Abstract
Chronic myelogenous leukemia (CML) arises from uncontrolled cell growth driven by a constitutively active BCR-ABL fusion protein tyrosine kinase, which is the product of the pathognomonic Philadelphia chromosomal translocation. Imatinib mesylate (Gleevec) is a BCR-ABL inhibitor used as a first line treatment of CML. Although imatinib is highly effective in chronic phase CML, in advanced disease patients frequently relapse due to the emergence of drug resistance. Approximately two-thirds of resistance is caused by point mutations in the BCR-ABL kinase domain, which give rise to active mutant forms of the enzyme that are insensitive to Gleevec. The T315I mutation represents one of the most common causes of resistance, is resistant to the second generation BCR-ABL inhibitors dasatinib and nilotinib, and represents an important and challenging target for discovery of next generation targeted CML treatments. We have applied X-ray crystallographic screening of our FAST™ fragment library and structure-guided hit-to-lead optimization to identify potent inhibitors of both wild-type and T315I mutant BCR-ABL. These efforts yielded a 7-azaindole compound series that exhibits binding to and inhibition of both wild-type and T315I BCR-ABL.
Methods: Wild-type (with Y393F) and T315I Abl kinase domain protein were expressed in E. coli and purified to homogeneity. These proteins were crystallized in the presence of a reference inhibitor followed by addition of the 7-azaindole series compounds soaked into the preformed crystals to displace the reference compound, giving the desired co-crystal. X-ray diffraction data were recorded at the company’s proprietary synchrotron beamline SGX-CAT at the Advanced Photon Source. Three-dimensional enzyme-inhibitor co-crystal structures were determined by molecular replacement and refined to permit modeling of bound ligand.
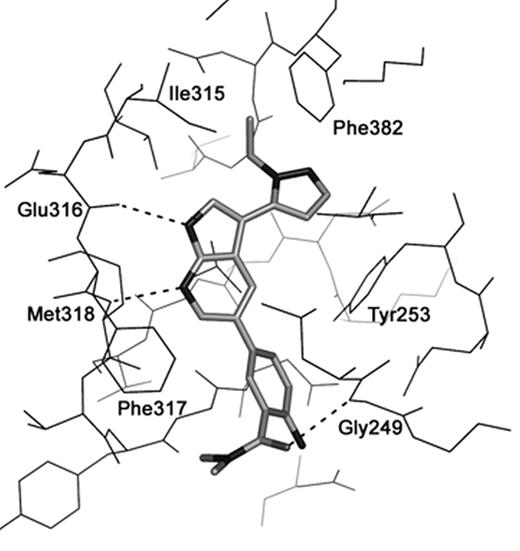
Results: Both wild-type and T315I Abl structures revealed enzyme in the active conformation with inhibitors bound to the kinase hinge region. The crystal structure of 2-amino-5-[3-(1-ethyl-1H-pyrazol-5-yl)-1H-pyrrolo[2,3-b]pyridin-5-yl]-N,N-dimethylbenzamide in complex with T315I, illustrates the typical binding mode which is independent of the 315 residue, and therefore accounts for the compound inhibiting the T315I mutant form of BCR-ABL (see figure). The inhibitor binds to the hinge region of ABL utilizing hydrogen bonding to backbone carbonyl of Glu316 and NH of Met318, with the pyrazole ring stacking in a lipophilic pocket between Phe382 and Tyr253. In addition, the benzamide carbonyl participates in a hydrogen bond interactioin with the backbone-NH of Glu249 of the p-loop.
Conclusions: X-ray crystallographic fragment screening and co-crystal structure studies have been successfully employed in discovery/optimization of 7-azaindole series compounds, yielding potent, selective inhibitors of both wild-type and imatinib-resistant forms of BCR-ABL.
Author notes
Disclosure:Employment: All of the authors are employed by SGX Pharmaceuticals. Ownership Interests:; The authors all own varying amounts of stock in the publically traded SGX Pharmaceuticals.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal