Abstract
Introduction: An intact respiratory burst is critical to the microbicidal activity of neutrophils. The respiratory burst is initiated by assembly and activation of the NADPH oxidase complex. We recently identified a 29 kDa neutrophil protein which binds p67phox, is identified as peroxiredoxin VI by sequence and activity, translocates to the plasma membrane of neutrophils during activation and enhances superoxide anion (O2−) production in a cell-free assay exhibiting saturable kinetics. The activity of p29 peroxiredoxin (Prx) requires cysteine residues of the protein. The present studies demonstrate the requirement of p29 Prx for optimal oxidase activity in intact cells.
Methods: K562 cells stably transfected with p47phox, p60phox, gp91phox and the low affinity fMLP receptor and expressing p22phox, p40phox and Rac were grown in tissue culture at 37°C in 5% CO2 with RPMI and 10% fetal calf serum. Three siRNA molecules (Q, A1, A2) as well as non-interfering RNA (Nsi) used as a control were obtained commercially. Transfection of K562 cells was completed with Nucleofector technology (Amaxa Biosystems, Gaithersburg, MD) using Solution L and protocol T020. Transfection was >90% by expression of a GFP plasmid; viability after the procedure was >90%. O2− was measured after stimulation with PMA (200 ng/ml) as SOD inhibitable cytochrome c reduction or in response to fMLP (1 μM) by the addition of Diogenes (National Diagnostics) and detection of chemiluminescence in a luminometer (FB12, Berthod Detection Systems, Pforzheim, Germany) as relative light units. Cell lysates were made with the addition of 10% Triton X-100 and stored at −70°C. Proteins (5–10μg of cell lysate) were separated on a 10% SDS-PAGE and blotted onto nitrocellulose. Detection of specific proteins was completed using polyclonal antibodies to p29 Prx and to other phox proteins and actin and secondary antibodies with a chemiluminescent technique.
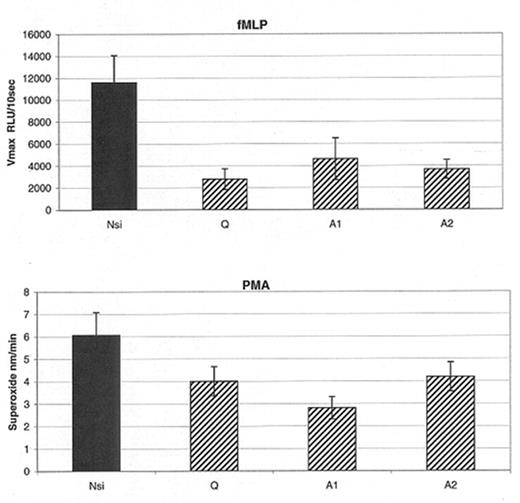
Results: Transfection of the K561 cells with Q, A1, and A2 resulted in a knockdown of p29 Prx (25–40%) compared to Nsi. Optimal knockdown occurred in the presence of 2μM siRNA, at 48 hours after transfection. No changes in actin, p67phox, p47phox, p40phox, p22phox or gp91phox were documented with nucleofection, siRNAs or Nsi. In 5 separate experiments, knockdown of p29 Prx resulted in a decrease (p<0.05) in O2− production (PMA) or O2− associated chemiluminescence (fMLP), see Figure (columns and bars represent mean ± SEM).
Conclusion: O2− production in response to PMA or fMLP was reduced in K562 cells with a knockdown of p29. These results extend previous studies and demonstrate the requirement of p29 Prx, peroxiredoxin VI, for optimal NADPH oxidase activity within intact cells.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal