Abstract
Survival of CLL cells requires sustained activation of the anti-apoptotic PI-3-kinase/Akt pathway, and many therapies for CLL cause leukemia cell death by triggering apoptosis. Blood lipoprotein particles are either pro- or anti-apoptotic. High density lipoprotein (HDL) particles are anti-apoptotic through sphingosine-1-phosphate receptor 3 (S1P3)-mediated activation of the PI-3-K/Akt pathway. We have noted that apoE4-very low density lipoprotein (VLDL) (but not apoE2- or apoE3-VLDL) particles increase apoptosis of endothelial cells by recruiting the phosphoinositol phosphatase SHIP-2 to the plasma membrane, thereby directly inhibiting the anti-apoptotic activity of HDL (
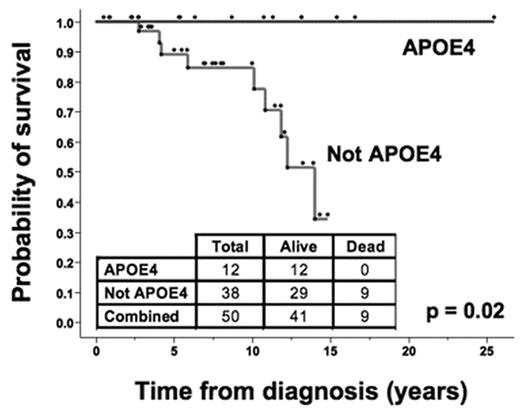
Men and women had the same time-to-treatment (treatment-free survival) irrespective of APOE genotype. VLDL is metabolized to LDL by the actions of LPL. Patients had shorter survival and time-to-treatment if their CLL cell lipoprotein lipase (LPL) mRNA levels were high (p = 0.002). In analyzing APOE genotype and LPL levels in the same patients, we demonstrated that APOE4 was a more important determinant of survival than was the LPL level (p = 0.0007). The beneficial effect of APOE4 in CLL survival is likely mediated through allele-specific influences of APOE4 on serum lipoproteins increasing leukemia cell apoptosis. The frequency of the APOE alleles in the CLL patient population was not significantly different than that of a control population (16 and 13%, respectively). APOE genotype therefore does not appear to affect susceptibility to CLL, but influences the clinical course of disease, particularly after therapy is initiated. In contrast, APOE genotype does influence susceptibility to other diseases, most notably Alzheimer’s disease in which APOE4 markedly increases risk. The beneficial impact of APOE4 in CLL and its deleterious impact on Alzheimer’s disease expression may relate to a common mechanism of APOE4 in enhancing apoptotic cell death. APOE genotyping of patients with CLL may provide important clinical prognostic information, particularly in women. Most importantly, the allele-specific influence of APOE on disease progression may provide important new insights into the mechanisms of disease and response to therapy, and lead to new agents for treatment.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal