Abstract
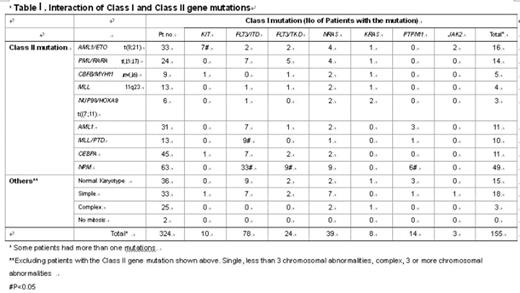
The development of acute myeloid leukemia (AML) is a multistep process. Gilliland and colleagues proposed a two hit theory of leukemogenesis that requires collaboration of at least two classes of gene mutations. The Class I gene mutations activate the signal transduction pathway and confer proliferation and survival advantage to hematopoietic cells. The Class II gene mutations affect transcriptional activators or coactivators and serve to impair cell differentiation. In this study, comprehensive analyses of a panel of gene mutations, their interactions and associations with antigen expression of leukemia cells were performed in 324 patients with primary AML, including 275 adults and 49 children(≤18years). The gene mutations included FLT3/ ITD (78 cases, 24.1%), FLT/ TKD (24 cases, 7.4%), NPM(63 cases, 19.4%), CEBPA(45 cases, 13.9%), NRAS (39 cases, 12%), AML1 (31 cases, 9.6%), PTPN11 (14 cases, 4.3%), MLL/PTD(13 cases, 4%), KIT(10 cases, 3.1%), KRAS (8 cases, 2.5%), and JAK2 (3 cases, 0.9%). In addition, 33 patients had t(8;21), 24 had t(15;17), 9 had inv(16) and 13 had 11q23 translocations. Totally, the Class I gene mutations were detected in 155 patients (47.8%), and Class II gene mutations, in 228 patients (70.4%). Most Class II mutation was associated with a distinct immunophenotype of leukemic cells, such as CEBPA mutation: HLADR(+)CD7(+)CD15(+)CD19(−)CD34(+) (p<0.05), NPM mutation: HLADR(−)CD19(−)CD34(−)CD33(+)(p<0.05), AML1 mutation: HLADR(+)(p<0.05), MLL/PTD: CD7(−)(p<0.05), AML1/ETO: HLADR(+)CD7(−)CD19(+)CD33(−)CD34(+)CD56(+)(p<0.05), PML/RARA: HLADR(−)CD2(+)CD7(−)CD11b(−)CD34(−)(p<0.05), CBFB/MYH11: CD11b(+)CD14(+), and translocation 11q23: CD19(+)CD33(−)CD34(−) (p<0.05). The interactions between Class I and Class II mutations are shown in table 1. Among Class I mutations, FLT3/ ITD could interact with each subtype of Class II gene mutations, but were particularly associated with NPM mutations (p<0.001) and MLL/PTD (p=0.001). FLT3/ TKD was closely related to NPM mutations (p=0.03). Most KIT mutation were detected in the core binding factor leukemia (p<0.001). PTPN11 mutations were more frequently detected in patients with NPM mutations than in others (p=0.035). Few patients with complex cytogenetics revealed mutations of the gene panel studied (Table 1), suggesting that leukemogenesis in these patients was through mechanism other than the known Class I and Class II mutations. In this study, the cooperative gene alterations of the NUP98/HOXA9 fusion gene were demonstrated (Table1) which, to the best of our knowledge, have not been reported before. In conclusion, the development of AML requires multistep genetic changes. Most Class II mutation is closely associated with a distinct pattern of antigen expression of leukemic cells. Exploring the interactions of gene mutations may help us more understand the pathogenesis of leukemia and benefit further therapeutic strategy.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal