Abstract
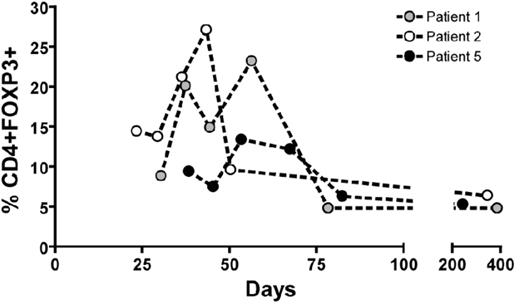
Preventing Graft-versus-Host Disease (GvHD) without impairment of immune reconstitution is a major goal in HLA-mismatched hematopoietic stem cell transplantation (HSCT). Many experimental strategies to selectively destroy or remove alloreactive donor T cells after allostimulation prior to infusion have been explored. An alternative approach is costimulatory blockade (CSB) during ex vivo allostimulation of donor T cells, rendering allospecific T cells within the donor cell pool alloanergized (i.e hyporesponsive to subsequent alloantigenic challenge). Murine and human data suggest effective induction of alloanergy by ex vivo CSB may involve an active cell-mediated suppression process requiring the presence of CD4+ CD25+ regulatory T cells (Tregs). We conducted a pilot clinical study of haploidentical HSCT after allospecific CSB with anti-B7.1 and -B7.2 antibodies and measured reconstitution of Treg by intracellular flow cytometry. 5 patients (pts; 4 high risk acute lymphoblastic leukemia, one marrow failure) underwent cyclophosphamide/TBI-conditioned haploidentical HSCT with cyclosporine and methotrexate as GvHD prophylaxis. Donor bone marrow was incubated with irradiated recipient peripheral blood mononuclear cells and 10μg anti-B7.1/2 antibodies/106 cells for 48 hours to induce alloanergy, washed and infused. All pts engrafted. All evaluable patients had a marked relative increase in peripheral blood CD4+ FOXP3+ T cells at D+20-60 (Figure 1). CD4+ FOXP3+ cells were CD25+ CD45RO+ intracellular CTLA4+ CD127lo consistent with a memory Treg phenotype. Treg were predominantly negative for HLA DR differentiating them from activated T cells. Despite receiving high doses of mismatched donor T cells (median 1.8 (CD4) and 3.1 (CD8) x 107/kg) and all pts achieving 100% donor chimerism, only 2 pts developed acute GvHD, both Grade II, resolving after short courses of corticosteroids. All evaluable patients also had an increase in CD4+ T effector (Teff) cells with an activated phenotype (CD25+ HLA DR+ FOXP3-) at D+30-50. Pts had very rapid immune reconstitution (CD4, CD8, NK and CD8-CMV-tetramer+ cell numbers and immunoglobulin levels) and have had normal vaccination responses. 2 pts died, at D+35 (bacterial sepsis) and D+71 (multi-organ failure), both without GvHD. 3 pts survive (median follow up 5 years) with normal performance status with no chronic GvHD or disease relapse. Conditioning-related cytokine secretion may have led to reversal of anergy in vivo and expansion of alloreactive cells within the Teff cell population. The marked in vivo expansion of Treg may represent one mechanism of suppression of alloreactive Teff and subsequent immunological control of acute GvHD without impairing immune reconstitution in pts receiving HLA-mismatched donor T cells after ex vivo allospecific CSB. We are using a modification of this strategy in a clinical trial of delayed infusion of escalating doses of alloanergized donor T cells after CD34-selected haploidentical HSCT, to determine the optimal dose of alloanergized donor T cells that controls acute GvHD without impairment of immune reconstitution.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal