Abstract
Background: Rituximab in combination with chemotherapy improves overall survival (OS) compared to chemotherapy alone when used for induction therapy, for patients with newly diagnosed and relapsed indolent lymphoma. Randomized controlled trials (RCTs) have demonstrated that maintenance treatment with rituximab (MR) prolongs progression free survival but evidence of effect on OS is lacking.
Objectives: to evaluate the effects of MR on OS in patients with follicular lymphoma (FL).
Methods: Systematic review and meta-analysis of RCTs that assessed MR for patients with B cell FL. Electronic databases of medical journals and ongoing trials were searched. Relative risks (RR) for dichotomous outcomes with 95% confidence intervals (CI) were pooled using Mantel-Haenszel method. Treatment effect on OS was estimated as hazard ratios (HRs) using methods described by Parmar et al. (
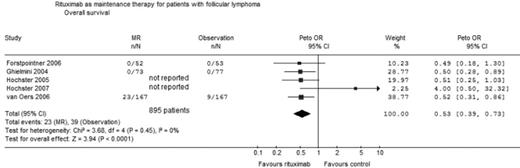
Results: 266 titles and abstracts were screened. Five trials fulfilled inclusion criteria (table). 1053 adult patients were randomized between the years 1998–2004. The median follow up ranged between 26 to 41 months. The minimal requirement for inclusion was either a stable disease after induction (3 trials) or partial remission (2 trials). Overall survival: Four trials (895 patients) were included in analysis of OS. The Hainsworth trial (
Conclusions: MR improves OS compared to observation in patients with refractory/relapsed FL who responded to induction therapy. Pooled HR of OS with MR treatment versus observation for patients with FL. n number of events; N number of patients evaluated.
Rituximab as maintenance therapy for patients with follicular lymphoma Overall survival
Rituximab as maintenance therapy for patients with follicular lymphoma Overall survival
Description of included trials
| Trial ID . | No. randomized patients . | Type of lymphoma . | Induction therapy . | Rituximab maintenance protocol . |
|---|---|---|---|---|
| *Separate analysis was possible for patients with FL. Y years, SLL small lymphocytic lymphoma, MCL mantle cell lymphoma, CVP cyclophosphamide, vincristine, prednisone, FC fludarabine, cyclophosphamide, FCM fludarabine, cyclophosphamide, mitoxantrone, CHOP cyclophosphamide, doxorubicin, vincristine, prednisone | ||||
| Hainsworth 2005 | 90 | Previously treated FL, SLL | Rituximab | Weekly for 4 weeks every 6 months for 2y |
| Hochster 2005 | 304 | Untreated FL, SLL* | CVP | Weekly for 4 weeks every 6 months for 2y |
| Hochster 2007 | 69 | Untreated FL, SLL | FC | Same as above |
| Forstpointner 2006 | 195 | Relapsed FL, MCL* | FCM+/− rituximab | Weekly for 4 weeks, at 3 and 9 months |
| Ghielmini 2004 | 151 | Newly diagnosed and relapsed FL | Rituximab | A single infusion every 2 months for four doses |
| van Oers 2006 | 334 | Relapsed FL | CHOP+/− rituximab | A single infusion every 3 months for 2y |
| Trial ID . | No. randomized patients . | Type of lymphoma . | Induction therapy . | Rituximab maintenance protocol . |
|---|---|---|---|---|
| *Separate analysis was possible for patients with FL. Y years, SLL small lymphocytic lymphoma, MCL mantle cell lymphoma, CVP cyclophosphamide, vincristine, prednisone, FC fludarabine, cyclophosphamide, FCM fludarabine, cyclophosphamide, mitoxantrone, CHOP cyclophosphamide, doxorubicin, vincristine, prednisone | ||||
| Hainsworth 2005 | 90 | Previously treated FL, SLL | Rituximab | Weekly for 4 weeks every 6 months for 2y |
| Hochster 2005 | 304 | Untreated FL, SLL* | CVP | Weekly for 4 weeks every 6 months for 2y |
| Hochster 2007 | 69 | Untreated FL, SLL | FC | Same as above |
| Forstpointner 2006 | 195 | Relapsed FL, MCL* | FCM+/− rituximab | Weekly for 4 weeks, at 3 and 9 months |
| Ghielmini 2004 | 151 | Newly diagnosed and relapsed FL | Rituximab | A single infusion every 2 months for four doses |
| van Oers 2006 | 334 | Relapsed FL | CHOP+/− rituximab | A single infusion every 3 months for 2y |
Author notes
Disclosure:Consultancy: Dr. Ghielmini has given consultancy services for Roche. Research Funding: Dr. Gheilmini has research funding from Roche. Dr. Dreyling received research funding for investigator-inititated (academic) trials from Roche. Honoraria Information: Dr. Dreyling received speaker honorarium from Roche.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal