Abstract
We assessed late mortality in 1479 individuals who had survived 2 or more years after allogeneic hematopoietic cell transplantation (HCT). Median age at HCT was 25.9 years and median length of follow-up was 9.5 years. The conditional survival probability at 15 years from HCT was 80.2% (SE = 1.9%) for those who were disease-free at entry into the cohort, and the relative mortality was 9.9 (95% confidence interval, 8.7-11.2). Relative mortality decreased with time from HCT, but remained significantly elevated at 15 years after HCT (standardized mortality ratio = 2.2). Relapse of primary disease (29%) and chronic graft-versus-host disease (cGVHD: 22%) were the leading causes of premature death. Nonrelapse-related mortality was increased among patients older than 18 years at HCT (18-45 years: relative risk [RR] = 1.7; 46+ years: RR = 3.7) and among those with cGVHD (RR = 2.7), and was lower among patients who received methotrexate for GVHD prophylaxis (RR = 0.5). HCT survivors were more likely to report difficulty in holding jobs (odds ratio [OR] = 13.9), and in obtaining health (OR = 7.1) or life (OR = 9.9) insurance compared with siblings. This study demonstrates that mortality rates remain twice as high as that of the general population among 15-year survivors of HCT, and that the survivors face challenges affecting their health and well-being.
Introduction
Liberalization in the indications for allogeneic hematopoietic cell transplantation (HCT) as well as an increase in options in the source of the hematopoietic stem cell are responsible for the increasing number of HCTs performed annually.1 Long-term survival has become an expected outcome for patients undergoing HCT. A limited number of studies that are either small in size or represent registry data have examined late mortality for patients undergoing allogeneic HCT.2-4 Some studies have also attempted to describe the health and functional status of individuals surviving allogeneic HCT.4-7 However, these studies have generally used passive rather than active follow-up methods and have not included evaluations using the National Death Index (NDI) or the Social Security Death Index (SSDI) to ensure completeness. Our study represents a comprehensive analysis of disease- and treatment-related factors associated with late mortality, using NDI, SSDI, and medical records, in a large cohort of allogeneic hematopoietic cell (HC) recipients. Furthermore, results presented here describe specific aspects of functional status such as marital status, employment, and problems with health and life insurance experienced by the long-term survivors of allogeneic HCT
Patients and methods
The Bone Marrow Transplant Survivor Study (BMTSS), a collaborative effort between City of Hope National Medical Center (COH) and the University of Minnesota (UMN), examines the long-term outcomes of individuals who have survived 2 or more years after undergoing HCT. The present report from BMTSS is restricted to individuals who met the following eligibility criteria: (1) primary diagnoses of chronic myelogenous leukemia (CML), acute myeloid leukemia (AML), acute lymphoblastic leukemia (ALL), non-Hodgkin lymphoma (NHL), severe aplastic anemia (SAA), or inborn errors of metabolism (IEM); (2) allogeneic HCT between 1974 and 1998; and (3) survival of at least 2 years from HCT. The Human Subjects Committee at the participating institutions approved the BMTSS protocol. Informed consent was obtained in accordance with the Declaration of Helsinki.
Mortality analysis
Information on primary diagnoses, therapeutic agents used for preparative regimens, and the source of donor stem cells (sibling vs unrelated donor), presence of chronic graft-versus-host disease (cGVHD), and the agents used for GVHD prophylaxis was obtained on all eligible cases from the institutional transplant databases.
Vital status information was obtained from 3 sources: medical records, NDI, and SSDI. Vital status of the BMTSS subjects was ascertained as of June 30, 2003. Information on the cause of death was obtained from the NDI Plus program and the medical records.
Standardized mortality ratio (SMR, the ratio of observed to expected deaths) was used to compare the mortality of this cohort to the age- (5-year interval), sex-, and calendar-specific (1975-1979, 1980-1984, 1985-1989, 1990-1994, 1995-1999) mortality of the US general population.8 Person-years at risk were computed from the date 2 years after HCT to either the date of death or date of censoring (June 30, 2003, for those still alive). The expected number of deaths was calculated9 by multiplying the number of person-years in each defined stratum by the corresponding US mortality rates reported by the National Center for Health Statistics.8 Ninety-five percent confidence interval of the SMR was calculated using the method described by Vandenbroucke.10 SMRs were calculated for deaths from all causes and also for deaths from specific causes, including secondary or subsequent cancer, cardiac toxicity, pulmonary complications, and external causes (accidents, suicides, homicides, etc).
Survival functions were estimated by the product-limit method by sex and age. To compare survival curves for this cohort with the age-comparable US population, an expected number of deaths for each year since transplantation was calculated based on the US age-, sex-, and calendar-specific mortality rates, yielding an expected survival function by sex.
Potential risk factors for late deaths were analyzed using Cox regression models.11 Factors evaluated included age at transplantation (categorized as younger than 18, 18 to 45, and older than 45 years), sex, race, primary diagnosis, type of donor stem cells, disease status at transplantation (standard risk of relapse at HCT and high risk of relapse at HCT), preparative regimens used, presence of cGVHD, and agents used for GVHD prophylaxis. Patients who underwent transplantation in first or second complete remission after acute leukemia and lymphoma or first chronic phase of CML were considered being at standard risk for relapse. All other patients were considered at high-risk for relapse. Analyses were performed for the entire cohort, for both relapse-related and nonrelapse-related mortality.
Analysis of marital status, employment, and health/life insurance
Data on specific aspects of functional well-being including marital status, employment, and problems with health and life insurance were collected from the surviving members of the study cohort who were 18 years or older at study participation, using the BMTSS questionnaire. This questionnaire was designed to capture a wide range of information including demographic characteristics, marital status, insurance coverage, education, income, and employment. For comparison purposes, 319 nearest-age siblings also completed the BMTSS questionnaire.
Chi-square test was used to compare distributions of functional well-being between survivors and siblings. Unconditional logistic regression was used to calculate odds ratios (ORs) and their 95% confidence intervals (CIs) after adjustment for age at study participation, sex, race and education. For analysis restricted to HCT survivors only, variables examined in the logistic regression were age at transplantation (younger than 18, 18-45, older than 45 years), sex, race (white, Hispanic, others), education (less than high school, high school graduate to some college, college graduate or higher), household income (> $60 000, $20 000-$60 000, < $20 000), primary diagnosis, cGVHD status (yes, no), length of follow-up (≤ 5, 6-10, 11 + years), and year of transplantation (1974-1979, 1980-1984, 1985-1989, 1990-1994, and 1995-1998).
Results
Patient characteristics
Table 1 summarizes the characteristics of the study population for the entire cohort, and by primary diagnosis. In this cohort, 1479 patients had undergone allogeneic HCT at COH or UMN, and survived 2 years, irrespective of disease status. Of these, 861 (58%) were male; the median age at HCT was 25.9 years (range, 0.2 to 71.5 years), and the cohort was followed for a median of 9.5 years (range, 2.0 to 28.4 years). Primary diagnoses included CML (31%), AML (29%), ALL (19%), SAA (12%), IEM (5%), and NHL (4%). The large majority (80.7%) of patients had received stem cells from a related donor, and bone marrow (96%) was the major source of stem cells. Total body irradiation (TBI) was used as part of the conditioning regimen in 87% of the cohort, while cyclophosphamide was used in 72% and etoposide in 27% of the cohort.
Demographic and clinical characteristics of 2-year survivors of allogeneic HCT
| Variables . | Entire cohort, n = 1479 . | Primary diagnosis . | |||||
|---|---|---|---|---|---|---|---|
| AML, n = 429 . | CML, n = 452 . | ALL, n = 287 . | NHL, n = 53 . | SAA, n = 175 . | IEM, n = 83 . | ||
| Median age at HCT, y (range) | 25.9 (0.2-71.5) | 26.2 (0.9-61.0) | 34.3 (1.1-71.5) | 17.8 (0.5-51.9) | 37.3 (5.7-53.3) | 16.9 (1.7-46.7) | 2.6 (0.2-32.9) |
| Age at transplantation, y (%) | |||||||
| Younger than 18 | 505 (34.1) | 142 (33.1) | 35 (7.7) | 145 (50.5) | 6 (11.3) | 97 (55.4) | 80 (96.4) |
| 18 to 45 | 853 (57.7) | 247 (57.6) | 356 (78.8) | 133 (46.3) | 37 (69.8) | 77 (44.0) | 3 (3.6) |
| Older than 45 | 121 (8.2) | 40 (9.3) | 61 (13.5) | 9 (3.1) | 10 (18.9) | 1 (0.6) | 0 (0) |
| Sex, no. (% male) | 861 (58.2) | 223 (52.0) | 261 (57.7) | 192 (66.9) | 33 (62.3) | 103 (58.9) | 49 (59.0) |
| Type of donor, no. (%) | |||||||
| Related donor | 1193 (80.7) | 373 (86.9) | 327 (72.3) | 241 (84.0) | 48 (90.6) | 159 (90.9) | 45 (54.2) |
| Unrelated donor | 286 (19.3) | 56 (13.1) | 125 (27.7) | 46 (16.0) | 5 (9.4) | 16 (9.1) | 38 (45.8) |
| Source of stem cell, no. (%) | |||||||
| BM | 1416 (95.7) | 412 (96.1) | 443 (98.0) | 277 (96.5) | 39 (73.6) | 171 (97.7) | 74 (89.2) |
| PBSCs | 35 (2.4) | 11 (2.6) | 8 (1.8) | 2 (0.7) | 14 (26.4) | 0 (0) | 0 (0) |
| Cord blood | 28 (1.9) | 6 (1.4) | 1 (0.2) | 8 (2.8) | 0 (0) | 4 (2.3) | 9 (10.8) |
| Year of transplantation, no. (%) | |||||||
| 1974 to 1979 | 55 (3.7) | 18 (4.2) | 0 (0) | 12 (4.2) | 4 (7.5) | 21 (12.0) | 0 (0) |
| 1980 to 1984 | 224 (15.1) | 73 (17.1) | 41 (9.1) | 62 (21.6) | 6 (11.3) | 40 (22.9) | 2 (2.4) |
| 1985 to 1989 | 293 (19.8) | 88 (20.5) | 92 (20.4) | 52 (18.1) | 5 (9.4) | 34 (19.4) | 22 (26.5) |
| 1990 to 1994 | 450 (30.4) | 120 (28.0) | 180 (39.8) | 80 (27.9) | 7 (13.2) | 38 (21.7) | 25 (.0.1) |
| 1995 to 1998 | 457 (30.9) | 130 (30.3) | 139 (30.8) | 81 (28.2) | 31 (58.5) | 42 (24.0) | 34 (41.0) |
| Median time since HCT, mo (range) | 9.5 (2.0-28.4) | 10 (2.0-26.5) | 9.4 (2.1-21.9) | 9.2 (2.0-26.3) | 6.3 (2.1-27.7) | 13.1 (2.0-28.4) | 8.0 (2.1-19.0) |
| Conditioning regimen, no. (%) | |||||||
| TLI | 1279 (86.5) | 368 (85.8) | 423 (93.6) | 280 (97.9) | 48 (90.6) | 119 (68.0) | 41 (49.4) |
| Cyclophosphamide | 1066 (72.1) | 316 (73.7) | 318 (70.4) | 188 (65.7) | 43 (81.1) | 157 (89.7) | 44 (53.0) |
| Busulfan | 164 (11.1) | 77 (17.9) | 31 (6.9) | 10 (3.5) | 4 (7.5) | 0 (0) | 42 (50.6) |
| VP16 | 403 (27.3) | 129 (30.1) | 138 (30.5) | 124 (43.4) | 12 (22.6) | 0 (0) | 0 (0) |
| Cytarabine | 72 (4.9) | 35 (8.2) | 2 (0.4) | 35 (12.2) | 0 (0) | 0 (0) | 0 (0) |
| Disease status at HCT, no. (%) | |||||||
| High risk of relapse | 364 (24.7) | 142 (33.3) | 133 (29.5) | 68 (23.7) | 21 (41.2) | 0 (0) | 0 (0) |
| Chronic GVHD | 730 (49.7) | 206 (48.6) | 286 (63.3) | 119 (41.9) | 35 (67.3) | 66 (37.7) | 18 (21.7) |
| Institutions (%) | |||||||
| COH | 722 (48.8) | 230 (53.6) | 241 (53.3) | 159 (55.4) | 34 (64.2) | 58 (33.1) | 0 (0) |
| UMN | 757 (51.2) | 199 (46.4) | 211 (46.7) | 128 (44.6) | 19 (35.8) | 117 (66.9) | 83 (100) |
| No. of deaths (%) | 320 (21.6) | 107 (24.9) | 98 (21.7) | 71 (24.7) | 13 (24.5) | 19 (10.9) | 12 (14.5) |
| Median age at death, y (range) | 33.4 (2.7-63.7) | 33.4 (5.2-62.6) | 42.7 (5.0-63.7) | 25.1 (5.0-53.8) | 44.2 (19.7-62.2) | 29.8 (7.8-55.5) | 7.6 (2.7-17.5) |
| Relapse in the first 2 y of HCT (ie, before entry into cohort), no. (%) | |||||||
| History of relapse | 127 (8.6) | 36 (8.4) | 46 (10.2) | 35 (12.2) | 8 (15.1) | 2 (1.1) | 0 (0) |
| No history of relapse | 1352 (91.4) | 393 (91.6) | 406 (89.8) | 252 (87.8) | 45 (84.9) | 173 (98.9) | 83 (100) |
| Variables . | Entire cohort, n = 1479 . | Primary diagnosis . | |||||
|---|---|---|---|---|---|---|---|
| AML, n = 429 . | CML, n = 452 . | ALL, n = 287 . | NHL, n = 53 . | SAA, n = 175 . | IEM, n = 83 . | ||
| Median age at HCT, y (range) | 25.9 (0.2-71.5) | 26.2 (0.9-61.0) | 34.3 (1.1-71.5) | 17.8 (0.5-51.9) | 37.3 (5.7-53.3) | 16.9 (1.7-46.7) | 2.6 (0.2-32.9) |
| Age at transplantation, y (%) | |||||||
| Younger than 18 | 505 (34.1) | 142 (33.1) | 35 (7.7) | 145 (50.5) | 6 (11.3) | 97 (55.4) | 80 (96.4) |
| 18 to 45 | 853 (57.7) | 247 (57.6) | 356 (78.8) | 133 (46.3) | 37 (69.8) | 77 (44.0) | 3 (3.6) |
| Older than 45 | 121 (8.2) | 40 (9.3) | 61 (13.5) | 9 (3.1) | 10 (18.9) | 1 (0.6) | 0 (0) |
| Sex, no. (% male) | 861 (58.2) | 223 (52.0) | 261 (57.7) | 192 (66.9) | 33 (62.3) | 103 (58.9) | 49 (59.0) |
| Type of donor, no. (%) | |||||||
| Related donor | 1193 (80.7) | 373 (86.9) | 327 (72.3) | 241 (84.0) | 48 (90.6) | 159 (90.9) | 45 (54.2) |
| Unrelated donor | 286 (19.3) | 56 (13.1) | 125 (27.7) | 46 (16.0) | 5 (9.4) | 16 (9.1) | 38 (45.8) |
| Source of stem cell, no. (%) | |||||||
| BM | 1416 (95.7) | 412 (96.1) | 443 (98.0) | 277 (96.5) | 39 (73.6) | 171 (97.7) | 74 (89.2) |
| PBSCs | 35 (2.4) | 11 (2.6) | 8 (1.8) | 2 (0.7) | 14 (26.4) | 0 (0) | 0 (0) |
| Cord blood | 28 (1.9) | 6 (1.4) | 1 (0.2) | 8 (2.8) | 0 (0) | 4 (2.3) | 9 (10.8) |
| Year of transplantation, no. (%) | |||||||
| 1974 to 1979 | 55 (3.7) | 18 (4.2) | 0 (0) | 12 (4.2) | 4 (7.5) | 21 (12.0) | 0 (0) |
| 1980 to 1984 | 224 (15.1) | 73 (17.1) | 41 (9.1) | 62 (21.6) | 6 (11.3) | 40 (22.9) | 2 (2.4) |
| 1985 to 1989 | 293 (19.8) | 88 (20.5) | 92 (20.4) | 52 (18.1) | 5 (9.4) | 34 (19.4) | 22 (26.5) |
| 1990 to 1994 | 450 (30.4) | 120 (28.0) | 180 (39.8) | 80 (27.9) | 7 (13.2) | 38 (21.7) | 25 (.0.1) |
| 1995 to 1998 | 457 (30.9) | 130 (30.3) | 139 (30.8) | 81 (28.2) | 31 (58.5) | 42 (24.0) | 34 (41.0) |
| Median time since HCT, mo (range) | 9.5 (2.0-28.4) | 10 (2.0-26.5) | 9.4 (2.1-21.9) | 9.2 (2.0-26.3) | 6.3 (2.1-27.7) | 13.1 (2.0-28.4) | 8.0 (2.1-19.0) |
| Conditioning regimen, no. (%) | |||||||
| TLI | 1279 (86.5) | 368 (85.8) | 423 (93.6) | 280 (97.9) | 48 (90.6) | 119 (68.0) | 41 (49.4) |
| Cyclophosphamide | 1066 (72.1) | 316 (73.7) | 318 (70.4) | 188 (65.7) | 43 (81.1) | 157 (89.7) | 44 (53.0) |
| Busulfan | 164 (11.1) | 77 (17.9) | 31 (6.9) | 10 (3.5) | 4 (7.5) | 0 (0) | 42 (50.6) |
| VP16 | 403 (27.3) | 129 (30.1) | 138 (30.5) | 124 (43.4) | 12 (22.6) | 0 (0) | 0 (0) |
| Cytarabine | 72 (4.9) | 35 (8.2) | 2 (0.4) | 35 (12.2) | 0 (0) | 0 (0) | 0 (0) |
| Disease status at HCT, no. (%) | |||||||
| High risk of relapse | 364 (24.7) | 142 (33.3) | 133 (29.5) | 68 (23.7) | 21 (41.2) | 0 (0) | 0 (0) |
| Chronic GVHD | 730 (49.7) | 206 (48.6) | 286 (63.3) | 119 (41.9) | 35 (67.3) | 66 (37.7) | 18 (21.7) |
| Institutions (%) | |||||||
| COH | 722 (48.8) | 230 (53.6) | 241 (53.3) | 159 (55.4) | 34 (64.2) | 58 (33.1) | 0 (0) |
| UMN | 757 (51.2) | 199 (46.4) | 211 (46.7) | 128 (44.6) | 19 (35.8) | 117 (66.9) | 83 (100) |
| No. of deaths (%) | 320 (21.6) | 107 (24.9) | 98 (21.7) | 71 (24.7) | 13 (24.5) | 19 (10.9) | 12 (14.5) |
| Median age at death, y (range) | 33.4 (2.7-63.7) | 33.4 (5.2-62.6) | 42.7 (5.0-63.7) | 25.1 (5.0-53.8) | 44.2 (19.7-62.2) | 29.8 (7.8-55.5) | 7.6 (2.7-17.5) |
| Relapse in the first 2 y of HCT (ie, before entry into cohort), no. (%) | |||||||
| History of relapse | 127 (8.6) | 36 (8.4) | 46 (10.2) | 35 (12.2) | 8 (15.1) | 2 (1.1) | 0 (0) |
| No history of relapse | 1352 (91.4) | 393 (91.6) | 406 (89.8) | 252 (87.8) | 45 (84.9) | 173 (98.9) | 83 (100) |
AML indicates acute myeloid leukemia; CML, chronic myeloid leukemia; ALL, acute lymphoblastic leukemia; NHL, non-Hodgkin lymphoma; SAA, severe aplastic anemia; IEM, inborn errors of metabolism; BM, bone marrow; PBSC, peripheral blood stem cells; and TLI, total body irradiation.
Overall survival
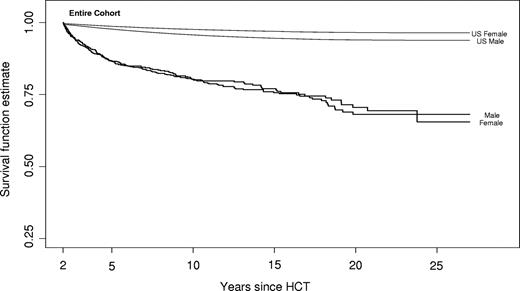
By June 30, 2003, 21.6% (n = 320) of the patients who had undergone HCT between 1974 and 1998, and survived at least 2 years, had died. Median age at death was 33.4 years. Most of the deaths (69%) occurred in the 2- to 5-year period after allogeneic HCT, although 43 deaths (13%) did occur after 10 years from HCT. For the entire cohort, the probability of surviving was 86.6% (SE = 0.9%) at 5 years, 80.4% (SE = 1.3%) at 10 years, and 76.3% (SE = 1.9%) at 15 years from HCT. All-cause mortality experience from 2 years after HCT for the entire cohort was compared with age-adjusted expected survival rates for the US population and is shown in Figure 1.
All-cause mortality in a cohort of 1479 2+-year survivors after allogeneic HCT. Sex-specific survival for hematologic malignancies, severe aplastic anemia, and inborn errors of metabolism for the entire cohort.
All-cause mortality in a cohort of 1479 2+-year survivors after allogeneic HCT. Sex-specific survival for hematologic malignancies, severe aplastic anemia, and inborn errors of metabolism for the entire cohort.
In this cohort of 1479 patients, a total of 127 patients had suffered at least one relapse in the first 2 years after HCT (ie, at entry into this cohort), and all subsequent analyses presented here excluded these 127 patients. Among the patients who did not suffer a relapse by 2 years after HCT, the probability of survival was 90.2% (SE = 0.8%) at 5 years, 84.6% (SE = 1.3%) at 10 years, and 80.2% (SE = 1.9%) at 15 years from HCT. Patients who underwent transplantation for SAA had a better probability of surviving than those who underwent transplantation for other diseases (Table 2). The 5-year and 10-year survival probability by diagnosis for patients with and without cGVHD is also presented in Table 2.
Five- and 10-year overall survival by primary diagnosis and cGVHD status in patients surviving disease-free for 2 years after allogeneic HCT
| Diagnosis . | Overall . | Without cGVHD . | With cGVHD . | 10-y . | ||
|---|---|---|---|---|---|---|
| 5-y . | 10-y . | 5-y . | 10-y . | 5-y . | ||
| Entire cohort | 90.2 (0.8) | 84.6 (1.3) | 95.1 (0.9) | 91.0 (1.4) | 85.5 (1.4) | 78.1 (2.1) |
| Acute myeloid leukemia | 89.3 (1.6) | 82.8 (2.4) | 95.5 (1.5) | 92.1 (2.3) | 83.0 (2.8) | 73.2 (4.2) |
| Chronic myeloid leukemia | 90.4 (1.5) | 83.3 (2.5) | 94.4 (1.9) | 89.5 (3.5) | 88.2 (2.1) | 79.8 (3.4) |
| Acute lymphoblastic leukemia | 88.5 (2.1) | 83.9 (3.0) | 93.6 (2.1) | 86.7 (3.5) | 82.5 (3.9) | 81.5 (5.2) |
| Non-Hodgkin lymphoma | 86.7 (5.5) | 70.7 (10.6) | 92.9 (7.9) | 80.0 (14.7) | 83.3 (7.3) | 64.8 (14.5) |
| Severe aplastic anemia | 95.9 (1.5) | 93.8 (2.2) | 99.1 (0.9) | 99.1 (1.2) | 90.7 (3.7) | 85.5 (4.9) |
| Inborn errors of metabolism | 89.1 (3.6) | 86.4 (5.6) | 92.3 (3.5) | 90.5 (5.8) | 77.8 (9.8) | 72.2 (12.7) |
| Diagnosis . | Overall . | Without cGVHD . | With cGVHD . | 10-y . | ||
|---|---|---|---|---|---|---|
| 5-y . | 10-y . | 5-y . | 10-y . | 5-y . | ||
| Entire cohort | 90.2 (0.8) | 84.6 (1.3) | 95.1 (0.9) | 91.0 (1.4) | 85.5 (1.4) | 78.1 (2.1) |
| Acute myeloid leukemia | 89.3 (1.6) | 82.8 (2.4) | 95.5 (1.5) | 92.1 (2.3) | 83.0 (2.8) | 73.2 (4.2) |
| Chronic myeloid leukemia | 90.4 (1.5) | 83.3 (2.5) | 94.4 (1.9) | 89.5 (3.5) | 88.2 (2.1) | 79.8 (3.4) |
| Acute lymphoblastic leukemia | 88.5 (2.1) | 83.9 (3.0) | 93.6 (2.1) | 86.7 (3.5) | 82.5 (3.9) | 81.5 (5.2) |
| Non-Hodgkin lymphoma | 86.7 (5.5) | 70.7 (10.6) | 92.9 (7.9) | 80.0 (14.7) | 83.3 (7.3) | 64.8 (14.5) |
| Severe aplastic anemia | 95.9 (1.5) | 93.8 (2.2) | 99.1 (0.9) | 99.1 (1.2) | 90.7 (3.7) | 85.5 (4.9) |
| Inborn errors of metabolism | 89.1 (3.6) | 86.4 (5.6) | 92.3 (3.5) | 90.5 (5.8) | 77.8 (9.8) | 72.2 (12.7) |
Data are all percentages (SE).
Relative mortality
Table 3 summarizes the relative mortality in this population, using age-, sex-, and calendar-specific rates from the general US population. Overall, premature death occurred 10 times more often than expected. High relative mortality rates were seen in both male (8-fold increased risk) and female (13-fold increased risk) patients. Relative mortality was elevated across all age groups, but was highest in subjects undergoing HCT when younger than 18 years (17-fold). High relative mortality was also observed across all diagnoses, with the highest relative mortality among individuals with a primary diagnosis of IEM (37-fold). Relative mortality was highest in the 2- to 5-year period after allogeneic HCT (78-fold), and declined dramatically in the 6- to 10-year period (8-fold). Relative mortality remained elevated (2-fold above that of the general population) in patients followed for more than 15 years after HCT.
Standardized mortality ratio (SMR) among 2-year survivors of allogeneic HCT
| Variables . | Entire cohort . | Standard risk of relapse . | High risk of relapse . | |||
|---|---|---|---|---|---|---|
| No. of deaths . | SMR (95% CI) . | No. of deaths . | SMR (95% CI) . | No. of deaths . | SMR (95% CI) . | |
| All patients | 241 | 9.9 (8.7-11.2) | 152 | 8.4 (7.1-9.8) | 88 | 14.5 (11.7-17.7) |
| Sex | ||||||
| Male | 147 | 8.2 (6.9-9.6) | 97 | 7.4 (6.0-8.9) | 50 | 10.8 (8.0-14.0) |
| Female | 94 | 12.8 (10.3-15.5) | 55 | 9.9 (7.4-12.6) | 38 | 21.5 (15.2-28.9) |
| Age at transplantation, y | ||||||
| Younger than 18 | 63 | 16.5 (12.7-20.8) | 48 | 14.6 (10.8-19.0) | 15 | 28.8 (16.1-45.3) |
| 18 to 45 | 150 | 9.1 (7.7-10.6) | 92 | 7.7 (6.2-9.3) | 57 | 12.9 (9.8-16.5) |
| Older than 45 | 28 | 7.1 (4.7-9.9) | 12 | 4.2 (2.2-7.0) | 16 | 14.2 (8.1-22.0) |
| Year of transplantation | ||||||
| 1974 to 1979 | 15 | 9.4 (5.2-14.7) | 13 | 9.4 (5.0-15.3) | 2 | 13.4 (1.3-38.5) |
| 1980 to 1984 | 49 | 7.9 (5.8-10.2) | 34 | 7.4 (5.1-10.1) | 15 | 9.2 (5.1-14.5) |
| 1985 to 1989 | 63 | 10.5 (8.1-13.3) | 39 | 8.9 (6.3-11.9) | 24 | 15.0 (9.6-21.6) |
| 1990 to 1994 | 71 | 11.2 (8.7-13.9) | 49 | 10.2 (7.5-13.2) | 21 | 13.8 (8.5-20.3) |
| 1995 to 1998 | 43 | 10.5 (7.6-13.8) | 17 | 5.8 (3.4-8.9) | 26 | 22.5 (14.7-31.9) |
| Survival after diagnosis | ||||||
| 2 to 5 y | 149 | 77.5 (65.6-90.5) | 92 | 73.5 (59.2-89.3) | 57 | 85.0 (64.4-108.5) |
| 6 to 10 y | 50 | 8.3 (6.1-10.7) | 29 | 6.3 (4.2-8.8) | 20 | 13.9 (8.5-20.7) |
| 11 to 15 y | 21 | 3.7 (2.3-5.4) | 15 | 3.7 (2.0-5.7) | 6 | 3.6 (1.3-7.2) |
| 16 to 20 y | 20 | 2.9 (1.8-4.3) | 15 | 3.0 (1.7-4.8) | 5 | 2.5 (0.8-5.2) |
| 21+ y | 1 | 0.3 (0.0001-1.1) | 1 | 0.3 (0.0001-1.2) | 0 | — |
| Chronic graft-versus-host disease | ||||||
| Yes | 158 | 12.4 (10.5-14.4) | 95 | 10.6 (8.6-12.9) | 62 | 16.8 (12.8-21.2) |
| No | 78 | 6.9 (5.4-8.5) | 53 | 5.9 (4.4-7.6) | 25 | 10.6 (6.9-15.2) |
| Primary diagnosis | ||||||
| AML | 82 | 10.8 (8.6-13.3) | 46 | 8.5 (6.2-11.1) | 36 | 17.3 (12.1-23.5) |
| CML | 74 | 8.2 (6.4-10.2) | 41 | 6.8 (4.9-9.1) | 33 | 11.0 (7.6-15.0) |
| ALL | 46 | 12.8 (9.4-16.8) | 32 | 10.9 (7.5-15.1) | 14 | 21.0 (11.5-33.5) |
| NHL | 10 | 12.5 (6.0-21.5) | 4 | 8.5 (2.2-18.9) | 5 | 16.1 (5.1-33.4) |
| SAA | 17 | 5.7 (3.3-8.8) | 17 | 5.7 (3.3-8.8) | 0 | — |
| IEM | 12 | 36.7 (18.9-60.4) | 12 | 36.7 (18.9-60.4) | 0 | — |
| Variables . | Entire cohort . | Standard risk of relapse . | High risk of relapse . | |||
|---|---|---|---|---|---|---|
| No. of deaths . | SMR (95% CI) . | No. of deaths . | SMR (95% CI) . | No. of deaths . | SMR (95% CI) . | |
| All patients | 241 | 9.9 (8.7-11.2) | 152 | 8.4 (7.1-9.8) | 88 | 14.5 (11.7-17.7) |
| Sex | ||||||
| Male | 147 | 8.2 (6.9-9.6) | 97 | 7.4 (6.0-8.9) | 50 | 10.8 (8.0-14.0) |
| Female | 94 | 12.8 (10.3-15.5) | 55 | 9.9 (7.4-12.6) | 38 | 21.5 (15.2-28.9) |
| Age at transplantation, y | ||||||
| Younger than 18 | 63 | 16.5 (12.7-20.8) | 48 | 14.6 (10.8-19.0) | 15 | 28.8 (16.1-45.3) |
| 18 to 45 | 150 | 9.1 (7.7-10.6) | 92 | 7.7 (6.2-9.3) | 57 | 12.9 (9.8-16.5) |
| Older than 45 | 28 | 7.1 (4.7-9.9) | 12 | 4.2 (2.2-7.0) | 16 | 14.2 (8.1-22.0) |
| Year of transplantation | ||||||
| 1974 to 1979 | 15 | 9.4 (5.2-14.7) | 13 | 9.4 (5.0-15.3) | 2 | 13.4 (1.3-38.5) |
| 1980 to 1984 | 49 | 7.9 (5.8-10.2) | 34 | 7.4 (5.1-10.1) | 15 | 9.2 (5.1-14.5) |
| 1985 to 1989 | 63 | 10.5 (8.1-13.3) | 39 | 8.9 (6.3-11.9) | 24 | 15.0 (9.6-21.6) |
| 1990 to 1994 | 71 | 11.2 (8.7-13.9) | 49 | 10.2 (7.5-13.2) | 21 | 13.8 (8.5-20.3) |
| 1995 to 1998 | 43 | 10.5 (7.6-13.8) | 17 | 5.8 (3.4-8.9) | 26 | 22.5 (14.7-31.9) |
| Survival after diagnosis | ||||||
| 2 to 5 y | 149 | 77.5 (65.6-90.5) | 92 | 73.5 (59.2-89.3) | 57 | 85.0 (64.4-108.5) |
| 6 to 10 y | 50 | 8.3 (6.1-10.7) | 29 | 6.3 (4.2-8.8) | 20 | 13.9 (8.5-20.7) |
| 11 to 15 y | 21 | 3.7 (2.3-5.4) | 15 | 3.7 (2.0-5.7) | 6 | 3.6 (1.3-7.2) |
| 16 to 20 y | 20 | 2.9 (1.8-4.3) | 15 | 3.0 (1.7-4.8) | 5 | 2.5 (0.8-5.2) |
| 21+ y | 1 | 0.3 (0.0001-1.1) | 1 | 0.3 (0.0001-1.2) | 0 | — |
| Chronic graft-versus-host disease | ||||||
| Yes | 158 | 12.4 (10.5-14.4) | 95 | 10.6 (8.6-12.9) | 62 | 16.8 (12.8-21.2) |
| No | 78 | 6.9 (5.4-8.5) | 53 | 5.9 (4.4-7.6) | 25 | 10.6 (6.9-15.2) |
| Primary diagnosis | ||||||
| AML | 82 | 10.8 (8.6-13.3) | 46 | 8.5 (6.2-11.1) | 36 | 17.3 (12.1-23.5) |
| CML | 74 | 8.2 (6.4-10.2) | 41 | 6.8 (4.9-9.1) | 33 | 11.0 (7.6-15.0) |
| ALL | 46 | 12.8 (9.4-16.8) | 32 | 10.9 (7.5-15.1) | 14 | 21.0 (11.5-33.5) |
| NHL | 10 | 12.5 (6.0-21.5) | 4 | 8.5 (2.2-18.9) | 5 | 16.1 (5.1-33.4) |
| SAA | 17 | 5.7 (3.3-8.8) | 17 | 5.7 (3.3-8.8) | 0 | — |
| IEM | 12 | 36.7 (18.9-60.4) | 12 | 36.7 (18.9-60.4) | 0 | — |
SMR indicates standardized mortality ratio; 95% CI, 95% confidence interval; and —, not applicable.
One subject with unknown disease status at the time of HCT was excluded from the analysis.
One subject with unknown cGVHD status was excluded from the analysis.
Specific causes of death
Two hundred forty-one deaths were observed in patients who had survived disease free for 2 years after HCT. Relapse of primary disease was the leading cause of death, with 70 deaths (29% of all deaths) attributed to recurrence of the primary disease. Forty-seven (67%) deaths due to primary disease occurred between the second and fifth year of follow-up; 19 (27%) occurred in the next 5 years, while only 4 deaths (6%) due to primary disease occurred after 10 years from HCT. cGVHD was the second most common cause of late mortality in this cohort, accounting for 53 deaths (22%), while late infection in the absence of cGVHD was the cause of 27 (11%) deaths. Late mortality was attributed to treatment-related causes in 61 patients (25%), including 17 (7%) due to subsequent malignancy, 12 (5%) due to pulmonary complications, 8 (3%) due to cardiac toxicity, and 17 (8.4%) due to other treatment-related sequelae. External causes accounted for late mortality in 7 patients (3%). The cause of death remained undetermined for 23 patients.
Relative to the US population, individuals in this cohort were 3.6 times more likely to have died of a subsequent new malignancy (95% CI, 2.1-5.5). Of the 17 deaths due to subsequent malignancies, 14 were due to solid nonhematopoietic malignancies, and 1 each was due to therapy-related leukemia, non-Hodgkin lymphoma, and a second malignancy of unknown etiology. The cohort was at a 15.1-fold increased risk of late deaths due to pulmonary dysfunction compared with the general population (95% CI, 7.8-24.9). Pulmonary dysfunction leading to death included interstitial pneumonitis (n = 7, 58%), pulmonary fibrosis (n = 3, 25%), and other lung pathology (n = 2, 17%). The risk of premature death due to cardiac complications was increased 2.3-fold compared with the general population (95% CI, 0.96-4.1).
Multiple regression analysis for late mortality
Table 4 summarizes the results from the multiple regression analysis for late mortality from any cause, as well as risk of late mortality related to relapse or nonrelapse-related causes (such as cGVHD, vital organ dysfunction, subsequent malignancies, etc).
Relative risk of late mortality among 2-year survivors of allogeneic hematopoietic cell transplantation
| Variables . | Death from any cause . | Relapse-related mortality . | Nonrelapse-related mortality . | |||
|---|---|---|---|---|---|---|
| Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | |
| Age at transplantation, y | ||||||
| Younger than 18 | 1.0 | — | 1.0 | — | 1.0 | — |
| 18 to 45 y | 1.31 (0.91-1.89) | .1 | 0.89 (0.49-1.64) | .7 | 1.72 (1.08-2.73) | .02 |
| Older than 45 y | 2.56 (1.51-4.35) | .001 | 1.43 (0.48-4.24) | .5 | 3.71 (1.97-6.98) | <.001 |
| Year of transplantation | ||||||
| 1974 to 1979 | 1.0 | — | 1.0 | — | 1.0 | — |
| 1980 to 1984 | 0.70 (0.35-1.39) | .3 | 0.33 (0.11-0.98) | .05 | 0.90 (0.37-2.23) | .8 |
| 1985 to 1989 | 0.67 (0.32-1.41) | .3 | 0.25 (0.08-0.81) | .02 | 0.95 (0.36-2.51) | .9 |
| 1990 to 1994 | 0.71 (0.33-1.55) | .4 | 0.31 (0.09-1.07) | .06 | 0.98 (0.36-2.71) | .999 |
| 1995 to 1998 | 0.60 (0.26-1.38) | .2 | 0.22 (0.06-0.88) | .03 | 0.87 (0.30-2.54) | .8 |
| Sex | ||||||
| Male | 1.0 | — | 1.0 | — | 1.0 | — |
| Female | 0.91 (0.70-1.20) | .5 | 0.97 (0.58-1.63) | .9 | 0.89 (0.64-1.22) | .5 |
| Donor type | ||||||
| Sibling | 1.0 | — | 1.0 | — | 1.0 | — |
| Unrelated | 1.39 (0.89-2.17) | .1 | 1.09 (0.47-2.51) | .8 | 1.60 (0.94-2.71) | .08 |
| Risk of relapse at HCT | ||||||
| Standard risk | 1.0 | — | 1.0 | — | 1.0 | — |
| High risk | 1.69 (1.25-2.27) | .001 | 3.69 (2.18-6.23) | <.001 | 1.23 (0.85-1.78) | .3 |
| Race | ||||||
| White | 1.0 | — | 1.0 | — | 1.0 | — |
| Hispanic | 0.77 (0.51-1.15) | .2 | 0.84 (0.37-1.88) | .7 | 0.71 (0.44-1.12) | .1 |
| Other | 1.10 (0.68-1.77) | .7 | 2.23 (1.07-4.65) | .03 | 0.77 (0.41-1.44) | .4 |
| CGVHD | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 2.26 (1.67-3.06) | <.001 | 1.70 (0.96-2.99) | .1 | 2.68 (1.85-3.87) | <.001 |
| Diagnosis | ||||||
| CML | 1.0 | — | 1.0 | — | 1.0 | — |
| AML | 1.18 (0.83-1.67) | .4 | 0.79 (0.40-1.55) | .5 | 1.45 (0.96-2.19) | .08 |
| NHL | 1.22 (0.59-2.51) | .6 | 0.59 (0.11-3.07) | .5 | 1.47 (0.65-3.34) | .4 |
| SAA | 0.45 (0.17-1.18) | .1 | — | — | 0.79 (0.28-2.25) | .7 |
| ALL | 1.16 (0.76-1.76) | .5 | 1.34 (0.66-2.73) | .4 | 1.03 (0.60-1.75) | .9 |
| IEM | 1.56 (0.67-3.62) | .3 | 1.21 (0.24-6.03) | .8 | 1.83 (0.67-4.98) | .2 |
| Conditioning | ||||||
| Cyclophosphamide | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 1.10 (0.70-1.72) | .7 | 0.79 (0.35-1.78) | .6 | 1.16 (0.67-2.02) | .6 |
| TBI | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.62 (0.27-1.44) | .3 | 0.16 (0.01-3.03) | .2 | 0.66 (0.26-1.68) | .4 |
| Etoposide | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 1.27 (0.77-2.08) | .3 | 0.77 (0.31-1.91) | .6 | 1.50 (0.83-2.73) | .2 |
| Busulfan | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.66 (0.28-1.55) | .3 | 0.14 (0.01-2.68) | .2 | 0.84 (0.33-2.16) | .7 |
| Cytarabine | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 1.29 (0.71-2.34) | .4 | 0.99 (0.36-2.74) | .999 | 1.44 (0.69-3.02) | .3 |
| Graft-versus-host disease prophylaxis | ||||||
| Methotrexate | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.55 (0.38-0.81) | .003 | 0.59 (0.27-1.30) | .2 | 0.52 (0.33-0.82) | .005 |
| CSA | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.68 (0.45-1.04) | .1 | 0.58 (0.27-1.25) | .2 | 0.70 (0.43-1.16) | .2 |
| Prednisone | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 1.07 (0.79-1.45) | .7 | 0.81 (0.46-1.41) | .4 | 1.17 (0.81-1.69) | .4 |
| T-cell depletion | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.73 (0.37-1.44) | .4 | 1.16 (0.37-3.71) | .8 | 0.55 (0.23-1.33) | .2 |
| Variables . | Death from any cause . | Relapse-related mortality . | Nonrelapse-related mortality . | |||
|---|---|---|---|---|---|---|
| Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | Relative risk (95% CI) . | P . | |
| Age at transplantation, y | ||||||
| Younger than 18 | 1.0 | — | 1.0 | — | 1.0 | — |
| 18 to 45 y | 1.31 (0.91-1.89) | .1 | 0.89 (0.49-1.64) | .7 | 1.72 (1.08-2.73) | .02 |
| Older than 45 y | 2.56 (1.51-4.35) | .001 | 1.43 (0.48-4.24) | .5 | 3.71 (1.97-6.98) | <.001 |
| Year of transplantation | ||||||
| 1974 to 1979 | 1.0 | — | 1.0 | — | 1.0 | — |
| 1980 to 1984 | 0.70 (0.35-1.39) | .3 | 0.33 (0.11-0.98) | .05 | 0.90 (0.37-2.23) | .8 |
| 1985 to 1989 | 0.67 (0.32-1.41) | .3 | 0.25 (0.08-0.81) | .02 | 0.95 (0.36-2.51) | .9 |
| 1990 to 1994 | 0.71 (0.33-1.55) | .4 | 0.31 (0.09-1.07) | .06 | 0.98 (0.36-2.71) | .999 |
| 1995 to 1998 | 0.60 (0.26-1.38) | .2 | 0.22 (0.06-0.88) | .03 | 0.87 (0.30-2.54) | .8 |
| Sex | ||||||
| Male | 1.0 | — | 1.0 | — | 1.0 | — |
| Female | 0.91 (0.70-1.20) | .5 | 0.97 (0.58-1.63) | .9 | 0.89 (0.64-1.22) | .5 |
| Donor type | ||||||
| Sibling | 1.0 | — | 1.0 | — | 1.0 | — |
| Unrelated | 1.39 (0.89-2.17) | .1 | 1.09 (0.47-2.51) | .8 | 1.60 (0.94-2.71) | .08 |
| Risk of relapse at HCT | ||||||
| Standard risk | 1.0 | — | 1.0 | — | 1.0 | — |
| High risk | 1.69 (1.25-2.27) | .001 | 3.69 (2.18-6.23) | <.001 | 1.23 (0.85-1.78) | .3 |
| Race | ||||||
| White | 1.0 | — | 1.0 | — | 1.0 | — |
| Hispanic | 0.77 (0.51-1.15) | .2 | 0.84 (0.37-1.88) | .7 | 0.71 (0.44-1.12) | .1 |
| Other | 1.10 (0.68-1.77) | .7 | 2.23 (1.07-4.65) | .03 | 0.77 (0.41-1.44) | .4 |
| CGVHD | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 2.26 (1.67-3.06) | <.001 | 1.70 (0.96-2.99) | .1 | 2.68 (1.85-3.87) | <.001 |
| Diagnosis | ||||||
| CML | 1.0 | — | 1.0 | — | 1.0 | — |
| AML | 1.18 (0.83-1.67) | .4 | 0.79 (0.40-1.55) | .5 | 1.45 (0.96-2.19) | .08 |
| NHL | 1.22 (0.59-2.51) | .6 | 0.59 (0.11-3.07) | .5 | 1.47 (0.65-3.34) | .4 |
| SAA | 0.45 (0.17-1.18) | .1 | — | — | 0.79 (0.28-2.25) | .7 |
| ALL | 1.16 (0.76-1.76) | .5 | 1.34 (0.66-2.73) | .4 | 1.03 (0.60-1.75) | .9 |
| IEM | 1.56 (0.67-3.62) | .3 | 1.21 (0.24-6.03) | .8 | 1.83 (0.67-4.98) | .2 |
| Conditioning | ||||||
| Cyclophosphamide | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 1.10 (0.70-1.72) | .7 | 0.79 (0.35-1.78) | .6 | 1.16 (0.67-2.02) | .6 |
| TBI | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.62 (0.27-1.44) | .3 | 0.16 (0.01-3.03) | .2 | 0.66 (0.26-1.68) | .4 |
| Etoposide | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 1.27 (0.77-2.08) | .3 | 0.77 (0.31-1.91) | .6 | 1.50 (0.83-2.73) | .2 |
| Busulfan | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.66 (0.28-1.55) | .3 | 0.14 (0.01-2.68) | .2 | 0.84 (0.33-2.16) | .7 |
| Cytarabine | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 1.29 (0.71-2.34) | .4 | 0.99 (0.36-2.74) | .999 | 1.44 (0.69-3.02) | .3 |
| Graft-versus-host disease prophylaxis | ||||||
| Methotrexate | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.55 (0.38-0.81) | .003 | 0.59 (0.27-1.30) | .2 | 0.52 (0.33-0.82) | .005 |
| CSA | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.68 (0.45-1.04) | .1 | 0.58 (0.27-1.25) | .2 | 0.70 (0.43-1.16) | .2 |
| Prednisone | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 1.07 (0.79-1.45) | .7 | 0.81 (0.46-1.41) | .4 | 1.17 (0.81-1.69) | .4 |
| T-cell depletion | ||||||
| No | 1.0 | — | 1.0 | — | 1.0 | — |
| Yes | 0.73 (0.37-1.44) | .4 | 1.16 (0.37-3.71) | .8 | 0.55 (0.23-1.33) | .2 |
— indicates not applicable.
The risk of late mortality due to any cause was 2.6-fold increased among individuals older than 45 years at the time of HCT, compared with those who were younger than 18 years; 2.3-fold increased among patients with cGVHD compared with those without cGVHD; and 1.7-fold increased among patients at high risk of relapse at the time of HCT, compared with those at standard risk. On the other hand, the risk of late mortality was significantly reduced among patients who received methotrexate as part of their GVHD prophylaxis regimens compared with those who did not.
Nonrelapse-related late mortality was higher among individuals who were older than 18 years at HCT (18 to 45 years: 1.7-fold; greater than 45 years: 3.7-fold), compared with patients who were younger, anded with patients who were younger, and among patients with cGVHD (2.7-fold increased risk) compared with those without. Finally, the risk of nonrelapse-related mortality was reduced by half among patients who received methotrexate as part of GVHD prophylaxis regimen compared with those who did not.
Relapse-related mortality was 3.7-fold higher in patients at high risk of relapse at HCT, compared with those at standard risk. Patients undergoing HCT after 1980 were less likely to suffer from relapse-related mortality, compared with those who underwent transplantation before 1980.
Multivariate analyses within the primary diagnostic categories of patients disease-free at entry into the cohort (2 years after HCT) are summarized in Table 5.
Multivariate analysis of risk factors associated with late mortality by primary diagnosis
| Risk factors . | Relative risk . | 95% confidence interval . |
|---|---|---|
| Severe aplastic anemia (all-cause late mortality) | ||
| cGVHD | 4.0 | 1.1-14.1 |
| Females | 0.2 | 0.1-0.8 |
| Methotrexate for GVHD prophylaxis | 0.1 | 0.01-0.6 |
| Acute lymphoblastic leukemia | ||
| Relapse-related late mortality (high risk of relapse) | 3.4 | 1.2-9.8 |
| Nonrelapse-related late mortality | ||
| Methotrexate for GVHD prophylaxis | 0.2 | 0.04-0.6 |
| Cyclosporine for GVHD prophylaxis | 0.2 | 0.03-0.9 |
| Acute myeloid leukemia | ||
| Relapse-related late mortality | ||
| High risk of relapse | 7.8 | 1.7-35.0 |
| cGVHD | 5.8 | 1.7-2.0 |
| Nonrelapse-related late mortality | ||
| 18 to 45 y at HCT | 2.7 | 1.2-5.9 |
| Older than 45 y at HCT | 3.2 | 1.2-8.9 |
| cGVHD | 3.4 | 1.8-6.3 |
| Chronic myeloid leukemia | ||
| Relapse-related late mortality | ||
| 18 to 45 y at HCT | 5.1 | 1-26.1 |
| Older than 45 y at HCT | 15.4 | 1.6-144.8 |
| High risk of relapse | 3.3 | 1.2-9.1 |
| Nonrelapse-related late mortality (cGVHD) | 2.4 | 1.1-5.1 |
| Risk factors . | Relative risk . | 95% confidence interval . |
|---|---|---|
| Severe aplastic anemia (all-cause late mortality) | ||
| cGVHD | 4.0 | 1.1-14.1 |
| Females | 0.2 | 0.1-0.8 |
| Methotrexate for GVHD prophylaxis | 0.1 | 0.01-0.6 |
| Acute lymphoblastic leukemia | ||
| Relapse-related late mortality (high risk of relapse) | 3.4 | 1.2-9.8 |
| Nonrelapse-related late mortality | ||
| Methotrexate for GVHD prophylaxis | 0.2 | 0.04-0.6 |
| Cyclosporine for GVHD prophylaxis | 0.2 | 0.03-0.9 |
| Acute myeloid leukemia | ||
| Relapse-related late mortality | ||
| High risk of relapse | 7.8 | 1.7-35.0 |
| cGVHD | 5.8 | 1.7-2.0 |
| Nonrelapse-related late mortality | ||
| 18 to 45 y at HCT | 2.7 | 1.2-5.9 |
| Older than 45 y at HCT | 3.2 | 1.2-8.9 |
| cGVHD | 3.4 | 1.8-6.3 |
| Chronic myeloid leukemia | ||
| Relapse-related late mortality | ||
| 18 to 45 y at HCT | 5.1 | 1-26.1 |
| Older than 45 y at HCT | 15.4 | 1.6-144.8 |
| High risk of relapse | 3.3 | 1.2-9.1 |
| Nonrelapse-related late mortality (cGVHD) | 2.4 | 1.1-5.1 |
Marital, employment, and health/life insurance status
The following aspects of the survivors' functional status were examined: marital status, health problems preventing the survivors from holding a job, difficulty in obtaining or retaining health insurance, and difficulty in obtaining or retaining life insurance. The HCT survivors were compared with a sibling cohort to describe the magnitude of problem. Furthermore, sociodemographic and clinical factors were identified among the HCT survivors that would define the group at the highest risk of vulnerability in terms of these 4 variables.
Of the 926 patients in this cohort who were alive and disease-free at 2 years after HCT, and 18 years or older at the time of study participation, 547 (59%) responded to the BMTSS questionnaire. Two hundred fifty-nine patients (28%) declined to participate; and current addresses and telephone numbers were not available for 115 (12%) patients. Thus, 67% of the individuals who were successfully contacted participated in the study. The participants did not differ significantly from the nonparticipants by race/ethnicity, risk of relapse at time of transplantation, or cGVHD status. Participants were more likely to be females (46% vs 39%, P = .05); and older at HCT (30.2 years vs 23.7 years, P < .001) and at study participation (41.5 years vs 36.3 years, P < .001). The median time from transplantation was shorter for participants than nonparticipants (8.6 vs 11.3 years, P < .001). Patients with CML were overrepresented among the participants (38% vs 29%), while those with SAA (10% vs 19%) were underrepresented. Finally, patients who received TBI (85% vs 75%, P < .001) or cyclophosphamide (68% vs 61%, P = .04) were more likely to participate in this study. Three hundred nineteen siblings also completed the BMTSS questionnaire and served as the comparison group. One hundred sixteen (36%) of the siblings were male, and their median age at participation was 44 years (range, 19-79 years). Table 6 summarizes a comparison of the functional well-being between survivors and siblings, and Table 7 describes the factors associated with impaired functional well-being in the cohort of allogeneic HCT survivors.
Comparison of functional well-being between survivors and siblings
| Functional well-being . | Survivors, no. (%) . | Siblings, no. (%) . | P . |
|---|---|---|---|
| Currently married* | |||
| No | 241 (45) | 97 (31) | — |
| Yes | 300 (55) | 220 (69) | <.001 |
| Difficulty holding a job† | |||
| No | 435 (81) | 311 (98) | — |
| Yes | 103 (19) | 7 (2) | <.001 |
| Difficulty obtaining health insurance‡ | |||
| No | 392 (74) | 299 (95) | — |
| Yes | 135 (26) | 15 (5) | <.001 |
| Currently have health insurance§ | |||
| No | 55 (10) | 16 (5) | — |
| Yes | 478 (90) | 299 (95) | .01 |
| Difficulty obtaining life insurance‖ | |||
| No | 190 (36) | 266 (84) | — |
| Yes | 156 (30) | 16 (5) | — |
| Never tried | 183 (34) | 35 (11) | <.001 |
| Currently have life insurance¶ | |||
| No | 232 (44) | 77 (24) | — |
| Yes | 296 (56) | 240 (76) | d<.001 |
| Functional well-being . | Survivors, no. (%) . | Siblings, no. (%) . | P . |
|---|---|---|---|
| Currently married* | |||
| No | 241 (45) | 97 (31) | — |
| Yes | 300 (55) | 220 (69) | <.001 |
| Difficulty holding a job† | |||
| No | 435 (81) | 311 (98) | — |
| Yes | 103 (19) | 7 (2) | <.001 |
| Difficulty obtaining health insurance‡ | |||
| No | 392 (74) | 299 (95) | — |
| Yes | 135 (26) | 15 (5) | <.001 |
| Currently have health insurance§ | |||
| No | 55 (10) | 16 (5) | — |
| Yes | 478 (90) | 299 (95) | .01 |
| Difficulty obtaining life insurance‖ | |||
| No | 190 (36) | 266 (84) | — |
| Yes | 156 (30) | 16 (5) | — |
| Never tried | 183 (34) | 35 (11) | <.001 |
| Currently have life insurance¶ | |||
| No | 232 (44) | 77 (24) | — |
| Yes | 296 (56) | 240 (76) | d<.001 |
— indicates not applicable.
Six survivors and 2 siblings with missing data were excluded.
Nine survivors and 1 sibling with missing data were excluded.
Twenty survivors and 5 siblings with missing data were excluded.
Fourteen survivors and 4 siblings with missing data were excluded.
Eighteen survivors and two siblings with missing data were excluded.
Nineteen survivors and 2 siblings with missing data were excluded.
Effects of demographic/treatment factors on survivors' functional well-being
| Risk factors . | Currently unmarried . | Difficulty holding job . | Difficulty obtaining health insurance . | Lack of health insurance . | Difficulty obtaining life insurance . | Lack of life insurance . |
|---|---|---|---|---|---|---|
| Age at hematopoietic cell transplantation, y | ||||||
| Younger than 18 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 18 to 45 | 0.16 (0.09-0.28) | 0.96 (0.46-2.00) | 1.25 (0.68-2.31) | 1.88 (0.70-5.04) | 2.83 (1.43-5.61) | 0.63 (0.36-1.10) |
| Older than 45 | 0.10 (0.04-0.22) | 1.81 (0.70-4.66) | 1.14 (0.45-2.90) | 0.87 (0.14-5.21) | 2.23 (0.88-5.68) | 0.43 (0.18-0.99) |
| Sex | ||||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 1.13 (0.78-1.65) | 1.69 (1.06-2.71) | 0.83 (0.53-1.28) | 0.44 (0.22-0.90) | 0.72 (0.47-1.10) | 0.95 (0.64-1.43) |
| Race | ||||||
| White | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Hispanic | 1.42 (0.88-2.30) | 1.77 (0.99-3.16) | 0.63 (0.33-1.20) | 1.93 (0.87-4.29) | 0.86 (0.47-1.58) | 1.73 (0.98-3.03) |
| Other | 1.41 (0.73-2.73) | 0.98 (0.42-2.32) | 0.81 (0.36-1.79) | 1.66 (0.55-5.04) | 0.84 (0.38-1.86) | 1.72 (0.84-3.52) |
| Education | ||||||
| Less than HS | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| HS graduate/some college | 0.96 (0.58-1.57) | 0.92 (0.51-1.68) | 1.47 (0.81-2.67) | 0.92 (0.39-2.21) | 1.23 (0.69-2.20) | 0.94 (0.55-1.60) |
| College graduate | 0.96 (0.59-1.57) | 0.77 (0.42-1.41) | 1.16 (0.65-2.08) | 0.83 (0.36-1.88) | 1.38 (0.79-2.41) | 0.95 (0.57-1.60) |
| Household income | ||||||
| More than $60 000 | — | — | 1.00 | 1.00 | 1.00 | 1.00 |
| $20 000 to $60 000 | — | — | 1.10 (0.67-1.81) | 6.98 (2.29-21.33) | 1.21 (0.76-1.92) | 1.78 (1.14-2.78) |
| Less than $20 000 | — | — | 2.65 (1.40-5.00) | 12.58 (3.67-43.09) | 0.72 (0.37-1.40) | 6.21 (3.31-11.67) |
| Primary diagnosis | ||||||
| CML | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| AML | 1.04 (0.66-1.65) | 0.61 (0.34-1.10) | 1.08 (0.63-1.86) | 1.40 (0.59-3.30) | 0.77 (0.46-1.27) | 2.15 (1.31-3.53) |
| NHL | 0.43 (0.14-1.29) | 0.51 (0.13-1.97) | 3.70 (1.25-10.92) | 1.76 (0.19-16.52) | 1.87 (0.65-5.38) | 0.80 (0.23-2.76) |
| ALL | 1.54 (0.87-2.72) | 0.96 (0.47-1.97) | 1.32 (0.69-2.51) | 1.14 (0.43-3.08) | 0.56 (0.29-1.10) | 1.46 (0.78-2.70) |
| AA/IEM | 1.04 (0.53-2.03) | 1.02 (0.45-2.30) | 0.94 (0.42-2.08) | 2.79 (1.00-7.78) | 0.48 (0.22-1.07) | 1.16 (0.57-2.37) |
| Chronic graft-versus-host disease | ||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.19 (0.80-1.76) | 3.21 (1.91-5.42) | 1.12 (0.71-1.76) | 1.01 (0.51-2.02) | 1.23 (0.80-1.91) | 0.72 (0.47-1.09) |
| Follow-up time, y | ||||||
| 5 y or fewer | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6 to 10 | 0.71 (0.31-1.63) | 1.27 (0.49-3.33) | 1.26 (0.46-3.47) | 0.98 (0.21-4.52) | 1.60 (0.59-4.29) | 0.91 (0.37-2.24) |
| 11+ | 0.86 (0.27-2.73) | 0.69 (0.15-3.12) | 1.02 (0.26-4.00) | 2.46 (0.38-15.94) | 1.95 (0.50-7.66) | 0.81 (0.23-2.86) |
| Risk factors . | Currently unmarried . | Difficulty holding job . | Difficulty obtaining health insurance . | Lack of health insurance . | Difficulty obtaining life insurance . | Lack of life insurance . |
|---|---|---|---|---|---|---|
| Age at hematopoietic cell transplantation, y | ||||||
| Younger than 18 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 18 to 45 | 0.16 (0.09-0.28) | 0.96 (0.46-2.00) | 1.25 (0.68-2.31) | 1.88 (0.70-5.04) | 2.83 (1.43-5.61) | 0.63 (0.36-1.10) |
| Older than 45 | 0.10 (0.04-0.22) | 1.81 (0.70-4.66) | 1.14 (0.45-2.90) | 0.87 (0.14-5.21) | 2.23 (0.88-5.68) | 0.43 (0.18-0.99) |
| Sex | ||||||
| Male | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Female | 1.13 (0.78-1.65) | 1.69 (1.06-2.71) | 0.83 (0.53-1.28) | 0.44 (0.22-0.90) | 0.72 (0.47-1.10) | 0.95 (0.64-1.43) |
| Race | ||||||
| White | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Hispanic | 1.42 (0.88-2.30) | 1.77 (0.99-3.16) | 0.63 (0.33-1.20) | 1.93 (0.87-4.29) | 0.86 (0.47-1.58) | 1.73 (0.98-3.03) |
| Other | 1.41 (0.73-2.73) | 0.98 (0.42-2.32) | 0.81 (0.36-1.79) | 1.66 (0.55-5.04) | 0.84 (0.38-1.86) | 1.72 (0.84-3.52) |
| Education | ||||||
| Less than HS | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| HS graduate/some college | 0.96 (0.58-1.57) | 0.92 (0.51-1.68) | 1.47 (0.81-2.67) | 0.92 (0.39-2.21) | 1.23 (0.69-2.20) | 0.94 (0.55-1.60) |
| College graduate | 0.96 (0.59-1.57) | 0.77 (0.42-1.41) | 1.16 (0.65-2.08) | 0.83 (0.36-1.88) | 1.38 (0.79-2.41) | 0.95 (0.57-1.60) |
| Household income | ||||||
| More than $60 000 | — | — | 1.00 | 1.00 | 1.00 | 1.00 |
| $20 000 to $60 000 | — | — | 1.10 (0.67-1.81) | 6.98 (2.29-21.33) | 1.21 (0.76-1.92) | 1.78 (1.14-2.78) |
| Less than $20 000 | — | — | 2.65 (1.40-5.00) | 12.58 (3.67-43.09) | 0.72 (0.37-1.40) | 6.21 (3.31-11.67) |
| Primary diagnosis | ||||||
| CML | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| AML | 1.04 (0.66-1.65) | 0.61 (0.34-1.10) | 1.08 (0.63-1.86) | 1.40 (0.59-3.30) | 0.77 (0.46-1.27) | 2.15 (1.31-3.53) |
| NHL | 0.43 (0.14-1.29) | 0.51 (0.13-1.97) | 3.70 (1.25-10.92) | 1.76 (0.19-16.52) | 1.87 (0.65-5.38) | 0.80 (0.23-2.76) |
| ALL | 1.54 (0.87-2.72) | 0.96 (0.47-1.97) | 1.32 (0.69-2.51) | 1.14 (0.43-3.08) | 0.56 (0.29-1.10) | 1.46 (0.78-2.70) |
| AA/IEM | 1.04 (0.53-2.03) | 1.02 (0.45-2.30) | 0.94 (0.42-2.08) | 2.79 (1.00-7.78) | 0.48 (0.22-1.07) | 1.16 (0.57-2.37) |
| Chronic graft-versus-host disease | ||||||
| No | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| Yes | 1.19 (0.80-1.76) | 3.21 (1.91-5.42) | 1.12 (0.71-1.76) | 1.01 (0.51-2.02) | 1.23 (0.80-1.91) | 0.72 (0.47-1.09) |
| Follow-up time, y | ||||||
| 5 y or fewer | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 | 1.00 |
| 6 to 10 | 0.71 (0.31-1.63) | 1.27 (0.49-3.33) | 1.26 (0.46-3.47) | 0.98 (0.21-4.52) | 1.60 (0.59-4.29) | 0.91 (0.37-2.24) |
| 11+ | 0.86 (0.27-2.73) | 0.69 (0.15-3.12) | 1.02 (0.26-4.00) | 2.46 (0.38-15.94) | 1.95 (0.50-7.66) | 0.81 (0.23-2.86) |
Data are odds ratio (95% confidence interval).
— indicates not applicable.
Marital rate (proportion currently married) for the survivors was significantly lower compared with siblings (55% vs 69%, P < .001) (Table 6). Compared with those who underwent transplantation when younger than 18 years, survivors that underwent transplantation at an older age were more likely to be married (Table 7). HCT survivors were 14-fold more likely to report a health problem preventing them from holding a job compared with siblings (19% vs 2%, [Table 6]). Presence of cGVHD (OR = 3.2; 95% CI, 1.9-5.4), and female sex (OR-1.7; 95% CI, 1.1-2.7) were associated with difficulty in holding a job (Table 7).
HCT survivors were 7-fold more likely to report difficulty in obtaining or retaining health insurance (26% vs 5%, P < .001 [Table 6]), and low annual household income (< $20 000) was associated with difficulty in obtaining health insurance (OR = 2.7; 95% CI, 1.4-5.0). However, 90% of the survivors did report health insurance coverage at the time of study participation, although the comparable rate was higher in siblings (95%, P = .01). Factors associated with lack of current health insurance included male sex (OR = 2.3) and low annual household income (< $20 000: OR = 13; $20 000-$60 000: OR = 7.0; Table 7).
A significantly greater proportion of HCT survivors reported difficulty in obtaining or retaining life insurance compared with siblings (30% vs 5%, P < .001) and the survivors were 10-fold more likely to report difficulty in obtaining or retaining life health insurance (Table 6). Age at HCT older than 18 years (18 to 45 years: OR = 2.8; older than 45 years: OR = 2.2) was associated with difficulty in obtaining life insurance. Only 56% of the survivors had life insurance at study participation, compared with 76% of the siblings (P < .001). Younger age at HCT (< 18: OR = 2.3) and low annual household income (< $20 000: RR = 1.8; $20 000-$60 000: OR = 6.2) were factors independently associated with lack of current life insurance (Table 7).
Discussion
Our goal was to use the services offered by the NDI, and the SSDI12 to complement the patient medical records and provide a comprehensive description of the late mortality experience in patients undergoing allogeneic HCT. The NDI is a central computerized index of death record information submitted under contractual agreement to the National Center for Health Statistics by the state vital statistics offices. The Social Security Administration Death master file is a database containing death notices for enrollees in the US Social Security program. With this study, we were able to identify allogeneic HC transplant recipients who would be at increased risk of experiencing late mortality, thus setting the stage for targeted interventions.
This study demonstrates that patients with ALL, AML, CML, NHL, SAA, and IEM who survive disease-free for 2 years after allogeneic HCT have a conditional probability of surviving for 5 years after transplantation of 90%, and for surviving 15 years after HCT of 80%. These results are consistent with those from a large International Bone Marrow Transplant Registry (IBMTR) study of patients with ALL, AML, CML, and SAA who were disease-free 2 years after allogeneic HCT; this study revealed that the probability of surviving for 7 years after transplantation was 89%.3
The current study also demonstrates that the cohort was at a 10-fold increased risk of experiencing late mortality compared with the general population. Of note, the mortality rate continued to be significantly increased compared with the general population among those who had survived 15 years after allogeneic transplantation. High relative mortality was observed in the youngest patients, and those with IEM. This probably could be a reflection of the low expected mortality in the age- and sex-matched general population.
In the current study, among patients who were disease-free 2 years after allogeneic HCT, 18% experienced late mortality. Twenty-nine percent of these deaths were due to relapse of the primary disease, accounting for the largest single cause of death in this population. Relapse-related mortality was significantly higher (3.7-fold) in patients at high risk of relapse at HCT, for the entire cohort, as well as in patients diagnosed with ALL (3.4-fold), AML (7.8-fold), or CML (3.3-fold), compared with those at standard risk of relapse.
As has been reported in earlier studies,3,13 cGVHD was the chief cause of nonrelapse-related death. The 10-year survival probability with and without cGVHD in this cohort was 79.8% vs 89.5% for CML, 73.2% vs 91.8% for AML, 80.9% vs 86.2% for ALL, 65.2% vs 80.0% for NHL, and 72.2% vs 90.5% for IEM. Furthermore, nonrelapse-related mortality was increased in patients with CML (2.4-fold) or AML (3.4-fold) with cGVHD compared with those without cGVHD. Use of methotrexate for GVHD prophylaxis was associated with a reduction in nonrelapse-related mortality, primarily due to a reduction in cGVHD. Methotrexate was one of the primary agents used for GVH prophylaxis during the period covered by the cohort (1974 to 1998). The association of methotrexate with a reduction in nonrelapse-related mortality therefore probably represents a surrogate indicator for effective GVH prophylaxis. Late infection in the absence of cGVHD accounted for 11% of premature deaths, again emphasizing the need for recognizing and treating infections effectively and aggressively in this population that is at risk for prolonged periods of immune suppression.
The incidence of subsequent cancer among survivors of transplantation is known to be significantly higher than that of the general population.14-20 The risk is related to exposure to radiation and chemotherapy; presence of GVHD; and use of antithymocyte globulin and cyclosporine for management of GVHD.14-18,20 The current study describes the mortality experience of allogeneic HC transplant recipients after being diagnosed with a subsequent cancer, and demonstrates that the risk was 3.5-fold increased, compared with the general population. The increased risk was seen primarily among patients with AML, CML, and ALL.
Chronic pulmonary complications affect at least 15% to 20% of patients after HCT, and pulmonary dysfunction is an important indicator for long-term outcome.21,22 The current cohort was at a 15-fold increased risk of late deaths due to pulmonary dysfunction compared with the general population, again emphasizing the need for preventive measures, such as limiting cumulative therapeutic exposure to agents with potential pulmonary toxicity, early and aggressive interventions in patients with pulmonary compromise, and caution against tobacco use in this vulnerable population.
An assessment of the long-term survival after allogeneic HCT must take into consideration the quality of the survival. Although there are several reports describing survival, few studies report the functional well-being and social reintegration of adult survivors of allogeneic HCT.4-7 Duell et al conducted a retrospective study on health and social reintegration in long-term survivors of allogeneic HCT, demonstrating that reduced clinical performance (as measured by Karnofsky score) is associated with cGVHD and relapse of primary disease.4 The study demonstrated that 89% of the survivors had returned to full-time work or school, and only 6% were unable to go to work or school. Factors associated with absence from work or school included older age, female sex, diagnosis of CML, presence of cGVHD, second cancer, and relapse of primary disease. In the current study, 19% of the survivors reported that health problems kept them from holding a job or attending school—a proportion that was significantly higher compared with sibling controls. Patients with cGVHD and female survivors were more likely to report difficulties in returning to work or school due to health problems.
Although previous investigations of the insurance coverage of survivors of childhood cancer found clear evidence of discrimination, particularly in employment-related health insurance,23-25 more recent studies of older survivors treated according to contemporary protocols indicate improved access to insurance and less economic discrimination,24,25 probably because of public awareness of the improved prognosis and because of legislation prohibiting discrimination in employment and promoting insurance portability. The current study aimed to identify the challenges faced by long-term survivors in reintegrating back into society after a high-intensity treatment regimen (ie, hematopoietic cell transplantation). The study describes that although the HCT survivors are significantly more likely to report difficulty in obtaining or retaining health insurance compared with siblings, the large majority (ie, 90%) does have health insurance. However, there is considerably greater difficulty in obtaining or retaining life insurance and only 56% of the HCT survivors had life insurance at study participation, while the comparable figure is 76% in siblings. The study also describes subpopulations that are most vulnerable in terms of difficulty in obtaining or retaining health or life insurance. However, the study did not evaluate in detail the challenges in obtaining or retaining health or life insurance faced by the survivors, and is therefore not geared to comment on the insurability of HCT survivors. On the other hand, this study aims to increase the awareness of the health care providers, policy makers, and third-party payers of the challenges faced by the long-term HCT survivors. It also sets the stage for further evaluation of the specific causes of lack of insurance in this population, as well as identification of high-risk populations that would benefit from interventions.
In conclusion, for several years after allogeneic transplantation, mortality rates in survivors remain higher than those expected in the general population, and the long-term survivors are at risk for disease- and treatment-related sequelae. This study extends the survival data, to describe specific aspects of functional well-being such as marital status, employment, and problems with health and life insurance experienced by long-term survivors of allogeneic HCT. To prevent and treat life-threatening late events, and to continue to address issues that impact the quality of survival, life-long follow-up of patients who undergo transplantation is recommended.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported in part by grants from the National Cancer Institute (R01 CA078938 and PO1 CA 30206 to S.B.), the Leukemia Lymphoma Society (2192 to S.B.), and the National Institutes of Health (K23 CA85503–01 to K.S.B.).
National Institutes of Health
Authorship
Contribution: S.B. designed the study, supervised and performed the research, analyzed the data, and wrote the paper; L.F. performed the research and analyzed data; A.C. performed the research and analyzed data; C-L.S. performed the analysis and helped write the paper; and K.S.B., J.G.G., P.B.M., A.N., M.O., N.K.C.R., L.L.R., D.S., A.S., S.J.F., and D.J.W. provided critical insight in the interpretation of data.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: S. Bhatia, City of Hope National Medical Center, 1500 East Duarte Rd, Duarte, CA 91010-3000; e-mail:sbhatia@coh.org.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal