Abstract
Inhibitory antibodies to factors VIII or IX have the potential to affect a broad range of outcomes among people with hemophilia; however, their possible effect on growth and maturation has not been explored. We evaluated skeletal maturation (bone age), pubertal progression, serum testosterone levels, height velocity, and stature in the multicenter Hemophilia Growth and Development Study. A total of 333 children and adolescents (mean age, 12.4 years) were enrolled from 1989 to 1990 and followed for 7 years. Of these, 18% (n = 60) had a history of inhibitors. Bone age among HIV− adolescents with a history of inhibitors lagged 9 or more months behind those without inhibitors at every age from 12 to 15 years. Those with a history of inhibitors were older at every Tanner stage transition, attained a lower maximum growth velocity, and their serum testosterone levels were significantly lower compared with those without inhibitors. Delays were greater among HIV+ patients with a history of inhibitors compared with those without inhibitors; however, the differences were generally small and not statistically significant. The results of this investigation underscore the importance of monitoring the growth and maturation of children and adolescents with hemophilia, particularly those with inhibitors.
Introduction
Inhibitory antibodies to factor VIII or IX represent a serious complication of replacement therapy for people with hemophilia. These antibodies have the potential to affect a broad range of physical, social, and academic activities among children and adolescents1-4 ; however, their possible effect on growth and maturation has not been systematically explored. The current investigation was inspired by clinical observations of slight build, muscle wasting due to lack of or inability to exercise among children with inhibitors, and an appearance of rapid maturation following successful induction of immune tolerance among adolescent boys. The multicenter natural history design of the Hemophilia Growth and Development Study (HGDS) makes it ideally suited for an epidemiologic investigation of associations between physical growth and inhibitors given the age of the study group, the duration, and the variety and standardization of growth measures. Thus, our objective was to address the question of whether a history of inhibitors, defined as ever having a Bethesda titer of 1 or more Bethesda units (BU), was associated with delays in physical growth and maturation among adolescents with hemophilia. We evaluated the following growth parameters: (1) skeletal maturation, (2) pubertal progression, (3) serum testosterone, and (4) height velocity and stature.
Previous reports from the HGDS have documented delays in physical growth and pubertal progression among the subset of participants (n = 207) who were infected with HIV through exposure to contaminated concentrates.5-8 In the current investigation, our attention was focused primarily on the HIV− participants (n = 126) in order to allow more specific delineation of the effects of inhibitors on maturation. Further, findings in this group are more clinically relevant to the adolescents with hemophilia currently under care. That being said, we were also curious whether differentials in maturation might be observed by inhibitor status among adolescents infected by HIV, and reasoned that if this were the case it would strengthen findings in the HIV− cohort. Thus, data for the entire HGDS were used in the current investigation, are presented in detail for the HIV− study group and summarized for HIV+ participants.
Patients, materials, and methods
Study population
Details of recruitment of the HGDS cohort have been reported by Hilgartner et al.9 In summary, a census was completed at participating centers for every individual receiving care in that center. Data collected included age, race/ethnicity, type and severity of hemophilia, and use of clotting factor concentrate in the 2 years prior to enrollment. A total of 9 or more infusions over that period, or 100 or more U/kg body weight of factor per year over 2 years were required for eligibility. On the basis of the census, a roster of all individuals meeting study criteria was prepared. In the event an eligible person was not recruited into the study, the center was requested to provide the reason for nonparticipation. Between 1989 and 1990, 14 hemophilia treatment centers in the United States enrolled 333 children and adolescents 6 to 19 years of age (mean, 12.4 years), a response rate of 69.2%. The enrolled cohort was representative of the total population identified in the census with regard to type and severity of hemophilia, racial and ethnic origin, and the total number of infusions of clotting factor concentrates in the 2 years prior to enrollment. Once enrolled, participants were followed at 6-month intervals for 7 years. In addition, baseline evaluations were conducted for a group of 47 brothers without hemophilia—sibling controls—who were identified as part of the center census. All but 4 HGDS participants with hemophilia were hepatitis C antibody positive at entry. All 47 sibling controls were hepatitis C antibody negative. The human-subjects committees of collaborating institutions approved the HGDS, informed consent was obtained from parents or legal guardians, and informed consent or assent was obtained from all participants, in compliance with the human-experimentation guidelines of the US Department of Health and Human Services and in accordance with the Declaration of Helsinki.
Clinical and laboratory data
Analyses were conducted using data on skeletal maturation (bone age), pubertal progression measured by Tanner stage, height, and serum testosterone. Skeletal maturation was determined centrally from X-rays of the hand-wrist by the FELS method,10 which was developed using 13 823 serial radiographs from boys and girls in the Fels Longitudinal Study. X-rays were collected during the first 4 years of the study, and those from participants not skeletally mature at baseline were included in the analysis. If skeletal maturity was reached during follow-up, subsequent X-ray measurements were excluded from analysis. In addition to X-rays for HGDS participants with hemophilia, baseline films were available for the sibling controls not already mature at entry. Of the 946 X-rays used in the analysis, 908 were from those with hemophilia and 38 were from sibling controls without hemophilia. Pubertal progression was evaluated at 6-month intervals throughout the 7-year period of follow-up in the method of Tanner and Whitehouse11 by study personnel centrally trained in the administration of a standardized protocol. A total of 2675 Tanner stage evaluations were available for analysis. Participants who had already achieved Tanner stage 5 (adult) at baseline were excluded from the analysis. Height was measured at 6-month intervals using a wall-mounted stadiometer. A total of 3104 height measurements collected over 7 years were used to calculate maximum height velocity, measured in centimeters (cm) per year, as well as the age at which maximum height velocity occurred. Serum testosterone levels were collected once during the study for 308 patients and measured centrally (New York Presbyterian Hospital–Cornell Medical Center, New York, NY) by a specific radioimmunoassay using OP/08 antibody (Pantex, Division of BioAnalysis, Santa Monica, CA). Measurement was done at baseline or during a subsequent visit, depending on the age of the participant.
While the vast majority of HGDS participants had hemophilia A or B (n = 324; 97.3%), a small number (n = 9) had von Willebrand disease or other bleeding disorders. All were included in the analyses.
Statistical methods
Skeletal maturation.
Longitudinal bone age measurements were modeled using a linear mixed effects model with race, HIV and inhibitor status, cubic polynomial terms in age, and their interactions as fixed effects and an unstructured covariance structure for the random error terms.
Pubertal progression.
A linear mixed effects model was used to model the participants' ages with baseline age, Tanner stage, HIV and inhibitor status, and their interactions as fixed effects and a compound symmetric covariance structure for the random error terms. For each HIV and inhibitor status combination, the mean ages at each Tanner stage were estimated from the model, and the mean age at the Tanner stage transitions 1 to 2, 2 to 3, 3 to 4, and 4 to 5 was calculated as the average of the estimated ages at the 2 corresponding Tanner stages.
Serum testosterone.
Serum testosterone values were square-root transformed to comply with assumptions of normality and analyzed using linear regression while controlling for age and HIV and inhibitor status.
Linear and nonlinear mixed effects models were fitted using PROC MIXED and PROC NLMIXED, respectively, in the SAS system (SAS, Cary, NC).
Growth velocity and stature.
A 4-parameter logistic nonlinear mixed effects model was fitted to the participants' height measurements as a function of age within each HIV and inhibitor status subgroup. Each subgroup's model was modeled with random intercept and random asymptote terms, and its growth velocity profile was then estimated using the first derivative of the corresponding 4-parameter logistic function.
Results
Demographic and hemophilia-related characteristics of the study group are presented by HIV and inhibitor status in Table 1. A total of 18% (n = 60) had a history of inhibitors, including one HIV− study participant and 3 HIV+ participants first diagnosed during follow-up. The maximum lifetime Bethesda titers ranged from 1 to 2048 BU. Approximately 32% of the inhibitors were low responding (< 5 BU), and 68% were high responding (5+ BU). At enrollment, participants with inhibitors had significantly more arthropathy measured by range-of-motion abnormalities, abnormalities in coordination and gait,1 and, during follow-up, more frequent hospitalizations than those without inhibitors. Infections related to central lines were an important cause of hospitalization among HIV− participants with inhibitors.12 In general, HGDS participants received on-demand therapy prior to and during study follow-up, although details of inhibitor titers and hemostasis treatment prior to enrollment and attempts to induce immune tolerance were not collected. Types of products in use at entry and at subsequent visits were recorded, as well as annual reports of inhibitor titers. During follow-up, approximately two-thirds of those with hemophilia A and low-responding (< 5 BU) inhibitors used factor VIII products exclusively; the other third used prothrombin complex concentrates (PCCs), activated prothrombin complex concentrates (aPCCs), or recombinant VIIa exclusively, or intermittently in conjunction with factor VIII products. Those with high-responding (≥ 5 BU) inhibitors used PCCs, aPCCs, or recombinant VIIa exclusively, or intermittently with factor VIII products.
Description of the HGDS cohort (n = 333)
| Characteristics . | HIV−, n = 126 . | HIV+, n = 207 . | All participants, n = 333 . | ||
|---|---|---|---|---|---|
| Without inhibitor, n = 106 . | With inhibitor, n = 20 . | Without inhibitor, n = 167 . | With inhibitor, n = 40 . | ||
| Median age, y | 10.2 | 10.4 | 13.2 | 13.4 | 12.3 |
| Type of bleeding disorder, no. (%) | |||||
| Hemophilia A | 73 (68.9) | 18 (90.0) | 145 (86.8) | 38 (95.0) | 274 (82.3) |
| Hemophilia B | 26 (24.5) | 2 (10.0) | 21 (12.6) | 1 (2.5) | 50 (15.0) |
| von Willebrand disease | 5 (4.7) | 0 (—) | 1 (0.6) | 1 (2.5) | 7 (2.1) |
| Other | 2 (1.9) | 0 (—) | 0 (—) | 0 (—) | 2 (0.6) |
| Clotting factor level (n=327), no. (%) | |||||
| Less than 0.01 IU/mL | 58 (56.3) | 16 (80.0) | 130 (78.8) | 38 (97.4) | 242 (74.0) |
| 0.01-0.05 IU/mL | 36 (35.0) | 3 (15.0) | 23 (13.9) | 1 (2.6) | 63 (19.3) |
| More than 0.05 IU/mL | 9 (8.7) | 1 (5.0) | 12 (7.3) | 0 (0.0) | 22 (6.7) |
| Ethnic composition, no. (%) | |||||
| European American | 77 (72.6) | 15 (75.0) | 117 (70.1) | 32 (80.0) | 241 (72.4) |
| Hispanic | 15 (14.2) | 4 (20.0) | 27 (16.2) | 4 (10.0) | 50 (15.0) |
| African American | 13 (12.3) | 1 (5.0) | 19 (11.4) | 3 (7.5) | 36 (10.8) |
| Other | 1 (0.9) | 0 (0.0) | 4 (2.4) | 1 (2.5) | 6 (1.8) |
| Characteristics . | HIV−, n = 126 . | HIV+, n = 207 . | All participants, n = 333 . | ||
|---|---|---|---|---|---|
| Without inhibitor, n = 106 . | With inhibitor, n = 20 . | Without inhibitor, n = 167 . | With inhibitor, n = 40 . | ||
| Median age, y | 10.2 | 10.4 | 13.2 | 13.4 | 12.3 |
| Type of bleeding disorder, no. (%) | |||||
| Hemophilia A | 73 (68.9) | 18 (90.0) | 145 (86.8) | 38 (95.0) | 274 (82.3) |
| Hemophilia B | 26 (24.5) | 2 (10.0) | 21 (12.6) | 1 (2.5) | 50 (15.0) |
| von Willebrand disease | 5 (4.7) | 0 (—) | 1 (0.6) | 1 (2.5) | 7 (2.1) |
| Other | 2 (1.9) | 0 (—) | 0 (—) | 0 (—) | 2 (0.6) |
| Clotting factor level (n=327), no. (%) | |||||
| Less than 0.01 IU/mL | 58 (56.3) | 16 (80.0) | 130 (78.8) | 38 (97.4) | 242 (74.0) |
| 0.01-0.05 IU/mL | 36 (35.0) | 3 (15.0) | 23 (13.9) | 1 (2.6) | 63 (19.3) |
| More than 0.05 IU/mL | 9 (8.7) | 1 (5.0) | 12 (7.3) | 0 (0.0) | 22 (6.7) |
| Ethnic composition, no. (%) | |||||
| European American | 77 (72.6) | 15 (75.0) | 117 (70.1) | 32 (80.0) | 241 (72.4) |
| Hispanic | 15 (14.2) | 4 (20.0) | 27 (16.2) | 4 (10.0) | 50 (15.0) |
| African American | 13 (12.3) | 1 (5.0) | 19 (11.4) | 3 (7.5) | 36 (10.8) |
| Other | 1 (0.9) | 0 (0.0) | 4 (2.4) | 1 (2.5) | 6 (1.8) |
(—) indicates 0.0%.
Skeletal maturation
Table 2 shows the crude (unadjusted) mean bone ages and their standard errors, along with the predicted bone ages by inhibitor status for chronological ages 11 through 15 years. The crude bone ages are generally similar to the predicted values, with an exception at age 14 years. This group contained only 5 measurements, reflected in the large standard error.
Crude and predicted bone age by inhibitor status among HIV− adolescents
| Chronological age, y . | Crude bone age . | Predicted bone age . | 95% CI, mo . | P . | |||
|---|---|---|---|---|---|---|---|
| Without inhibitor, y ± SE . | With inhibitor, y ± SE . | Without inhibitor, y . | With inhibitor, y . | Difference, mo . | |||
| 11 | 10.5 ± 0.3 | 9.8 ± 0.4 | 10.7 | 10.0 | 8.7 | 2.3, 15.1 | .008 |
| 12 | 12.2 ± 0.2 | 11.3 ± 0.4 | 12.0 | 11.2 | 9.5 | 3.0, 16.1 | .005 |
| 13 | 13.0 ± 0.3 | 11.7 ± 0.5 | 13.2 | 12.4 | 9.9 | 3.2, 16.6 | .004 |
| 14 | 14.3 ± 0.3 | 14.7 ± 0.6 | 14.4 | 13.6 | 9.8 | 2.9, 16.7 | .005 |
| 15 | 15.8 ± 0.3 | 14.8 ± 0.3 | 15.5 | 14.8 | 9.2 | 2.2, 16.2 | .01 |
| Chronological age, y . | Crude bone age . | Predicted bone age . | 95% CI, mo . | P . | |||
|---|---|---|---|---|---|---|---|
| Without inhibitor, y ± SE . | With inhibitor, y ± SE . | Without inhibitor, y . | With inhibitor, y . | Difference, mo . | |||
| 11 | 10.5 ± 0.3 | 9.8 ± 0.4 | 10.7 | 10.0 | 8.7 | 2.3, 15.1 | .008 |
| 12 | 12.2 ± 0.2 | 11.3 ± 0.4 | 12.0 | 11.2 | 9.5 | 3.0, 16.1 | .005 |
| 13 | 13.0 ± 0.3 | 11.7 ± 0.5 | 13.2 | 12.4 | 9.9 | 3.2, 16.6 | .004 |
| 14 | 14.3 ± 0.3 | 14.7 ± 0.6 | 14.4 | 13.6 | 9.8 | 2.9, 16.7 | .005 |
| 15 | 15.8 ± 0.3 | 14.8 ± 0.3 | 15.5 | 14.8 | 9.2 | 2.2, 16.2 | .01 |
A total of 319 films from 120 HIV− participants were used in the analysis to estimate the predicted bone ages; 10 films from 6 participants were excluded from the analysis because they were skeletally mature at baseline.
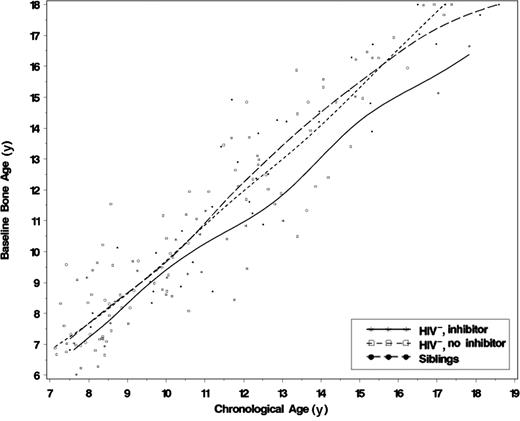
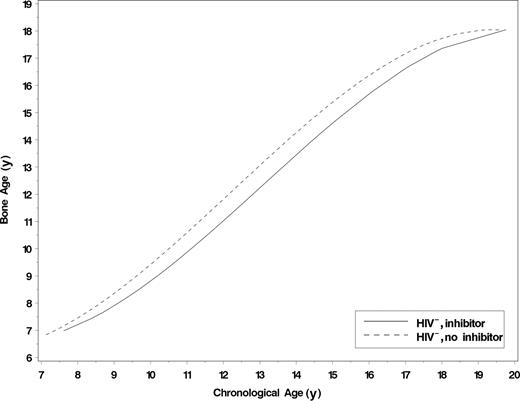
The predicted bone age was significantly lower among HIV− adolescents with a history of inhibitors compared with those without inhibitors at every chronological age examined. The greatest differences occurred from 12 through 15 years of age. Figure 1 shows the baseline bone age for chronological age among 3 groups: HIV− with hemophilia and inhibitors, HIV− with hemophilia and no inhibitors, and the sibling control participants without hemophilia. The bone ages for chronological age are quite similar regardless of hemophilia status, except where there is a history of inhibitors. Figure 2 displays predicted bone ages for chronological age by inhibitor status throughout the entire age span evaluated. The bone ages of HIV− adolescents without a history of inhibitor are similar to, or exceed, chronological age throughout adolescence.
Cubic spline interpolation of baseline bone age among HIV− participants with hemophilia (with and without a history of inhibitors) and a group of siblings without hemophilia. A total of 158 baseline films were used in the analysis: 19 participants had hemophilia and an inhibitor, 101 participants had hemophilia without an inhibitor, and 38 were sibling control participants. Altogether, 6 participants with hemophilia and 6 siblings were excluded from the analysis because they were skeletally mature at baseline, and 3 siblings lacked bone age measurements.
Cubic spline interpolation of baseline bone age among HIV− participants with hemophilia (with and without a history of inhibitors) and a group of siblings without hemophilia. A total of 158 baseline films were used in the analysis: 19 participants had hemophilia and an inhibitor, 101 participants had hemophilia without an inhibitor, and 38 were sibling control participants. Altogether, 6 participants with hemophilia and 6 siblings were excluded from the analysis because they were skeletally mature at baseline, and 3 siblings lacked bone age measurements.
Predicted bone age by inhibitor status for HIV− study participants. A total of 319 films from 120 HIV− participants were used in the analysis. A total of 10 films from 6 participants were excluded from the analysis because they were skeletally mature at baseline.
Predicted bone age by inhibitor status for HIV− study participants. A total of 319 films from 120 HIV− participants were used in the analysis. A total of 10 films from 6 participants were excluded from the analysis because they were skeletally mature at baseline.
Pubertal progression
As shown in Table 3, HIV− participants with a history of inhibitors were older at every Tanner stage transition (from stage 1 [preadolescent] to 2, stage 2 to 3, stage 3 to 4, and stage 4 to 5 [adult]) than adolescents without inhibitors. The differences were greatest in the early transitions; the smallest difference, 3.7 months, was observed at the time of transition from Tanner stage 4 to 5.
Predicted mean age at Tanner stage transitions by inhibitor status for HIV− adolescents
| Tanner stage . | Age without inhibitor, y . | Age with inhibitor, y . | Difference, mo . | 95% CI, mo . | P . |
|---|---|---|---|---|---|
| 1-2 | 12.5 | 13.2 | 9.2 | 3.1, 15.2 | .003 |
| 2-3 | 13.4 | 14.1 | 8.0 | 1.1, 14.9 | .02 |
| 3-4 | 14.6 | 15.2 | 7.5 | 0.6, 14.3 | .03 |
| 4-5 | 16.0 | 16.3 | 3.7 | −2.8, 10.2 | .27 |
| Tanner stage . | Age without inhibitor, y . | Age with inhibitor, y . | Difference, mo . | 95% CI, mo . | P . |
|---|---|---|---|---|---|
| 1-2 | 12.5 | 13.2 | 9.2 | 3.1, 15.2 | .003 |
| 2-3 | 13.4 | 14.1 | 8.0 | 1.1, 14.9 | .02 |
| 3-4 | 14.6 | 15.2 | 7.5 | 0.6, 14.3 | .03 |
| 4-5 | 16.0 | 16.3 | 3.7 | −2.8, 10.2 | .27 |
A total of 1051 evaluations from 114 HIV− participants were used in the analysis; 89 evaluations from 12 participants were excluded from the analysis because they were already Tanner stage 5 at baseline.
Serum testosterone
The age at time of sample collection ranged from 11 to 19 years (median, 13.8 years). Serum testosterone level was significantly lower in participants with a history of inhibitor compared with those without an inhibitor (4.35 nM vs 8.13 nM [125.4 vs 234.2 ng/dL]; P < .001) respectively, after adjustment for age and HIV status.
Growth velocity and stature
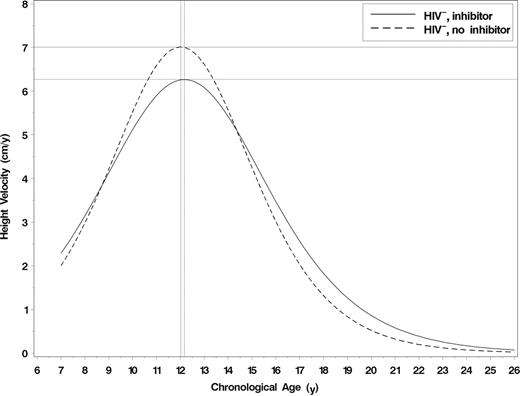
Within the HIV− group, the maximum growth velocity in adolescents without an inhibitor was 7.0 cm/year compared with 6.3 cm/year (95% confidence interval [CI] for difference: 0.2-1.3; P = .01) in those with an inhibitor, although the age at maximum velocity did not differ between the groups (12.0 years vs 12.2 years, respectively; 95% CI for difference: −0.3-0.6; P = .47). This is illustrated in Figure 3.
Predicted height velocity curves by inhibitor status among HIV− study participants. A total of 1188 height measurements for 126 HIV− participants were used in the analysis. Horizontal line indicates maximum growth velocity; and vertical line, age at maximum growth velocity.
Predicted height velocity curves by inhibitor status among HIV− study participants. A total of 1188 height measurements for 126 HIV− participants were used in the analysis. Horizontal line indicates maximum growth velocity; and vertical line, age at maximum growth velocity.
Stature was significantly lower for HIV− adolescents with inhibitors throughout adolescence. At age 18, the difference was 7.1 cm (95% CI: 3.2-11.0; P < .001). Those without inhibitors had a mean height of 177.1 cm compared with 170.0 in those with inhibitors. By age 24 years, there was still a difference of 5.4 cm in height between the inhibitor groups. Although these are substantial differences, the possible confounding effects of arthropathy on the accuracy of height measurements must be considered.
HIV+ study participants
Similar, though less pronounced differences in maturation and growth were observed among HIV+ adolescents by inhibitor status. Delays in skeletal maturation were greater among participants with an inhibitor compared with those without an inhibitor throughout adolescence; however, the differences were generally small (1-2 months) and not statistically significant. Progression through Tanner stages was slower for those with an inhibitor, from 1 to 3 months at every stage, but not significantly different. Both HIV status and inhibitor status were significant independent predictors of testosterone level, although the effect of inhibitor status was stronger. Neither the maximum growth velocity nor the age at maximum velocity differed significantly by inhibitor status. Differences in stature by inhibitor status were also observed among HIV+ adolescents, although these were generally small, from 1 to 2 cm, and not statistically significant.
Discussion
The data presented here indicate a physiologic disruption of normal development for adolescents with a history of inhibitors. Growth disorders have long been recognized to occur in other systemic conditions,13,14 particularly when the disease process is not satisfactorily controlled. Hemophilia complicated by inhibitors easily fits the description of a chronic disease not under control, evidenced by more frequent and difficult-to-manage bleeding, pain, and other complications. Reports from the HGDS confirm that study participants with inhibitors had a greater degree of arthropathy measured by range-of-motion abnormalities; were more likely to have abnormalities in coordination and gait resulting from either acute episodes of pain or discomfort, loss of range of motion and joint swelling, or chronic joint disease1 ; and had more frequent hospitalizations12 than those without inhibitors. The HGDS is uniquely suited to an investigation of associations between inhibitors and delays in maturation for a number of reasons, including (1) the age range of the study group, (2) the duration of follow-up, (3) the variety of growth outcomes examined, (4) the standardized methods for data collection and interpretation, and (5) the fact that it is a multicenter study with participants representative of the population from which they were enrolled. The latter characteristic increases the generalizability of results to other adolescents with hemophilia and inhibitors.
We began our analysis with an examination of skeletal maturation, a measurement of biologic maturity more closely related to other maturational processes that occur during puberty than chronological age.15 The FELS method, used to assess skeletal age in the HGDS, is a computer-assisted procedure that incorporates both graded (up to 85) and metric (up to 13) indicators. It has been shown to be a more appropriate method for assessing skeletal age of children in the United States than either the Greulich-Pyle or Tanner-Whitehouse methods.15 The differences in bone age for chronological age between the HIV− adolescents with and without a history of inhibitor were statistically significant as well as clinically significant: in excess of 9 months between the ages of 12 and 15 years. Further, bone age for chronological age appeared quite similar for adolescents with hemophilia uncomplicated by inhibitors and a group of siblings of study participants who did not have hemophilia. Consistent with delays in skeletal maturation, pubertal progression was slower in adolescents with a history of inhibitors compared with those without a history at every Tanner stage transition, significantly so through each of the transitions from Tanner stages 1 to 4. The method of assessing pubertal progression used in the original work of Tanner ensured consistency of ratings by the use of photographs of the same boy taken throughout adolescence.16 In a clinical setting, however, variability in measurement is common,16 can be problematic, and results in loss of statistical power and efficiency.17 Even with central training and a standardized protocol designed to reduce errors in classification, we observed variability and occasional inconsistency in measurement of Tanner stage. Nonetheless, we were able to observe important differences between the inhibitor groups. These ranged from greater than 9 months at transition from Tanner stage 1 to 2 to more than 7 months in the timing of transition from Tanner stage 3 to 4. Not surprisingly, mean levels of serum testosterone were also significantly lower among adolescents with a history of inhibitors. Analysis of the rate of growth in the HIV− population demonstrated differences between the inhibitor groups in maximum growth velocity, although the ages at which peak velocity occurred were quite similar. We are mindful that a greater degree of arthropathy among adolescents with inhibitors, particularly that affecting knee joints, could interfere with accurate measurement of height and potentially introduce a bias favoring detection of differences. HIV− adolescents with inhibitors did not “catch up” with the noninhibitor cohort; their stature upon reaching adulthood was an average of 5.4 cm less than that of the adolescents without inhibitors.
Confounding factors that might account for the observed associations were considered. Our prior work has documented the effect of HIV on each of the measures of growth examined; thus, our analyses were stratified by, or controlled for, HIV status. Within the HIV+ study group, we observed what appeared to be effects of inhibitor status across all growth measures. These were small and generally not statistically significant, perhaps due to the intervening effects of HIV. We interpret the consistency of the differentials in maturation among those in the HIV+ group, although not significant, as further support of our findings among HIV− adolescents. The skeletal maturation analyses controlled for race. Associations between race and risk for the development of inhibitors have been reported, as well as relationships between skeletal maturation and race.18,19 In addition to race, other possible confounding factors were considered, including type and severity of hemophilia, socioeconomic status (measured by parents' education), and hepatitis C viral load. Controlling for these variables did not change our conclusions, even though controlling for hepatitis C viral load reduced our available Tanner stage and height measurements by approximately 40%. Removal from the analysis of the HIV− study participant who developed his inhibitor during follow-up did not affect the results.
The HGDS dataset lacks information about the natural history of study participants' inhibitors prior to enrollment or attempts, successful or unsuccessful, at tolerization. Therefore, we defined our at-risk group in the broadest possible way, including anyone with a history of inhibitor of 1 BU or more, regardless of duration, response (low or high), or impact on method of treatment for their hemophilia. We reasoned that the effect of inclusion of a subgroup with transient inhibitors, or those whose inhibitors had been eradicated by immune tolerance, would be to underestimate rather than overestimate findings of relationships with growth measures.
Our goal was to address the question of whether an association exists between inhibitors and delays in physical maturation. We believe that our findings provide compelling evidence of such a link across a variety of growth measures. Indeed, they indicate a sizable shift in mean levels for the indicators of maturation that were examined. Differences in skeletal maturation and ages at Tanner stage transition strongly suggest pubertal delay. These findings have important implications for adolescents with inhibitors.
While we are not able, with existing data, to offer an explanation for the relationship between delays in maturation and inhibitors, possible mechanisms can be suggested, including restriction in movement and exercise, resulting in a lack of the stimulation necessary for normal growth processes; immune response genes (eg, those directing cytokine responses) may be affecting tissue or bone maturation; or the chronic up-regulation of inflammatory gene products associated with persistent bleeding may adversely influence bone metabolism. Anemia associated with more frequent bleeding, or infections, may also be contributors. In the absence of direct support for these or other mechanisms, definitive recommendations for interventions cannot be made. Nonetheless, it is intuitive that early and aggressive attempts to minimize inhibitors or eradicate them completely in order to maximize the efficiency of therapy to suppress bleeding, prevent chronic pain, and achieve overall control of the disease process, is a logical goal. This investigation opens an avenue of inquiry into another dimension of hemophilia-related morbidity, and underscores the importance of carefully monitoring the growth and maturation of children and adolescents with hemophilia, particularly those with inhibitors.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We recognize Dr Margaret W. Hilgartner and Prof Alex F. Roche and thank Aime Grimsley and Erika Menius for their contributions to the initiation and completion of this investigation.
This work was supported by the National Institutes of Health, National Institute of Child Health and Human Development grant 1 R01 HD41224 (The Hemophilia Growth and Development Study).
National Institutes of Health
Authorship
Contribution: S.M.D. designed and performed research, collected data, prepared the analysis plan, and drafted the manuscript; H.S.L. prepared the analysis plan, performed statistical analysis and interpretation of the results, and drafted the manuscript; A.E.L. performed statistical analysis and interpretation of the results; W.K.H. designed and performed research, collected data, and drafted the manuscript; E.B. developed the research plan and drafted the manuscript; and E.D.G. designed and performed research, collected data, and drafted the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
A complete list of the members of the Hemophilia Growth and Development Study Group is provided in Document S1, available on the Bloodwebsite; see the Supplemental Materials link at the top of the online article.
Correspondence: Sharyne M. Donfield, Rho, Inc, 6330 Quadrangle Dr, Chapel Hill, NC 27517; e-mail:sdonfield@rhoworld.com.