Abstract
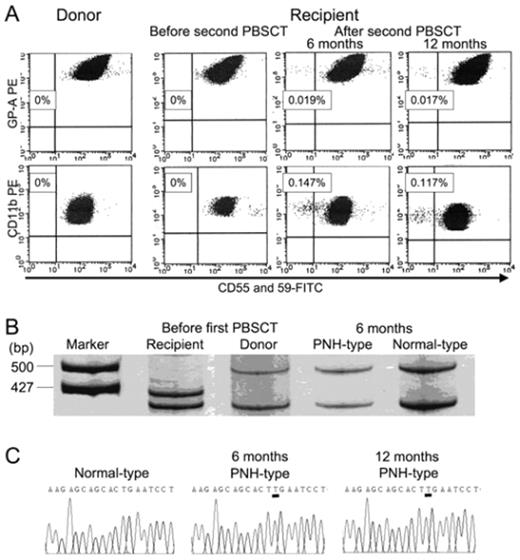
Acquired aplastic anemia (AA) is thought to be caused by the immune system attack against hematopoietic stem cells. However, there is no direct evidence that an immune system attack against normal hematopoietic stem cells leads to development of AA. The immune system attack may be directed toward abnormal stem cells given the fact that some patients with myelodysplastic syndrome respond to immunosuppressive therapy. Although the presence of a small population of CD55−CD59− blood cells represents a reliable marker for the immune pathophysiology of AA, little is known regarding when and how such paroxysmal nocturnal hemoglobinuria (PNH)-type cells appear in patients with AA. The development of AA with a small population of PNH-type cells was recently observed in an allogeneic stem cell transplant (SCT) recipient. This patient, a 59-year-old male, who had been treated with allogeneic peripheral blood stem cell transplantation (PBSCT) from an HLA-compatible sibling for treatment of very severe AA in March 2002, developed severe pancytopenia in December 2005. Late graft failure (LGF) without residual recipient cells was diagnosed based on the results of a chimerism analysis. Sensitive flow cytometry failed to reveal any increase in the proportion of CD55−CD59− PNH-type blood cells. The patient underwent a second PBSCT from the original donor without preconditioning in February 2006. Although his pancytopenia was completely resolved by day 20, his blood counts gradually decreased from day 60 without any apparent complications. Flow cytometry revealed small populations of PNH-type granulocytes in his peripheral blood (Figure 1). Both the PNH-type and normal phenotype granulocytes were of donor origin. PIG-A gene analyses showed the PNH-type granulocytes in the patient to be a clonal stem cell with an insertion of thymine at position 593 (codon 198). Similar results were obtained from the sorted PNH-type granulocytes obtained 6 months later. The patient was treated with horse antithymocyte globulin and cyclosporine. The patient required no further transfusions after 88 days of the therapy and remains well as of August, 2007. The small population of PNH-type cells was not detectable in any of 50 SCT recipients showing stable engraftment or in an AA patient suffering graft rejection after a SCT. These findings suggest that some factors expressed by the patient induced an immune system attack against autologous hematopoietic cells, leading to de novo development of donor-cell derived AA. This is the first evidence that an immune system attack against normal hematopoietic stem cells results in AA associated with a clonal expansion of a PIG-A mutant which may originally be present in the donor bone marrow.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal