Abstract
Mesenchymal stem cells (MSCs) are multipotential nonhematopoietic progenitor cells capable of differentiating into multiple lineages of the mesenchyme. MSCs have emerged as a promising therapeutic modality for tissue regeneration and repair. Further clinical interest has been raised by the observation that MSCs are immunoprivileged and, more importantly, display immunomodulatory capacities. Although the mechanisms underlying the immunosuppressive effects of MSCs have not been clearly defined, their immunosuppressive properties have already been exploited in the clinical setting. The aim of this review is to critically discuss the immunogenicity and immunomodulatory properties of MSCs, both in vitro and in vivo, the possible underlying mechanisms, the potential clinical use of MSCs as modulators of immune responses in vivo, and to indicate clinical safety concerns and recommendations for future research.
Introduction
Bone marrow (BM) stromal cells were first identified by Friedenstein, who described an adherent fibroblast-like population able to differentiate into bone that he referred to as osteogenic precursor cells.1 Subsequent studies demonstrated that these cells have the ability to differentiate into various other mesodermal cell lineages, including chondrocytes, tenocytes, and myoblasts (reviewed in Prockop2 ). Based on this multilineage differentiation capacity, Caplan introduced the term mesenchymal stem cells (MSCs),3 although many other terms have been introduced to describe a nonhomogenous population of multipotent cells. Although MSCs at a population level fulfill stem-cell criteria (ie, self renewal and multilineage differentiation capacity), it remains questionable whether the qualification “stem cell” is legitimate for MSCs at the single cell level. It was therefore recently proposed to use the term multipotent mesenchymal stromal cells (with the acronym MSCs) to describe fibroblast-like plastic-adherent cells.4 Recently, Bonnet et al demonstrated that single cell–derived populations of murine BM-derived MSCs characterized by stage-specific embryonic antigen-1 expression, were capable of differentiation in vivo,5 thus showing their true stem-cell properties. In this review, we will refer to the multipotent mesenchymal stromal cells with the acronym MSCs.
Although MSCs originally were isolated from BM,6 similar populations have been isolated from other tissues, including adipose tissue,7 placenta,8 amniotic fluid,9 and fetal tissues such as fetal lung and blood.10 In addition, umbilical cord blood (UCB) has been identified as a source of MSCs.11,12 Probably as a result of their low frequency in UCB, conflicting reports initially have been published on the presence of MSCs in UCB. It has now become clear that the volume and storage time of the cord blood are important parameters for successful isolation of MSCs from UCB.11
At present no specific marker or combination of markers has been identified that specifically defines MSCs. Phenotypically, ex vivo expanded MSCs express a number of nonspecific markers, including CD105 (SH2 or endoglin), CD73 (SH3 or SH4), CD90, CD166, CD44, and CD29.6,13 MSCs are devoid of hematopoietic and endothelial markers, such as CD11b, CD14, CD31, and CD45.6
The capacity to differentiate into multiple mesenchymal lineages, including bone, fat, and cartilage, is being used as a functional criterion to define MSCs.2 Recent studies indicated the identification of pluripotent cells that not only differentiate into cells of the mesoderm lineage, but also into endoderm and neuroectoderm lineages, including neurons,14 hepatocytes,15 and endothelia.16 Such pluripotent stem cells have been identified in BM and referred to as multipotent adult progenitor cells (MAPCs),17 human BM-derived multipotent stem cells (hBMSCs),18 marrow-isolated adult multilineage inducible (MIAMIs) cells,19 or very small embryonic-like stem cells (VSELs).20 Similar pluripotent cell types have been found in UCB (unrestricted somatic stem cells; USSCs),12 in adipose tissue,21 and recently in amniotic fluid.22 These primitive cell types require specific and stringent culture conditions, including embryonic stem cell–specific fetal calf serum (FCS), coated culture dishes (a.o. fibronectin), medium with specific growth factor requirements, specific type or culture dish, and prolonged culture duration at low cell density. Culturing these cells at higher cell density promotes differentiation toward a mesenchymal progenitor cell with restricted differentiation potential.23 It has not been possible to prospectively isolate these cells from freshly obtained tissues, blood, or BM. Therefore, it is still unclear to what extent they are primary cells that play a physiological role or are the result of prolonged culture expansion under specific culture conditions.
The identification of MSCs in vivo and the prospective isolation of MSCs from primary tissues is hampered by the availability of a specific MSC marker. To date, MSC isolation still relies on their adherence to plastic, resulting in a heterogeneous population of adherent cells, popularly defined as MSCs. Currently, direct proof that these multipotent stem cells play a physiological role in vivo is lacking. Specific markers to prospectively isolate these cells could help to further resolve this issue.
Several cell surface antigens have been reported to enrich for MSCs. For human cells, selection of Stro-1–positive cells has been demonstrated to result in a 10- to 20-fold enrichment of CFU-F relative to their incidence in unseparated human BM.24 Further enrichment of mesenchymal progenitor cells with capacity for differentiation into multiple lineages was obtained by combination of Stro-1 with CD106 (vascular adhesion molecule-1; VCAM-1).25 Caplan and colleagues described additional antibodies to identify human BM cells, including CD105 (SH2) and CD73 (SH3/SH4).26 The low-affinity nerve growth factor receptor (LNGFR; CD271) also has been shown to enrich for marrow stromal cells, and MSCs selected by CD271 expression were shown to have a 10- to 1000-fold higher proliferative capacity in comparison to MSCs isolated by plastic adherence.27 Recently, Buhring et al developed new antibodies for prospective isolation of MSCs, including W8B2 and frizzled-9 (FZD9), for the isolation of human MSCs from BM and placenta, respectively.28
Immunosuppressive properties of MSCs
MSC-mediated immunosuppression in vitro
The interaction between MSCs and T cells.
The first indications for the immunosuppressive nature of MSCs were derived from studies with human,31-34 baboon35 and murine MSCs36,37 that demonstrated that MSCs were able to suppress T lymphocyte activation and proliferation in vitro. This inhibition affects the proliferation of T cells induced by alloantigens,32-34 mitogens31 as well as activation of T cells by CD3 and CD28 antibodies.34,37 MSCs have been reported to inhibit the cytotoxic effects of antigen-primed cytotoxic T cells (CTLs),33 that might be due to suppression of the proliferation of CTLs, rather than to a direct inhibition of cytolytic activity.38,39 Suppression of T cell proliferation by MSCs has no immunological restriction, similar suppressive effects being observed with cells that were autologous or allogeneic to the responder cells.32,37 Most studies agree that soluble factors are involved because separation of MSCs and PBMCs by a semi permeable membrane (transwell) does not prevent inhibition of proliferation.31,34,39 Supernatants from human and mouse MSCs cultures show no inhibitory effect,33,41,42 unless MSCs have been cocultured with lymphocytes,36 suggesting that the suppressive factor(s) are not constitutively secreted by MSCs, but require a dynamic cross talk between MSCs and T-lymphocytes. A role for TGF-β and hepatocyte growth factor (HGF) as mediators for suppression of T-cell proliferation in a mixed lymphocyte reaction has been suggested by Di Nicola et al, who found that neutralizing antibodies against TGF-β and HGF restored the proliferative response of T cells.31 Others demonstrated that these factors do not play a role in the suppressive effect by MSCs on T cells stimulated with mitogens42,43 and suggest that different mechanisms are involved depending on the stimuli. Prostaglandin E2 (PGE2) represents another candidate molecule. MSCs constitutively produce PGE2, a process that is enhanced upon coculture with PBMCs.34 Although inhibition of PGE2 synthesis was demonstrated to mitigate MSC-mediated suppression of T-cell proliferation and cytokine production by T cells,44 other studies contradict these findings.34,43 More recently, the tryptophan catabolizing enzyme indoleamine 2,3-dioxygenase (IDO) has been suggested to play a role in the suppression of T-cell proliferation by MSCs.45 Upon stimulation with IFN-γ, MSCs express IDO activity that degrades essential tryptophan and results in kynurenine breakdown products, resulting in reduced lymphocyte proliferation. However, the role of IDO in MSC-mediated suppression is not very clear.31,34,44 While one recent study indicated that MSCs induce apoptosis of T cells due to the conversion of tryptophan to kynurenine,46 several other studies however found no effect of addition of MSC on apoptosis of T cells.29,32,42 MSC-induced T-cell anergy has been proposed as another potential mechanism of immune suppression. MSCs lack surface expression of costimulatory molecules, such as CD80 (B7-1) and CD86 (B7-2), and it is believed that MSCs can render T cells anergic. Several studies have shown that the unresponsiveness of T cells in the presence of MSCs was transient and could be restored after removal of MSCs,31,37 whereas others have demonstrated in murine models that T-cell tolerance was induced.47 Alternatively, MSCs have been demonstrated to induce a condition of anergy due to divisional arrest in T cells (ie, following removal of MSCs); IFN-γ production but not proliferation of murine PBMCs was restored, despite addition of exogenous IL-2.48 The discrepancy between these studies may be due to different experimental conditions or the origin of the MSCs.
Another level at which MSCs may modulate immune responses is through the induction of regulatory T cells (Treg). MSCs have been reported to induce formation of CD8+ regulatory T cells that were responsible for inhibition of allogeneic lymphocyte proliferation.36 Furthermore, an increase in the population of CD4+CD25+ cells, displaying a regulatory phenotype (ie, Fox P3 positivity) has been demonstrated in mitogen-stimulated PBMC cultures in the presence of MSC,38,44 although the functional properties (ie, the suppression of T-cell proliferation) of these cells have not yet been demonstrated. In contrast, depletion of CD4+CD25+ regulatory T cells had no effect on the inhibition of T-cell proliferation by MSCs.37
Although these data are generally interpreted to indicate an immunosuppressive role of MSCs, the current evidence shows that MSCs merely suppress T-cell proliferation in vitro and thereby primarily affects the effector arm of T-cell immune response. In support of the hypothesis that the “immunosuppressive” effect of MSCs might be ascribed to a nonspecific antiproliferative effect is a recent study by Ramasamy et al, who described that MSCs also inhibited the proliferation of malignant cells of different lineages.40
Antigen-presenting cells are directed toward a regulatory phenotype by MSCs.
Dendritic cells (DCs) play a key role in the induction of immunity and tolerance, depending on the activation and maturation stage and, as recently proposed, the cytokine milieu at sites of inflammation.49 MSCs have been demonstrated to interfere with DC differentiation, maturation and function. Addition of MSCs results in inhibition of differentiation of both monocytes and CD34+ progenitors into CD1a+-DCs, skewing their differentiation toward cells with features of macrophages. DCs generated in the presence of MSCs were impaired in their response to maturation signals and exhibited no expression of CD83 or up-regulation of HLA-DR and costimulatory molecules.50-52 Consistent with these findings, immature DCs generated in the presence of MSCs were strongly hampered in their ability to induce activation of T cells. In addition, an altered cytokine production pattern, ie decreased production of proinflammatory cytokines tumor necrosis factor (TNF)-α, interferon (IFN)-γ, and interleukin (IL)-12 and increased production of the anti-inflammatory cytokine IL-10 in MSC/monocyte culture, was also observed.44,50,53 Taken together, these results suggest that MSCs suppress the differentiation of dendritic cells, resulting in the formation of immature DCs that exhibit a suppressor or inhibitory phenotype.
Transwell experiments have indicated that the suppressive effect of MSCs on DC differentiation is mediated by soluble factors.51 The production of IL-6 and M-CSF by MSCs may contribute to the inhibitory effect of MSCs on DC differentiation, although blocking studies indicate that these factors are not solely responsible for the inhibitory effect. Alternatively, PGE2 might be an intriguing candidate factor. Inhibition of PGE2 synthesis restored the secretion of TNF-α and IFN-γ by DCs cultured in the presence of MSCs.44 The increased production of IL-10 by DCs upon coculture with MSCs may also contribute to the suppressive effects of MSCs. Neutralizing antibodies to IL-10 indeed restored T-cell proliferation,53 although not completely. In addition to direct suppression of T-cell proliferation, the induction of regulatory antigen-presenting cells (APCs) might thus be a key mechanism by which MSCs indirectly suppress proliferation of T cells.
MSCs modulate B-cell functions.
In murine studies, MSCs have been reported to inhibit the proliferation of B cells, stimulated with anti-CD40L and IL-4,48 or with pokeweed mitogen.41 Allogeneic MSCs have been shown to inhibit the proliferation, activation and IgG secretion of B cells from BXSB mice that are used as an experimental model for human systemic lupus erythematosus.54 Consistent with the murine studies, human MSCs have been shown to inhibit proliferation of B cells activated with anti-Ig antibodies, soluble CD40 ligand and cytokines.55 In addition, differentiation, antibody production and chemotactic behavior of B cells was affected by MSCs.55 Krampera et al showed that MSCs only reduced the proliferation of B cells in the presence of IFN-γ. The suppressive effect of IFN-γ was possibly related to its ability to stimulate the production of IDO by MSCs, which in turn suppresses the proliferative response of effector cells through the tryptophan pathway.56 Although the mechanisms involved in these activities are not yet fully understood, transwell experiments indicated that soluble factors released by MSCs were sufficient to inhibit proliferation of B cells.55 In contrast, culture supernatant from MSCs had no effect, suggesting that the release of inhibitory factors requires paracrine signals from B cells.
Interaction between MSCs and natural killer cells.
Natural killer (NK) cells exhibit spontaneous cytolytic activity that mainly targets cells that lack expression of HLA class I molecules. Killing by NK cells is regulated by a balance of signals transmitted by activating and inhibitory receptors interacting with HLA molecules on target cells. However, NK cells are also able to lyse autologous tumor cells regulated by their activating receptors.57 It has been suggested that MSCs suppress IL-2 or IL-15 driven NK-cell proliferation and IFN-γ production.38,39,44,58 MSCs do not inhibit the lysis of freshly isolated NK cells,39 whereas NK cells cultured for 4 to 5 days with IL-2 in the presence of MSCs have a reduced cytotoxic potential against K562 target cells.56 Furthermore, Sotiropoulou et al demonstrated that short term culture with MSCs only affect NK-cell cytotoxicity against HLA class I-positive tumor cells but not against HLA class I-negative targets.58 These data indicate that MSCs exert an inhibitory effect on the NK-cell cytotoxicity against HLA class I-positive targets that are less susceptible to NK-mediated lysis than HLA class I-negative cells.
Experiments with transwell culture systems have indicated that MSCs are able to suppress the proliferation and cytokine production of IL-15 stimulated NK cells via soluble factors. In contrast, the inhibitory effect of MSCs on NK-cell cytotoxicity required cell-cell contact, suggesting the existence of different mechanisms for MSC-mediated NK-cell suppression.58 PGE2 secretion by MSCs was demonstrated to partially affect NK-cell proliferation, CD56 expression and cytotoxicity, but did not interfere with cytokine production or expression of activating receptors.58 Inhibition of TGF-β partially restores NK-cell proliferation, whereas blocking both PGE2 and TGF-β completely restored the proliferation capacity of NK cells, indicating that these factors suppress NK-cell activity by different mechanisms.
Until recently, MSCs were considered immunoprivileged and previous studies reported that MSCs were not lysed by freshly isolated NK cells.39,59 However, recent data indicate that activated NK cell are capable of effectively lysing MSCs.58,60 Although MSCs express normal levels of MHC class I that should protect against NK-mediated killing, MSCs express different ligands that are recognized by activating NK receptors that trigger NK alloreactivity.60 Treatment of MSCs with IFN-γ decreased their susceptibility to NK cell–mediated lysis due to up-regulation of HLA class I molecules.60
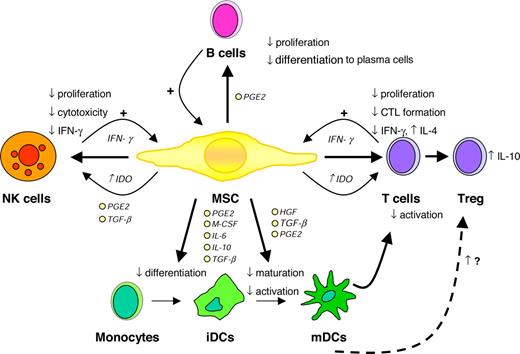
Taken together, numerous studies convincingly demonstrate that MSCs are able to modulate the function of different immune cells in vitro, particularly involving the suppression of T-cell proliferation and the inhibition of DC differentiation (Figure 1). The mechanisms underlying the immunosuppressive effects of MSCS are still unclear and several different, sometimes contradictionary results have been proposed. Finally, the in vivo biological relevance of these in vitro observations has yet to be shown.
Immunomodulatory effects of MSCs. CTL indicates cytotoxic T cell; HGF, hepatocyte growth factor; IDO, indoleamine 2,3-dioxygenase; PGE2, prostaglandin E2; and TGF-β, transforming growth factor β. Illustration by Paulette Dennis.
Immunomodulatory effects of MSCs. CTL indicates cytotoxic T cell; HGF, hepatocyte growth factor; IDO, indoleamine 2,3-dioxygenase; PGE2, prostaglandin E2; and TGF-β, transforming growth factor β. Illustration by Paulette Dennis.
Immunosuppressive properties of MSCs in vivo
Animal models.
The immunomodulatory effects of MSCs have been examined in a variety of animal models related to alloreactive immunity (organ and stem cell transplantation), autoimmunity or tumor immunity (Table 1). One of the first in vivo studies demonstrated that systemic infusion of allogeneic MSCs derived from the BM of baboons prolonged the survival of allogeneic skin grafts to 11 days compared with 7 days in animals not receiving MSCs.35 In addition, we have recently demonstrated that infusion of syngeneic host-derived MSCs resulted in decreased rejection of allogeneic stem cell grafts in a murine allogeneic BM transplantation model,61 although we did not address possible immunological mechanisms underlying these observations.
In vivo immunosuppressive effects of MSCs
| Animal, model . | MSCs . | Outcome . | Reference no. . |
|---|---|---|---|
| Mouse | 36 | ||
| Melanoma | Allogeneic IV and SC infusion | Promotion of tumor growth | — |
| GVHD | Allogeneic multiple IV infusions | Prevention of GVHD | 62 |
| GVHD | Allogeneic single IV infusion | No effect on development of GVHD | 63 |
| Graft rejection | Syngeneic | Decreased graft rejection | 61 |
| EAE | Allogeneic IV infusion | Prevention of EAE development | 47 |
| CIA | Allogeneic IV infusion | No effect | 65 |
| Baboon: Skin graft transplantation | Allogeneic IV infusion | Prolonged skin graft survival | 35 |
| Rat: Ischemia/reperfusion injury | Allogeneic IV infusion | Protection against renal ischemia/reperfusion injury | 66 |
| Human | |||
| Acute myeloid leukemia | Haploidentical IV infusion | HSC engraftment with no GVHD | 91 |
| Severe acute GVHD | Haploidentical IV infusion | Resolution of grade IV acute GVHD | 71, 73 |
| Leukemia | Haploidentical IV infusion | Rapid platelet engraftment, low incidence of GVHD | 70 |
| Animal, model . | MSCs . | Outcome . | Reference no. . |
|---|---|---|---|
| Mouse | 36 | ||
| Melanoma | Allogeneic IV and SC infusion | Promotion of tumor growth | — |
| GVHD | Allogeneic multiple IV infusions | Prevention of GVHD | 62 |
| GVHD | Allogeneic single IV infusion | No effect on development of GVHD | 63 |
| Graft rejection | Syngeneic | Decreased graft rejection | 61 |
| EAE | Allogeneic IV infusion | Prevention of EAE development | 47 |
| CIA | Allogeneic IV infusion | No effect | 65 |
| Baboon: Skin graft transplantation | Allogeneic IV infusion | Prolonged skin graft survival | 35 |
| Rat: Ischemia/reperfusion injury | Allogeneic IV infusion | Protection against renal ischemia/reperfusion injury | 66 |
| Human | |||
| Acute myeloid leukemia | Haploidentical IV infusion | HSC engraftment with no GVHD | 91 |
| Severe acute GVHD | Haploidentical IV infusion | Resolution of grade IV acute GVHD | 71, 73 |
| Leukemia | Haploidentical IV infusion | Rapid platelet engraftment, low incidence of GVHD | 70 |
EAE indicates experimental acute encephalomyelitis; IV, intravenous; SC, subcutaneous; and —, not applicable.
One of the most impressive in vivo effects of MSCs has been observed in the treatment of graft-versus-host disease (GVHD) after allogeneic stem cell transplantation. Systemical infusion of ex-vivo expanded MSCs derived from adipose tissue was able to control lethal GVHD in mice transplanted with haploidentical hematopoietic stem cells grafts.62 This study demonstrated that only infusions of MSCs early after transplantation were effective in controlling GVHD. Moreover, it was suggested that repeated infusions of MSCs are required to ameliorate GVHD and this might explain a recent observation that the infusion of a single dose of MSCs at the time of an allogeneic BM transplantation did not affect the incidence and severity of GVHD in mice.63
Modulation of autoimmunity is considered a potential novel target for MSC treatment. Recently, 3 reports on the effects of MSCs in animal models of autoimmunity have appeared. Murine MSCs have been demonstrated to ameliorate experimental autoimmune encephalomyelitis (EAE), a model of human multiple sclerosis, through the induction of peripheral T cell tolerance against the pathogenic antigen.47,64 The infusion of MSCs was only effective at disease onset and at the peak of the disease, but not after disease stabilization. In contrast, infusion of MSCs had no beneficial effects on collagen-induced arthritis (CIA) as tested in a murine model of rheumatoid arthritis (RA).65
Djouad et al demonstrated that MSCs prevented the rejection of allogeneic tumor cells in immunocompetent mice. MSCs infused systemically or adjacent to subcutaneously implanted B16 melanoma cells resulted in enhanced tumor formation, whereas melanoma cells injected alone were eliminated by the host immune system.36 MSCs have also been demonstrated to provide tissue protective effects in a rat kidney model of ischemia/reperfusion injury, possibly mediated by the secretion of soluble immunomodulating factors.66 Moreover, infusion of rat MSCs in an experimental rat model of glomerulonephritis was shown to accelerate glomerular healing, probably related to the release of growth factors.67 In line with this are recent observations demonstrating that MSCs preferentially engraft at sites of tissue damage or tumor growth.68
Clinical experience.
The immunosuppressive capacities of MSCs have generated clinical interest in the field of solid organ of hematopoietic stem cell (HSC) transplantation in order to prevent graft rejection and to prevent or control graft-versus-host disease following HSC transplantation. Due to their immunosuppressive properties, MSCs are considered a potential cellular therapy for prevention of graft rejection and GVHD. The first clinical studies were performed to assess the safety of MSC infusion. A clinical study in breast cancer patients69 showed that the infusion of MSCs was safe and resulted in a rapid hematopoietic recovery. In a multicenter clinical trial, culture-expanded MSCs derived from the bone marrow of HLA-identical siblings were coinfused with HLA-identical hematopoietic stem-cells in 46 patients undergoing allogeneic stem-cell transplantation after a myeloablative conditioning regimen.70 MSCs were infused 4 hours before infusion of the stem cell graft without any infusion-related adverse events, ectopic tissue formation, or increase in the incidence or severity of GVHD. In comparison with historical controls, no acceleration of hematopoietic engraftment or prevention of graft rejection was observed.
In an European phase I-II study, 13 children received a haploidentical stem-cell graft in combination with expanded MSCs derived from the marrow of the stem-cell donor.71 No immediate adverse effects were observed after infusion of MSCs, while the incidence of graft failure or rejection in a control cohort of 52 patients was 20%, all patients engrafted and the preliminary data demonstrated an accelerated leukocyte recovery, although platelet and neutrophil engraftment kinetics were similar. Further support for the possible clinical benefit of MSCs has been presented by several case reports. The results from such case reports suggests that MSCs may not only exert preventive effects on the development of GVHD, but also exhibit therapeutic effects in established GVHD of the gut after allogeneic stem-cell transplantation.72 At present, additional patients with acute and chronic GVHD have been treated with MSCs. No side effects were seen after MSC infusions. Among the 40 patients treated for severe acute GVHD, 19 had complete responses, 9 patients showed improvements, 7 patients did not respond, 4 had stable disease, and 1 patient was not evaluated due to short follow-up. Twenty-one patients are alive between 6 weeks up to 3.5 years after transplantation. Of these patients 9 developed extensive chronic GVHD.73 Two prospective randomized European phase III studies recently have been launched to further explore the therapeutic usefulness of MSCs for the treatment or prevention of acute GVHD following allogeneic stem- cell transplantation.
The mechanisms underlying the possible in vivo immunomodulatory effects of MSCs remain a critical and unresolved question. It has been difficult to recover MSCs from BM of recipients who received transplants. Therefore it is conceivable that MSCs home to other tissues or organs to mediate immune suppression. In line with this hypothesis are recent observations in animal studies demonstrating that MSCs migrate to lymphoid organs47 and engraft at sites of tissue damage or tumor growth.68 Several studies indicated that after systemic administration, MSCs lodge nonspecifically in the capillary beds of various tissues, mainly the lungs.74 Interestingly, a murine study demonstrated that ex vivo expansion of MSCs dramatically reduced their homing and engraftment capacity.75
The ability of MSCs to prevent or reverse GVHD may be via secretion of soluble factors or direct cell-cell contact on alloreactive T cells or by suppressing DC function. Alternatively or additionally, MSCs might increase the healing of wounded tissue by providing soluble factors, transdifferentiation, or cell fusion.76
Safety concerns
Little is know regarding the in vivo survival of MSCs, and there are no clinical studies reporting whether MSCs remain present after transplantation. Although the few clinical studies performed to date confirm safety of infusion of MSCs, the lack of adverse effects might be due to the limited survival of MSCs. Therefore, concerns remain over the potential of systemic immune suppression, ectopic tissue formation, malignant transformation, and immunogenicity. Further controlled studies are required to address these concerns.
Systemic administration
The disadvantage of current immunosuppressive drugs is that they do not distinguish between pathological immune responses and protective immune responses. Systemic immunosuppression may therefore also depress host immune responses against infections caused by bacteria, fungi, and viruses. This is particularly important in allogeneic stem cell transplant or solid organ transplant recipients and for patients with autoimmune diseases, since in these patients the immune system is already compromised. It is therefore of importance to critically examine the effects of immunosuppression induced by MSCs and to compare with immunosuppressive agents currently used in the clinic. MSCs have been demonstrated to home to sites of injury and may therefore provide site-specific and local immunosuppression. This may serve as a potential mechanism to induce organ or tissue specificity. In contrast, a recent animal study demonstrated that local as well as systemic infusion of MSCs systemically suppressed the host antitumor immune response, thereby favoring allogeneic tumor formation.36 The immunosuppressive effects of MSCs are antigen nonspecific and independent of MHC expression: similar inhibitory effects being reported with MSCs that were autologous, allogeneic, or xenogeneic to the responder cells.35,36,38 Infusion of MSCs might therefore provide nonspecific systemic immunosuppression.
Ectopic tissue formation.
MSCs have the ability to differentiate into several mesenchymal lineages and therefore are considered attractive candidates for tissue repair. Horwitz et al have reported the use of MSCs for the repair of bone in patients with osteogenesis imperfecta (OI).78 It is assumed that the differentiation of MSCs toward a particular tissue lineage is primarily driven by the tissue-specific microenvironment. If true, this may serve as a mechanism protecting against cross-differentiation toward other mesenchymal tissue lineages. Recently, however, calcifications were observed in the infarcted hearts at mice that received local MSC treatment.77 In view of the paucity of available clinical data, ectopic tissue formation after MSC treatment therefore remains an important clinical safety concern.
Malignant transformation of MSCs.
Although MSCs have emerged as a promising tool for clinical applications due to the relatively simple requirements of ex vivo expansion without loss of their differentiation potential, culture expansion may (negatively) alter the functional in vivo characteristics. No immortalization and transformation has been observed after expansion of human MSCs, although it has been recently demonstrated that adipose tissue-derived MSCs undergo spontaneous transformation upon prolonged ex vivo expansion under stressful conditions.79 Murine cells are described to be more susceptible to chromosomal aberration under in vitro cultivation. Furthermore, the growth characteristics of murine MSCs differ from human MSCs in that they exhibit a significantly prolonged lag phase. The exponential growth observed after this phase might be due to the proliferation of transformed cells with a potential growth advantage. Emerging evidence in mice suggest that tumors may originate from spontaneous mutations of mesenchymal stem cells.80 In line with these observations are results from our own group demonstrating that even short-term culture was sufficient for the transformation of murine MSCs into a cell population with autonomous growth and biological characteristics of osteosarcoma.81 While no in vivo transformation or tumor formation has been observed in patients, these observations underscore the requirement for further studies on the genetic stability of expanded MSCs. It seems appropriate to test expanded MSC products for the presence of a normal karyotype before administration.
Immunogenicity of MSCs.
MSCs are considered to be hypoimmunogenic, displaying low expression levels of human leukocyte antigen (HLA) major histocompatibility complex (MHC) class I and, importantly, no expression of costimulatory molecules.34 An emerging body of data indicate that MSCs do not elicit a proliferative response by allogeneic lymphocytes.33-35,37 However, MSCs directly suppress T-cell proliferation in an antigen-independent fashion, and therefore it is not appropriate to use proliferation as a read-out for immunogenicity. In vivo studies demonstrated that MSCs avoid normal alloresponses.82 These characteristics support the possibility of exploiting universal donor MSCs for therapeutic applications. However, recent evidence indicates that MSCs can function as APCs and activate immune responses under appropriate conditions.83,84 MSCs are able to take up antigens, and after stimulation with IFNγ, to induce T-cell responses to recall antigens.83 Stagg et al reported that IFNγ-stimulated MSCs induced antigen-specific responses of primary ovalbumin (OVA)–specific transgenic T cells.84 Moreover, when mice were immunized with OVA-loaded, IFNγ-stimulated MSCs, they developed antigen-specific cytotoxic CD8+ T cells and rejected OVA-expressing tumor cells. We have demonstrated that infusion of allogeneic MSCs can prime naive T cells in immunocompetent mice.61 Furthermore, in a recent study it was demonstrated that subcutaneously implanted allogeneic MSCs were rejected in nonimmunosuppressed recipient mice. Splenocytes isolated from mice that had been implanted with allogeneic MSCs displayed a significant IFN-γ response against allogeneic MSCs in vitro.85 Xenotransplantation studies with human MSCs suggest that MSCs are not intrinsically immunoprivileged. Intracoronary injection of adult human MSCs in rat myocardium was associated with rejection and significant infiltration of mainly macrophages, whereas persistent engraftment of adult human MSCs was observed in immunological incompetent rats.86 These studies support the notion that allogeneic MSCs can engraft in immunocompromised hosts or at immunoprivileged sites but trigger an immune response in hosts with an intact immune system. Although further studies of the immunogenicity of MSCs are needed, rejection of MSCs and its clinical consequences should therefore be carefully considered in clinical trials. On the other hand, rejection of allogeneic MSCs might be profitable: in this way MSCs only temporary suppress the immune system, thereby reducing the risk of infection, malignant transformation, or suppression of a graft-versus-tumor effect.
Physiological role for MSCs in immunosuppression
The current knowledge about MSCs is primarily derived from studies performed on ex vivo expanded cells. There is a general consensus that MSCs are residents of the microenvironment and play a role in supporting hematopoiesis. However, whether the immunosuppressive properties of MSCs play a physiological role in maintaining immune homeostasis has not been established.
Recent observations suggested that MSCs from patients with autoimmune diseases are affected. MSCs derived from the BM of patients with severe aplastic anemia are deficient in their ability to suppress T-cell proliferation and cytokine release.87 Whether these defects are relevant for the pathogenesis of aplastic anemia remains to be shown. Both stromal and endothelial progenitors in patients with systemic sclerosis also have been reported to be functionally impaired, showing reduced proliferation and differentiation capacity. It has been suggested that the functional impairment of the BM microenvironment might play a role in the impaired vasculogenesis in scleroderma.88 It may therefore be hypothesized that the immunosuppressive capacities of MSCs might play a role in the BM microenvironment to create an immunoprivileged site that protects primitive stem cells against bystander effects of local immune responses.
The observation that human MSCs can be isolated from decidua,8 amniotic fluid,9 fetal blood,10 and umbilical cord blood11,12 may indicate a role for MSCs in fetal tolerance. Fetal immune responses to paternal antigens are suppressed by a phenomenon called “immune privilege.”89 The emerging data on the mechanisms contributing to immune privilege in the pregnant uterus show striking similarity to the immunosuppressive effects of MSCs, including the production of IDO,45 and support the hypothesis that MSCs are involved in fetal tolerance.90 Further studies are required to dissect the potential of MSCs in providing immune privilege in the pregnant uterus and to gain more insight in the mechanisms of immune inhibition.
Conclusions
The current body of data on the immunosuppressive properties of MSCs holds great promise for treating immune-mediated disorders. Despite the fact that relatively little is known about their in vivo biology, MSCs already have been introduced in the clinic. Preliminary clinical results are encouraging, and randomized studies are under way. However, it is important not to overestimate the potential therapeutic effects of MSCs, and many questions need to be addressed before the putative therapeutic promise of these cells can be realized. At present, MSCs are extensively characterized in a culture-expanded state, and relatively little is known of their biological properties in an unmanipulated state. Culture expansion of MSCs may alter their fundamental biological properties and changes may occur, including the accumulation of molecular alterations. In addition, the use of different isolation methods and culture conditions has led to multiple populations described as “MSCs” with different, sometimes conflicting, characteristics. There is an obvious need for standardization, and although several markers have been examined to prospectively identify and isolate human MSCs, there is yet no defined universal marker.
Directions for future research include (i) standardization and validation of the isolation and culture expansion method of MSCs used in animal and clinical studies, to facilitate comparisons between cell products generated at different sites; (ii) identification of cell-surface specific markers in order to dissect the hierarchy within MSC populations and facilitate the generation of homogenous cell populations; (iii) animal studies to unravel the mechanism underlying the immunosuppressive effects of MSCs in order to optimize the potential therapeutic application; (iv) in vivo tracking studies to examine the in vivo survival and homing of MSCs; and (v) multicenter randomized clinical trials to further assess safety and efficacy. Finally, in vivo tracking of MSCs in patients will allow directed diagnostic interventions to further study their local effects that may explain their biological activities. A better understanding of this fascinating cell population might realize a novel therapeutic strategy to modulate immune responses in a variety of immune-mediated diseases.
Acknowledgments
This manuscript was supported by research grant 03-3014 from the Dutch Cancer Society, the Netherlands, and EuroCord Nederland Foundation.
Authorship
Contribution: A.J.N. and W.E.F. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Willem E. Fibbe, Department of Immunohematology and Blood Transfusion, E3-Q, Leiden University Medical Center, Albinusdreef 2, 2333 ZA Leiden, the Netherlands; e-mail:w.e.fibbe@lumc.nl.