorithm in HLA-identical sibling HSCT, or matched unrelated donors might be preferred over a high-risk HLA-identical sibling in some cases.
Authorship
Correspondence: Christoph Bucher, Pediatrics BMT, University of Minnesota, MMC 360 Mays, 420 Delaware St SE, Minneapolis, MN 55455. e-mail: christoph.bucher@gmail.com.
The study has been approved by the local ethics committee (ref. no. 312/06) and was supported by a grant from the Swiss National Research Foundation (3200B0-106105).
Contribution: C.B. collected and analyzed data and wrote the paper; M.S. analyzed data and wrote the paper; A.G. designed the study and wrote the paper; J.P. analyzed data; A.B., A.T., S.M.-M., M.P., J.H., D.H., D.T., and A.D. collected data and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal