Abstract
Introduction: stem-cell-transplantation (SCT) aims to combine tumor cytoreduction and host replacement with donor cells. For patients with no matched sibling available for allogeneic SCT (approximately 70% of patients) an alternative option is SCT from a relative carrying only one identical HLA haplotype (haplo-SCT), which may provide a donor for almost every patient at optimal timing. In the small number of multi-cord blood transplants reported, it was found that usually only one unit engrafted faster, while the others were rejected. We hypothesized that multi-donors may improve the engraftment following haplo-SCT. Patients and methods: Fifteen ultra high-risk patients (median age 20y, 9–52, mostly after 1–2 previous SCTs and 13 of them with refractory disease) were included. Conditioning regimen was fludarabine based. All grafts were T cell depleted.
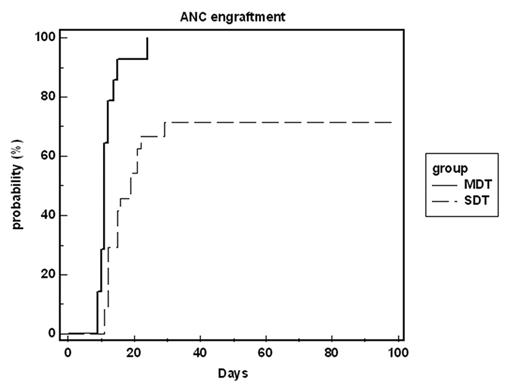
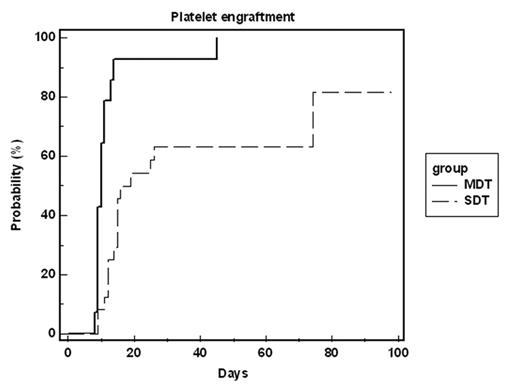
Results: All 14 evaluable patients engrafted but one of them later rejected the graft. The time for neutrophil engraftment (0.5 & 1.0x109/L) was fast, 11d (9–24) and 12d (10–28) respectively. Platelets engrafted even earlier (20 & 100x109/L), 10d (8–45) and 14d (12–32) respectively. Three patients demonstrate continuous bi-donor hematopoiesis (follow up 72, 128 and 168d) while 11 patients converted to single donor hematopoiesis within a median of 21d (14–39). Comparison of MDT patients to an earlier patient group receiving a similar protocol with a single donor (SDT) showed better granulocyte and platelet engraftment (p<0.001 for both, figures 1 and 2). Additionally, no primary graft failure occurred in patients undergoing MDT, while in SDT it occurred in 9/38 patients (p<0.001). One patient in-which leukemia persisted throughout the conditioning, leukemic cells disappeared upon the conversion to single donor chimerism. Despite major blood type differences, no hemolysis related side-effects were noted. Eight patients developed GVHD (mostly grade 1/2).
Conclusion: MDT is safe and induces fast and durable engraftment of both myeloid and thrombocyte lineages in patients undergoing haplo-SCT. Further studies should follow this report.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal