Abstract
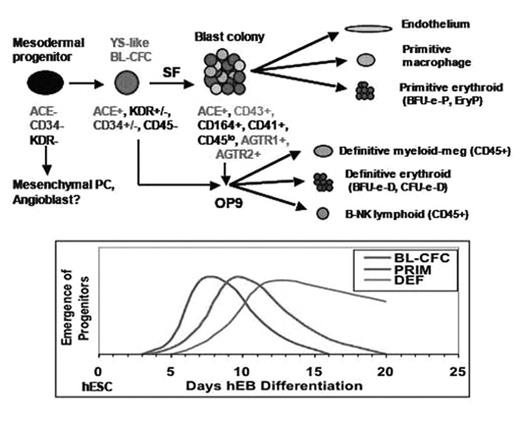
We have defined serum-free (SF) culture conditions for efficiently generating clonogenic hemangioblasts from pluripotent human embryonic stem cells (hESC). We also report that angiotensin-converting enzyme (ACE), a critical physiologic regulator of blood pressure, angiogenesis, and inflammation, is a novel marker for identifying and purifying hemangioblastic stem-progenitors from differentiating hESC. Using in vitro clonal assays of primitive and definitive hematopoietic potential, we demonstrated that ACE+ human embryoid body (hEB) cell populations contain a common yolk sac (YS)-like progenitor for not only endothelium, but also both primitive and definitive hematopoieses. Thrombopoietin (TPO) and basic fibroblast growth factor (FGF2) were identified as critical growth factors for expanding ACE+ hemangioblasts. ACE+CD34-CD45- human embryoid body (hEB) cells contained the highest number of these progenitors. Further maturation of ACE+ hemangioblasts in a stromal environment produced definitive-type lympho-hematopoietic progenitors, albeit with a late YS, and not AGM phenotype. The developmental kinetics of YS-like hematopoiesis generated from hEB cells was remarkably in parallel to the actual timeline of human YS development, which occurs during weeks 2–6 of human gestation. We also showed that ACE and the renin-angiotensin system (RAS) axis plays a direct role in hESC-derived hemangioblastic differentiation, and is directly regulated via signaling through the two major angiotensin peptide receptors (AGTR1 and AGTR2). Moreover, directed differentiation of hemangioblasts toward either endothelium, or multipotent primitive hematopoietic progenitors was efficiently enhanced via modulation of either AGTR1 or AGTR2 signaling. RAS axis manipulation may therefore be exploited for directing the differentiation of transplantable hESC-derived hemangioblastic stem-progenitors, thus providing novel opportunities for human tissue engineering. The key molecular and cellular events of human hemato-endothelial genesis can now be delineated in a manner that was previously impossible due to inaccessibility of human embryonic tissue.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal