Abstract
MRI is a noninvasive method for detection of transfusional siderosis in the liver and heart of children with thalassemia major (TM) and sickle cell disease (SCD). Previous studies from our laboratory demonstrated that cardiac siderosis and cardiac failure are relatively uncommon in chronically transfused SCD, despite heavy somatic iron burdens. Others have reported correspondingly low rates of endocrinopathies. We used the MRI relaxation rate R2* as a surrogate for pancreatic iron to test the hypothesis that TM patients develop greater pancreatic iron load than SCD patients having comparable liver iron burdens.
Methods We studied 70 TM and 31 chronically-transfused SCD patients. Pancreatic R2* was assessed in several mid pancreatic slices using a multi-echo, gradient echo pulse sequence with 8 echo times spaced from 2 to 16 ms. Pixelwise, monoexponential R2* reconstruction was performed with offset correction. Mean R2* was calculated from a region of interest (ROI) drawn around the body and tail of the pancreas. Control values for pancreatic R2* were derived from 14 normal volunteers, ages 19–31.
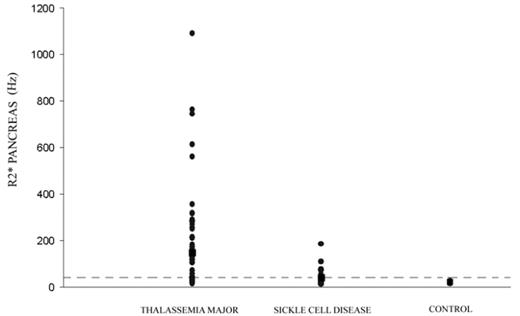
Results Patient demographics are summarized in Table 1. TM patients were an average of 5 years older but less heavily iron loaded (liver iron 5 mg/g lower and ferritin 1800 ng/mL lower). Despite this, transferrin saturations trended higher in the TM patients (76% versus 58%). SCD patients had higher high sensitivity CRP and white blood cell count, consistent with greater inflammation. Figure 1 demonstrates pancreatic R2* distribution in TM, SCD, and control subjects; dotted line indicates the 95th confidence interval for the control subjects. Pancreatic R2* was elevated in 74% of the TM patients (R2* 148.9±200.9), compared with 48% of the SCD patients (R2* 39.6±37, p<0.021). In the TM patients, pancreatic R2* was correlated to cardiac R2*, but not to liver R2*.
Discussion Pancreatic R2* is more common and more severe in TM compared to SCD, consistent with previously reported endocrinopathy rates. The younger age (decreased transfusion exposure) of the SCD patients and their lower transferrin saturations (secondary to inflammation) probably contribute to this observation. Prospective trials are necessary to determine the functional correlations and predictive value of pancreatic R2* measurements in these populations.
Patient Demographics
| Parameter . | Thalassemia (n=70) . | Sickle Cell Disease (n=31) . | P Value . |
|---|---|---|---|
| Age | 19.4 ± 10 | 14.5 ± 5.6 | 0.013 |
| Gender | 40M, 30F | 17M, 14F | NS |
| Height(cm) | 145 ± 29 | 147 ± 20 | NS |
| Weight(kg) | 45.4±17 | 43.8 ± 19 | NS |
| Reticulocyte | 2.0±1.9 (n=34) | 9.6 ± 7.8 | <0.001 |
| Iron(mcg/dL) | 193±99 (n=64) | 146 ± 89 | 0.003 |
| Ferritin(ng/mL) | 4064±5661 | 5905 ± 6320 | 0.15 |
| Transferrin (mg/dL) | 174±70 | 169 ± 87 | NS |
| HIC(mg/g) | 10.9 ± 12.2 (n=68) | 16.1 ± 11.9 (n=30) | 0.06 |
| Iron % Sat(%) | 76 ± 39 (n=38) | 58 ± 29 (n=14) | 0.11 |
| TIBC(mcg/dL) | 255 ± 119 | 219 ± 104 (n=13) | 0.06 |
| AST(U/L) | 62.7 ± 63 (n=34) | 72 ± 71 | NS |
| ALT(U/L) | 77.6 ± 94.4 (n=34) | 72 ± 99 | NS |
| hs-CRP(mg/L) | 1.6 ± 1.9 (n=46) | 6.2 ± 7 | <0.001 |
| WBC | 10 ± 7 | 12.5 ± 7 | 0.04 |
| Parameter . | Thalassemia (n=70) . | Sickle Cell Disease (n=31) . | P Value . |
|---|---|---|---|
| Age | 19.4 ± 10 | 14.5 ± 5.6 | 0.013 |
| Gender | 40M, 30F | 17M, 14F | NS |
| Height(cm) | 145 ± 29 | 147 ± 20 | NS |
| Weight(kg) | 45.4±17 | 43.8 ± 19 | NS |
| Reticulocyte | 2.0±1.9 (n=34) | 9.6 ± 7.8 | <0.001 |
| Iron(mcg/dL) | 193±99 (n=64) | 146 ± 89 | 0.003 |
| Ferritin(ng/mL) | 4064±5661 | 5905 ± 6320 | 0.15 |
| Transferrin (mg/dL) | 174±70 | 169 ± 87 | NS |
| HIC(mg/g) | 10.9 ± 12.2 (n=68) | 16.1 ± 11.9 (n=30) | 0.06 |
| Iron % Sat(%) | 76 ± 39 (n=38) | 58 ± 29 (n=14) | 0.11 |
| TIBC(mcg/dL) | 255 ± 119 | 219 ± 104 (n=13) | 0.06 |
| AST(U/L) | 62.7 ± 63 (n=34) | 72 ± 71 | NS |
| ALT(U/L) | 77.6 ± 94.4 (n=34) | 72 ± 99 | NS |
| hs-CRP(mg/L) | 1.6 ± 1.9 (n=46) | 6.2 ± 7 | <0.001 |
| WBC | 10 ± 7 | 12.5 ± 7 | 0.04 |
Figure
Author notes
Disclosure:Consultancy: Dr. Wood and Dr. Coates have consulted for Novartis. Research Funding: Dr. Wood and Dr Coates receive research funding from Novartis. Honoraria Information: Dr. Wood and Dr. Coates have received honoraria from Novartis and Apotex. Membership Information: Dr Wood is an advisor to the Exjade Speaker’s Bureau. Dr. Coates is a member of the Exjade Speaker’s Bureau. Off Label Use: R2* assessment of pancreas is not FDA-approved.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal