Abstract
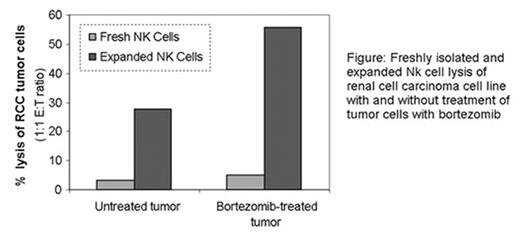
The activation of NK cell inhibitory receptors may limit the antitumor efficacy of adoptive autologous and allogeneic NK cell infusions. Recently, we found that exposing malignant cells to the proteosome inhibitor bortezomib upregulated surface expression of death receptors for TRAIL, resulting in significant enhancement of autologous NK cell tumor cytotoxicity in vitro and in vivo. Here we show that NK cells expanded in vitro in the presence of IL-2 and EBV-LCL feeder cells upregulate surface expression of TRAIL which significantly augments bortezomib-induced tumor sensitization to NK cell killing. CD56+/CD3– NK cells were isolated from normal donors by immuno-magnetic bead selection and were co-cultured with irradiated EBV-LCL feeder cells in X-VIVO 20, 10% human AB serum, and 500 IU/ml hrIL-2 for up to 21 days. Depending on culture conditions, a 300 to 10,000 fold increase in NK cell numbers was achieved. Non-expanded and expanded NK cells were analyzed by flow cytometry for the expression of CD56, CD16, TRAIL, FasL, NKG2D, LFA-1, perforin, and granzymes A and B at baseline and ≥ 10 days following in vitro expansion. Chromium release assays were performed to assess fresh vs. expanded NK cell cytotoxicity of renal cell carcinoma (RCC) tumor targets treated with 10 nM bortezomib for 18 hr vs. untreated RCC controls. Freshly-isolated NK cells did not express TRAIL or FasL; in contrast NKG2D, LFA-1, perforin and granzymes A and B were constitutively expressed in fresh NK cells. After expansion, there was a dramatic increase in surface expression of TRAIL and NKG2D; on fresh vs. expanded NK cells from 3 different donors, TRAIL expression increased from 0% to 80.8±15.4% (mean fluorescence intensity [MFI] of TRAIL increased from 6.0±5.1 to 37.9±3.2). The MFI of NKG2D surface expression also increased following NK cell expansion (432.0±70.9 from 48.3±16.3). Expression of LFA-1 and perforin did not change, although there was a small increase in surface and intracellular expression of FasL and granzymes A and B respectively. At a 1:1 effector to target ratio, fresh NK cells lysed 3.4± 2.1% and 5.0± 2.7% of untreated and bortezomib-treated RCC tumor cells respectively. In contrast, there was a dramatic increase in bortezomib-treated tumor susceptibility to killing by expanded NK cells; NK cells expanded for 12-18 days killed 27.6± 9.3% and 55.8± 8.3% of untreated vs. bortezomib-treated RCC tumor cells respectively.
Conclusion: In vitro-expanded NK cells are phenotypically and functionally different from non-expanded NK cells. Expanded cells have increased NKG2D and TRAIL expression and greatly enhanced TRAIL-mediated tumor cytotoxicity compared to non-expanded NK cells. Based on these findings, a phase I trial investigating the safety and anti-tumor effects of escalating doses of adoptively-infused ex vivo-expanded autologous NK cells following bortezomib treatment in patients with advanced metastatic tumors and hematological malignancies has recently been initiated.
Freshly isolated and expanded Nk cell lysis of renal cell carcinoma cell line with and without treatment of tumor cells with bortezomib
Freshly isolated and expanded Nk cell lysis of renal cell carcinoma cell line with and without treatment of tumor cells with bortezomib
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal