A prospective, multicenter, nonrandomized phase 2 trial was conducted to evaluate the efficacy and safety of a single dose of yttrium-90 (90Y) ibritumomab tiuxetan in elderly patients in first relapsed or primary refractory diffuse large B-cell lymphoma (DLBCL) ineligible for stem-cell transplantation. Patients had been previously treated with chemotherapy (group A, n = 76) or chemotherapy plus rituximab (group B, n = 28). Patients in group A were further divided into patients in whom induction therapy had failed (stratum AI, n = 33) and patients who had relapsed after achieving complete response (CR; stratum AII, n = 43). The overall response rate (ORR) was 52% and 53% in strata AI and AII, respectively, and 19% in group B, with CR/CRu rates of 24%, 39.5%, and 12%, respectively. Median progression-free survival was 5.9 months and 3.5 months in strata AI and AII, respectively, and 1.6 months in group B. Median overall survival was 21.4, 22.4, and 4.6 months in stratum AI, stratum AII, and group B, respectively. Two patients died from thrombocytopenic cerebral bleeding following administration of therapy. Nonhematologic adverse events were mild to moderate. 90Y-ibritumomab is active in patients with relapsed and refractory diffuse large B-cell lymphoma (DLBCL) and its further evaluation in phase 3 studies is ongoing.
Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most frequent non-Hodgkin lymphoma (NHL) subtype, accounting for 30% to 35% of all cases. Approximately 40% of patients with advanced-stage DLBCL can be cured with cyclophosphamide, doxorubicin, vincristine, and prednisone (CHOP) or CHOP-like chemotherapeutic regimens.1,2 Over the past 5 years, the addition of the anti-CD20 monoclonal antibody, rituximab, to CHOP (R-CHOP) has resulted in significantly improved complete response (CR) rates, progression-free survival (PFS), and overall survival (OS) in first-line therapy and has now become the standard of care for these patients.3,4 Despite this improvement, the treatment of DLBCL remains unsatisfactory for patients with high-intermediate and high International Prognostic Index (IPI) score at diagnosis,5 where the benefits of the addition of rituximab are less pronounced and new treatment strategies are thus required.6 One such approach is the radioimmunoconjugate yttrium-90 (90Y) ibritumomab tiuxetan (Zevalin; Bayer Schering Pharma, Berlin, Germany, and Biogen Idec, San Diego, CA), which is a murine IgG1 anti-CD20 monoclonal antibody (IDEC-2B8) and is covalently linked to the high-energy, pure beta-emitting 90Y nuclide (2.3 MeV) by the chelator tiuxetan (MX-DTPA). The radiometal 90Y has a range of up to 5 mm in tissue, which equates to between 200 and 300 cell paths, allowing for the radiation crossfire effect, whereby tumor cells are killed at a distance from the antibody-binding antigen. Yttrium-90 ibritumomab has produced overall responses in 74% to 83% of patients with indolent NHL and durable CR/unconfirmed CR (CRu) in 34% of cases.7,–9 As part of these studies, 12 patients with aggressive NHL were treated with 90Y-ibritumomab; in this patient group, a 58% overall response rate (ORR) and a median time to progression of 4.6 months (range, 0.9 to 68.6 + months) were found,10,11 with 2 of 5 patients with DLBCL remaining in remission beyond 5 years
Given these early data, we have now further assessed the safety and efficacy of 90Y-ibritumomab in a larger patient population and report the activity of 90Y-ibritumomab in a group of patients with first relapsed or refractory DLBCL older than 60 years ineligible for autologous stem-cell transplantation (SCT). We demonstrate high response rates to 90Y-ibritumomab in relapsed DLBCL patients initially sensitive to chemotherapy and responses also in patients who were chemorefractory. Given these data, studies integrating 90Y-ibritumomab into first-line immunochemotherapy regimens are currently under way, and will define the potential role of this agent in the overall management of DLBCL.
Patients and methods
Eligibility criteria
Using the Revised European American Lymphoma or World Health Organization (WHO) classification system, we included histologically confirmed CD20+ first relapse or primary refractory DLBCL patients (at least 60 years of age) with measurable disease in whom myeloablative therapy with SCT was not deemed to be an appropriate subsequent treatment option due to age or frailty. Central pathology review was performed after patient inclusion, excluding patients with transformed DLBCL. Eligibility criteria included one prior treatment with CHOP or a CHOP-like regimen, WHO performance status of 0 to 2, life expectancy of at least 3 months, less than 25% bone marrow involvement, absolute neutrophil count (ANC) of at least 1.5 × 109/L, hemoglobin level of at least 90 g/L (9 g/dL), and platelet count of at least 150 × 109/L. In accordance with the Declaration of Helsinki, patients provided written informed consent to participate in the protocol, which was approved by the participating institutions' institutional review boards and radiation safety committees.
Exclusion criteria were as follows: prior myeloablative therapies, prior radiotherapy to more than 25% of active bone marrow, any specific antilymphoma therapy less than 4 weeks before study treatment (prednisone at doses of no greater than 20 mg/d or the equivalent within 2 weeks of the study treatment was allowed), granulocyte colony-stimulating factor or granulocyte-macrophage colony-stimulating factor within 2 weeks of the study treatment, known HIV positivity, central nervous system lymphoma, abnormal liver or renal function, concurrent severe and/or uncontrolled medical disease, human anti-murine antibody (HAMA) reactivity, or known sensitivity to murine antibodies.
Study design
At the time we designed the study, data demonstrating the benefit of adding rituximab to CHOP chemotherapy were only just beginning to emerge.12 Therefore, we divided the patients into 2 main groups depending on prior therapy: those who had previously received CHOP or a CHOP-like regimen alone (group A) or rituximab plus CHOP or CHOP-like regimen (group B). The target number of patients in group B was lower because the shift from CHOP to R-CHOP had not occurred in clinical practice at the time the recruitment period started. Group A patients were further stratified based on whether they were induction failures (had not achieved a CR/CRu to first-line therapy, stratum AI) or relapsed after having achieved a CR (stratum AII). The dosimetry methodology used as part of the regulatory studies in follicular lymphoma was not mandatory in this trial, but was performed at some centers according to local regulations or physician preferences. In the absence of imaging, the first rituximab infusion was given alone. One week later, a second infusion of rituximab was immediately followed by a single dose of 14.8 MBq/kg (0.4 mCi/kg) 90Y-ibritumomab calculated based on actual body weight (maximum dose: 1184 MBq [32 mCi]), given as a slow intravenous push over 10 minutes, as described by Cheson.13 Yttrium-90 ibritumomab was routinely administered on an outpatient basis in view of the lack of gamma emissions.
End points
The primary end points were the ORR and CR rate. Response to treatment was evaluated by serial examinations at 6, 12, and 24 weeks after 90Y-ibritumomab treatment by computed tomography (CT) scans following International Workshop Response Criteria.14 Secondary study end points included PFS, OS, safety and tolerability, and quality of life. The follow-up period continued until 1 year after the last patient received 90Y-ibritumomab.
Safety and tolerability were assessed by monitoring incidence, severity, and type of adverse event. Adverse events were graded according to the National Cancer Institute common toxicity criteria, version 2.0. Laboratory studies included baseline and end of treatment (week 24) electrocardiogram, chest x-ray, immunoglobulin, and HAMA measurements. During the treatment period, physical examinations and assessments of disease-related symptoms were performed weekly. Complete differential blood and platelet counts were obtained before 90Y-ibritumomab administration, weekly thereafter for 12 weeks, and again at week 24. When platelets dropped below 30 × 103/μL, thrice weekly monitoring of hematologic parameters was performed. Platelet transfusions to maintain platelet levels at 10 × 103/μL, or 20 × 103/μL in patients with a higher risk of bleeding were recommended.
Statistical analysis
Overall response rate, CR and PR, demographic and background characteristics, vital signs, and safety measurements were tabulated using descriptive statistics. Secondary parameters—PFS and OS—were analyzed by the Kaplan-Meier method.
Results
Patient characteristics
A total of 104 patients were enrolled in the study between September 2001 and October 2003 from 26 centers in 4 countries. The patient demographics and baseline characteristics are listed in Table 1. Two patients did not receive 90Y-ibritumomab because the platelet counts were too low (n = 1), or a technical problem occurred with the radiolabeling (n = 1). Efficacy data (full analysis set) are available from 102 patients (stratum AI, n = 33; stratum AII, n = 43; group B, n = 26).
Patient demographic and baseline characteristics
| Baseline characteristics . | Group A . | Group B previous therapy: chemotherapy + rituximab*n = 28 . | |
|---|---|---|---|
| Previous therapy: chemotherapy alone . | |||
| Stratum AI, n = 33 . | Stratum AII, n = 43 . | ||
| Male, no. | 20 | 23 | 12 |
| Female, no. | 13 | 20 | 16 |
| Age, years (mean ± st dev) | 72.7 ± 7.3 | 71 ± 5.6 | 72.1 ± 6.0 |
| Weight, kg (mean ± st dev) | 70.6 ± 10.7 | 73.4 ± 15.7 | 65.3 ± 15.2 |
| WHO performance status, %; | |||
| 0 | 50.0 | 58.1 | 36.0 |
| 1 | 40.6 | 37.2 | 41.0 |
| 2 | 9.4 | 4.7 | 9.0 |
| Bone marrow involvement, no. (%) | 0 (0) | 2 (4.7) | 2 (7) |
| Bulky tumor greater than 5 cm, % | 37.5 | 37.2 | 52.0 |
| IPI at initial diagnosis, no. (%) | |||
| 1 (low) | 8 (24.2) | 11 (25.6) | 0 (0.0) |
| 2 (low-intermediate) | 9 (27.3) | 14 (32.6) | 8 (28.6) |
| 3 (high-intermediate) | 8 (24.2) | 8 (18.6) | 9 (32.1) |
| Higher than 4 (high) | 3 (9.1) | 7 (16.3) | 8 (28.6) |
| Unknown | 5 (15.2) | 3 (7.0) | 3 (10.7) |
| Response to first-line therapy, no. (%) | n = 33 | n = 43 | n = 26 |
| ORR | 22 (67) | 43 (100) | 17 (65) |
| CR | — | 40 (93) | 10 (38.5) |
| CRu | — | 3 (7) | 3 (11.5) |
| PR | 22 (67) | — | 4 (15) |
| SD | 3 (9) | — | — |
| PD | 8 (24) | — | 9 (35) |
| Baseline characteristics . | Group A . | Group B previous therapy: chemotherapy + rituximab*n = 28 . | |
|---|---|---|---|
| Previous therapy: chemotherapy alone . | |||
| Stratum AI, n = 33 . | Stratum AII, n = 43 . | ||
| Male, no. | 20 | 23 | 12 |
| Female, no. | 13 | 20 | 16 |
| Age, years (mean ± st dev) | 72.7 ± 7.3 | 71 ± 5.6 | 72.1 ± 6.0 |
| Weight, kg (mean ± st dev) | 70.6 ± 10.7 | 73.4 ± 15.7 | 65.3 ± 15.2 |
| WHO performance status, %; | |||
| 0 | 50.0 | 58.1 | 36.0 |
| 1 | 40.6 | 37.2 | 41.0 |
| 2 | 9.4 | 4.7 | 9.0 |
| Bone marrow involvement, no. (%) | 0 (0) | 2 (4.7) | 2 (7) |
| Bulky tumor greater than 5 cm, % | 37.5 | 37.2 | 52.0 |
| IPI at initial diagnosis, no. (%) | |||
| 1 (low) | 8 (24.2) | 11 (25.6) | 0 (0.0) |
| 2 (low-intermediate) | 9 (27.3) | 14 (32.6) | 8 (28.6) |
| 3 (high-intermediate) | 8 (24.2) | 8 (18.6) | 9 (32.1) |
| Higher than 4 (high) | 3 (9.1) | 7 (16.3) | 8 (28.6) |
| Unknown | 5 (15.2) | 3 (7.0) | 3 (10.7) |
| Response to first-line therapy, no. (%) | n = 33 | n = 43 | n = 26 |
| ORR | 22 (67) | 43 (100) | 17 (65) |
| CR | — | 40 (93) | 10 (38.5) |
| CRu | — | 3 (7) | 3 (11.5) |
| PR | 22 (67) | — | 4 (15) |
| SD | 3 (9) | — | — |
| PD | 8 (24) | — | 9 (35) |
st dev indicates standard deviation; SD, stable disease; and PD, progressive disease.
Patient responses to previous first-line chemotherapy or immunochemotherapy are shown in Table 1. Of the 33 patients in whom induction therapy failed (stratum AI), 22 (67%) had previously achieved only a PR, 3 (9%) had stable disease, and 8 (24%) had progressed on first-line therapy. The 26 patients in group B had received a mean number of 6 cycles of R-CHOP or R-CHOP–like induction therapy and included 13 (50%) patients who had failed to achieve a CR, 9 (35%) of whom had progressed on this therapy.
Therapeutic responses
The assessment of response to a single dose of 90Y-ibritumomab is shown in Tables 2–3. ORR was 53% in group A with ORRs of 52% and 53% in strata AI and AII, and CR/CRu rates of 24% and 40%, respectively. The ORR was 19% in group B. Kaplan-Meier analyses of the OS for each group and stratum are presented in Figure 1A. Median follow-up time was 21.7 months; median survival was 21.4, 22.4, and 4.6 months for stratum AI, stratum AII, and group B, respectively. After a median follow-up of 21.7 months, 13 patients in group A (17%), including those who failed induction therapy, had a durable response lasting longer than 20 months (Figure 1B), with 4 patients still in remission at 30 months. Progression-free survival curves for strata AI and AII were similar, suggesting that the differences in responsiveness to chemotherapy between the groups may not influence the durability of response to radioimmunotherapy (RIT) in responding patients.
Best response to a single dose of 90Y-ibritumomab following first-line treatment with chemotherapy alone (group A)
| Response . | In patients pretreated with chemotherapy alone, no. (%) . | ||
|---|---|---|---|
| Group A overall, n = 76 . | Stratum AI, n = 33* . | Stratum AII, n = 43 . | |
| CR | 21 (28) | 7 (21) | 14 (32.5) |
| CRu | 4 (5) | 1 (3) | 3 (7) |
| PR | 15 (20) | 9 (27) | 6 (14) |
| ORR | 40 (53) | 17 (52) | 23 (53) |
| Response . | In patients pretreated with chemotherapy alone, no. (%) . | ||
|---|---|---|---|
| Group A overall, n = 76 . | Stratum AI, n = 33* . | Stratum AII, n = 43 . | |
| CR | 21 (28) | 7 (21) | 14 (32.5) |
| CRu | 4 (5) | 1 (3) | 3 (7) |
| PR | 15 (20) | 9 (27) | 6 (14) |
| ORR | 40 (53) | 17 (52) | 23 (53) |
Includes 22 PRs after first-line chemotherapy treatment.
Best response to a single dose of 90Y-ibritumomab following first-line treatment with or chemotherapy with rituximab (group B)
| Response . | In patients pretreated with chemotherapy plus rituximab, no. (%) . | ||
|---|---|---|---|
| Group B overall, n = 26 . | Induction failure, n = 13* . | Relapsed from CR, n = 13 . | |
| CR | 1 (4) | — | 1 (7.7) |
| CRu | 2 (8) | 1 (7.7) | 1 (7.7) |
| PR | 2 (8) | 1 (7.7) | 1 (7.7) |
| ORR | 5 (19) | 2 (15) | 3 (23) |
| Response . | In patients pretreated with chemotherapy plus rituximab, no. (%) . | ||
|---|---|---|---|
| Group B overall, n = 26 . | Induction failure, n = 13* . | Relapsed from CR, n = 13 . | |
| CR | 1 (4) | — | 1 (7.7) |
| CRu | 2 (8) | 1 (7.7) | 1 (7.7) |
| PR | 2 (8) | 1 (7.7) | 1 (7.7) |
| ORR | 5 (19) | 2 (15) | 3 (23) |
Includes 4 PRs after first-line chemotherapy + rituximab treatment.
Kaplan-Meier analysis of OS and PFS after 90Y-ibritumomab separated by group and stratum. (A) With a median follow-up time of 21.7 months, median survival was 21.4 months (95% confidence interval [CI] = 9.3, NC) for stratum AI, 22.4 months (95% CI = 17.6, NC) for stratum AII, and 4.6 months (95% CI = 3.0, NC) for group B. The follow-up period continued until 1 year after the last patient received 90Y-ibritumomab. (B) The median PFS was 5.9 months for stratum AI (95% CI = 3.3-15.0) and 3.5 months for stratum AII (95% CI = 2.8-10.2). In group B, the median PFS was 1.6 months (95% CI = 1.4-1.7). NC indicates not calculated.
Kaplan-Meier analysis of OS and PFS after 90Y-ibritumomab separated by group and stratum. (A) With a median follow-up time of 21.7 months, median survival was 21.4 months (95% confidence interval [CI] = 9.3, NC) for stratum AI, 22.4 months (95% CI = 17.6, NC) for stratum AII, and 4.6 months (95% CI = 3.0, NC) for group B. The follow-up period continued until 1 year after the last patient received 90Y-ibritumomab. (B) The median PFS was 5.9 months for stratum AI (95% CI = 3.3-15.0) and 3.5 months for stratum AII (95% CI = 2.8-10.2). In group B, the median PFS was 1.6 months (95% CI = 1.4-1.7). NC indicates not calculated.
Adverse events
90Y-ibritumomab therapy resulted in only mild to moderate nonhematologic toxicity. Gastrointestinal toxicity was the most common type of grade 3 or 4 nonhematologic adverse event and was found in 10% of patients. The percentage of patients with grade 1 or 2 toxicity was less than 25% for nervous, renal, respiratory, and skin toxicities and was similar to that reported in rituximab-treated patients.7 The incidence of adverse events was similar in all groups and strata.
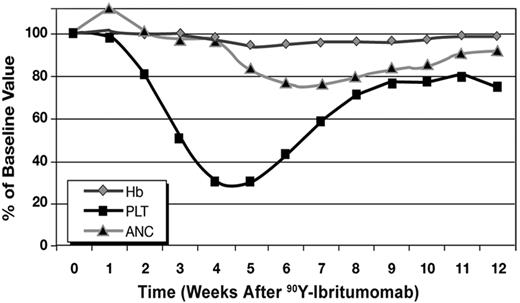
During the treatment phase, 60% of patients experienced hematologic toxicity (43.3% grade 3 or 4). Declines in hemoglobin (4% to 5%), ANC (17% to 24%), and platelet count (76% to 79%) as a function of time after 90Y-ibritumomab treatment are shown in Figure 2. Twenty-eight patients (26.9%) required platelet transfusions, but recovery from thrombocytopenia was near complete by week 9 of the study. Two patients with normal baseline platelet counts died of cerebral hemorrhage during a period of grade 4 thrombocytopenia between 5 and 8 weeks after 90Y-ibritumomab treatment. One additional patient died of cerebral hemorrhage, but during progression of the underlying lymphoma. Two other patients with normal platelet counts died of causes unrelated to progression, 1 of bleeding from a duodenal ulcer approximately 9 months after the start of study medication and the other of gastric adenocarcinoma diagnosed 3 months after 90Y-ibritumomab treatment. A further patient died 2.3 years after study treatment (reason unknown). All other reported deaths followed further relapse or progression of lymphoma. The incidence of severe infection was low, with 7% of patients being hospitalized for infection during the study.
Mean ANC, hemoglobin (Hb) concentration, and platelet count (PLT). Hemoglobin declined 4% to 5% between weeks 5 and 9 after RIT, and ANC decreased 17% to 24% between weeks 5 and 8 after RIT. Mean platelet count declined from 2.5 × 106/L at baseline to less than 0.8 × 106/L at weeks 4 to 5 after RIT. Complete differential blood and platelet counts were obtained before 90Y-ibritumomab administration and weekly thereafter for 12 weeks.
Mean ANC, hemoglobin (Hb) concentration, and platelet count (PLT). Hemoglobin declined 4% to 5% between weeks 5 and 9 after RIT, and ANC decreased 17% to 24% between weeks 5 and 8 after RIT. Mean platelet count declined from 2.5 × 106/L at baseline to less than 0.8 × 106/L at weeks 4 to 5 after RIT. Complete differential blood and platelet counts were obtained before 90Y-ibritumomab administration and weekly thereafter for 12 weeks.
Discussion
Here we report the data from the largest prospective, multicenter study using 90Y-ibritumomab as single agent in the treatment of relapsed DLBCL. 90Y-ibritumomab yielded high response rates in patients who had received prior chemotherapy alone (group A), with an ORR of 53%. The results in this larger patient population are similar to those obtained in the pilot study reported by Gordon et al10 and confirm that 90Y-ibritumomab has single-agent activity in DLBCL. The CR/CRu rate of 40% achieved in patients relapsing after first-line therapy is noteworthy, as is the response in those who failed to achieve a CR after 6 cycles of CHOP; here, there was a 52% ORR and a 24% CR/CRu rate.
Some patients who responded to 90Y-ibritumomab had durable responses, particularly those who achieved a CR/CRu; these patients had a response duration ranging from 24 months to more than 36 months. These results are in keeping with the responses observed in the pilot study in aggressive NHL11 and the proportion of long-term responders among the patients with indolent NHL who achieved a CR/CRu with 90Y-ibritumomab.7
In patients who had previously received R-CHOP chemotherapy (group B), 90Y-ibritumomab was less effective, with an ORR of 19%. A potential explanation for this lower response rate relates to antibody resistance, but seems unlikely given the high response rates seen with anti-CD20 radioimmunotherapy in follicular lymphoma patients refractory to rituximab.15,16 One alternative explanation is that R-CHOP failure is a predictor of refractory disease.
In support of this notion, this group contained many patients with a poor prognosis: 50% of the patients were primarily refractory to R-CHOP, and 35% of the patients had progressive disease while receiving therapy. Group B also contained more patients in the high/high-intermediate risk groups according to the IPI, with 61% having high/high-intermediate IPI compared with 34% in group A (33% and 35% for strata AI and AII, respectively) (Table 1). Furthermore, 52% of patients in group B, compared with only 37% in group A, had bulky disease with lymph nodes larger than 5 cm that are associated with lower response rates to RIT in follicular lymphoma.17
90Y-ibritumomab was generally well tolerated, with significant early-onset thrombocytopenia being the most important side effect. While the magnitude and duration of neutropenia and thrombocytopenia were similar to what was observed with 90Y-ibritumomab in patients with indolent NHL, there appeared to be a discernible difference in the kinetics of myelosuppression with the fall in platelet count typically occurring 3 weeks after 90Y-ibritumomab administration, approximately 2 weeks earlier than that observed in the registration studies conducted mainly in low-grade B-cell lymphoma populations.18 In this DLBCL study, all patients had received only 1 prior therapy and had normal blood counts, and only 4 had low levels of lymphomatous bone marrow infiltration. The reason for this apparent early myelosuppression observed is not understood, but the implications of this finding suggest that very close hematologic monitoring is required in the posttreatment period, with prophylactic platelet support administered to patients who require it.
In conclusion, 90Y-ibritumomab induced high response rates in previously treated patients with DLBCL and in patients refractory to CHOP chemotherapy. Lower responses were observed after failure of R-CHOP than after failure of CHOP alone, although patients in the R-CHOP group generally had poorer prognostic features than those in the chemotherapy-alone group, and durable responses lasting longer than 2 years occurred in all patient groups. Given these data, larger studies to establish the potential role of 90Y-ibritumomab in patients receiving R-CHOP seem appropriate, and a prospective randomized trial comparing 6 cycles of R-CHOP followed by 90Y-ibritumomab with R-CHOP alone as first-line therapy for DLBCL is now under way.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Authorship
Contribution: R.M., F.M. and T.I. designed and performed the study, analyzed the data, and wrote the paper; H.S. reviewed the pathology; J.K. designed the trial; S.R., G.H., D.H., G.M., G.P., P.-l.Z., A.-M.L. and N.P. contributed patients, reviewed the data, and amended this paper.
Conflict-of-interest disclosure: JK. is an employee of and stockholder in Schering AG. T.I., G.H., R.M., and F.M. have received honoraria and have acted as consultants to Schering AG. R.M. and N.P. have acted as consultants to Roche Basel. The other authors declare no competing financial interests.
Correspondence: Robert Marcus, Department of Haematology, Level 3, Addenbrooke's Hospital, Hills Road, Cambridge CB2 2QQ, United Kingdom; e-mail: robert.marcus@addenbrookes.nhs.uk.

![Figure 1. Kaplan-Meier analysis of OS and PFS after 90Y-ibritumomab separated by group and stratum. (A) With a median follow-up time of 21.7 months, median survival was 21.4 months (95% confidence interval [CI] = 9.3, NC) for stratum AI, 22.4 months (95% CI = 17.6, NC) for stratum AII, and 4.6 months (95% CI = 3.0, NC) for group B. The follow-up period continued until 1 year after the last patient received 90Y-ibritumomab. (B) The median PFS was 5.9 months for stratum AI (95% CI = 3.3-15.0) and 3.5 months for stratum AII (95% CI = 2.8-10.2). In group B, the median PFS was 1.6 months (95% CI = 1.4-1.7). NC indicates not calculated.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/1/10.1182_blood-2007-01-068056/2/m_zh80130703460001.jpeg?Expires=1764974285&Signature=zazvUJvTyNzxy6nl9Nr-L0KUVez36sji97SmQMdCC5NRPahFWbRNWwh2dEiwiE~xP9dO~zRVBOOfVNch5mmjC85OJ8OiF4r-lsMby91blLC9h5TBTfM89ieTHdfnO4~RRe62ALDqIUHuLr3UFu0pGXZBuv9QWjRMU0EyhCiigM-Vz2Aws~C0BifE0wVU3U5qAUlrwI4RcvQgM358TWmv3nmTFv1y~xbnCndfIYo59ZiWzU3sZqsMB-IcTiFRUAZHVRZmXXZA-Jn-A1R-PiCjRm1MKUGtqFnYF8cyTOLGJ5ZKCkNVVwYjmP7Af63fxhPgS1VXzS0lWy8dS6ucthn4sA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal