Abstract
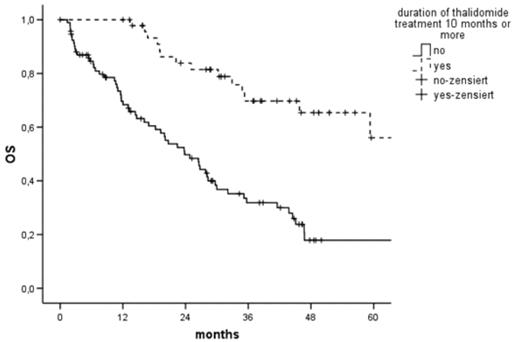
Although efficacy of thalidomide (thal) monotherapy in relapsed/refractory Multiple Myeloma (MM) is proven, the optimal duration of treatment is unknown. Here, we analysed individual patient data of 852 patients (pts.) with relapsed/refractory MM from 21 centres who received thal monotherapy in a trial or in a compassionate use program. To assess the influence of treatment duration, pts. who discontinued thal because of lack of efficacy, progression or death were excluded, so we analysed only pts. who continued on thal as long as they tolerated it or until the study they were enrolled in was complete. 162 pts. were evaluable; 32 completed the trial, 57 had severe neurotoxicity, 2 severe somnolence, 72 other adverse events leading to discontinuation of thal. The median age was 65 years (interquartile range 58–72), 92 (56%) were male, 96 (59%) had relapsed MM. Sixteen (10%) achieved complete remission (CR), 64 (39%) partial remission (PR), 29 (18%) minimal response, 34 (21%) no change, 9 (6%) had progressive disease, 9 (6%) were unevaluable for response. The median daily dose at 3 months was 200 mg/d (100–400), the cumulative dose after 3 months 23200 mg (9025–34150). Median time to response was 3.6 months (3–7), median treatment duration 5 months (2.3–12). Median event-free survival (EFS) was 16 months (95%CI 12–21), median overall survival (OS) 33 months (95%CI 22–44). In univariate analysis, prognostic factors for EFS/OS were leukocyte/platelet count, haemoglobin, cumulative dose at 3 months, duration of treatment and CR/PR. In multivariate analysis, duration of treatment, CR/PR and platelet count remained independent prognostic factors for EFS/OS. If pts. took thal for ≥ 10 months (n=51), median EFS was 31 (95%CI 14–48) vs 12 (9–14, p<0.001) months and median OS 83 (95%CI 49–116) vs 24 (95%CI 17–31, figure 1, p<0.001) months. We analysed responding pts. (n=80) separately to assess the role of treatment duration in maintenance. Patients in CR/PR who took thal ≥ 10 months (n=41) had a longer EFS (39 (95%CI 19–59) vs 18 (95%CI 9–28, p<0.001) and OS (83 (95%CI 49–116) vs 32 (95%CI 20–44, p<0.001) months). This analysis of patients whose duration of treatment was determined by factors independent of disease demonstrates a clear survival benefit of longer duration of thal treatment for relapsed/refractory MM and supports its value as maintenance therapy.
Author notes
Disclosure: Employment: Axel Glasmacher is an employee of Celgene GmbH. Research Funding: Marie von Lilienfeld-Toal and Gordon Cook have received research funding from Pharmion. Honoraria Information: Marie von Lilienfeld-Toal received honoraria from Celgene. Ibrahim Yakoub-Agha received honoraria from Pharmion. Membership Information: Roland Fenk: Pharmion advisory board; Pieter Sonneveld: Celgene and Ortho-Biotech advisory board; Anders Waage: Pharmion advisory board.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal