Abstract
NK cells play a major role in the activity of graft-versus-host (GVL) effect after an HLA-mismatched stem cell transplantation. In unrelated cord blood transplantation (CBT) where there is often an HLA mismatch between the donor and recipient, NK cells may also play a vital role, though their roles have not been extensively studied. Cord blood (CB) is known to have a unique subset of NK cells characterized by a CD16+CD56− phenotype. CD16+CD56− NK cells in CB are thought to be progenitors of CD16+CD56+ NK cells because CD16+CD56− NK cells acquires CD56 expression after in vitro culture in the presence of IL-2. However, the function of this immature NK cell subset after CBT remains unknown. A marked increase in the number of CD16+CD56- NK cells in the peripheral blood of an HLA-mismatched CBT recipient with acute myeloid leukemia (AML) was recently observed. A 56-year old male, who received a reduced intensity CBT following a full relapse after allogeneic stem cell transplantation from an HLA-matched sibling donor, showed an increase in the copy number of WT-1 mRNA in the peripheral blood around day 80 after the CBT, but the WT-1 copy number decreased from 1500/microliter RNA to 230/microliter RNA in association with the increase in the number of CD16+CD56- NK cells, and his molecular remission lasted more than 1.5 years thereafter. This case prompted an investigation of CD16+CD56− NK cells in the peripheral blood after allogeneic stem cell transplantation. A similar increase in the proportion of CD16+CD56− NK cells (20% or more) in the peripheral blood CD16+ NK cells was observed in 64% (7/11) of CBT recipients, all of whom maintained remission, but in none of the 11 bone marrow and 8 peripheral blood stem cell transplant recipients examined (Figure 1). CD16+CD56− NK cells in CBT recipients expressed receptors specific to NK cells such as NKp30 and NKp46 same level as CD16+CD56− NK cells of fresh CB cells. CD16+CD56− NK cells isolated from CBT recipients became CD56+ when they were cultured in the presence of IL-2 with or without K562-mb15-4-1BBL. When cultured NK cells derived from the CD16+CD56− NK cells were separated into CD158b+ and CD158b− cells, CD158b+ cells failed to kill 721–221 cells transfected with HLA-C*0301 while they killed untransfected or HLA-C*0401-transfected 221 cells. Despite the presence of the corresponding KIR ligand (C*0304), cultured CD16+CD56− NK cells showed cytotoxicity against the patient’s leukemic cells. These findings suggest that an increase in the proportion of CD16+CD56− NK cells is unique to recipients of CBT and that this immature NK-cell subset in CBT recipients may undergo differentiation into mature NK cells in vivo capable of killing residual leukemic cells, thereby contributing to the GVL effect regardless of the presence of the KIR ligand.
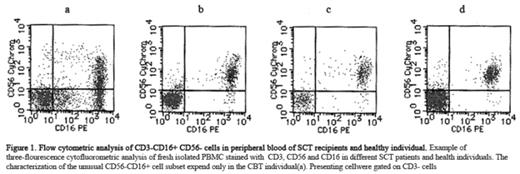
Flow cytometric analysis of CD3-CD16+CD56-cells in peripheral blood of SCT recipients and healthy individual.Examples of three-flourescence cytofluorometric analysis of fresh isolated PBMC stained with CD3,CD56 and CD16 in different SCT patients and health individuals. The characterization of the unusual CD56-CD16+ cell subset expend only in the CBT individual(a). Presenting cellware gated on CD3-cells
Flow cytometric analysis of CD3-CD16+CD56-cells in peripheral blood of SCT recipients and healthy individual.Examples of three-flourescence cytofluorometric analysis of fresh isolated PBMC stained with CD3,CD56 and CD16 in different SCT patients and health individuals. The characterization of the unusual CD56-CD16+ cell subset expend only in the CBT individual(a). Presenting cellware gated on CD3-cells
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal