Abstract
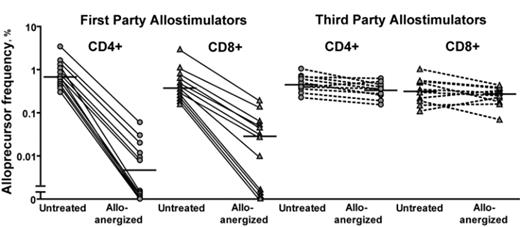
The prevention of severe acute Graft-versus-Host Disease (GvHD) without impairment of immune reconstitution is the major challenge in HLA-mismatched hematopoietic stem cell transplantation (HSCT). One alternative to experimental strategies to selectively destroy or remove alloreactive T cells from the donor T cell pool is to induce hyporesponsiveness (anergy) in alloreactive T cells within the donor T cell pool and thus preserve the vast majority of T cell repertoire. We previously reported early clinical data of HLA-mismatched HSCT after alloanergization of donor bone marrow via ex vivo allostimulation in the presence of co-stimulatory blockade (CSB) with Cytotoxic T Lymphocyte Antigen-4 Immunoglobulin (CTLA4-Ig). Analysis of a larger cohort of such patients revealed a low rate of severe acute GvHD and very few clinically significant viral infections, with over 30% of patients (pts) surviving long-term without disease relapse. This suggested that CSB might indeed be controlling alloreactivity with preservation of pathogen-specific immunity and a graft-versus-leukemia (GvL) effect. We therefore sought to directly determine the effect of alloanergization of human donor T cells on alloreactivity, pathogen- and leukemia-antigen-specific immunity. After alloanergization via blockade of CD28-mediated co-stimulation with clinical-grade humanized anti-B7.1 and anti B7.2 antibodies, HLA-mismatched alloproliferative responses were reduced by 2 logs, a more efficient reduction in alloreactivity than previously reported with the use of CTLA4 Ig. Using CFSE-based labeling of human responder T cells we have demonstrated directly for the first time that alloanergization efficiently abrogates stimulator-specific alloproliferation in both CD4 and CD8 donor T cells, whereas third party responses are retained (Figure 1). Importantly, the strategy does not diminish the capacity of donor CD4 and CD8 T cells to mount a range of functional immune responses, including proliferation, cytokine production and cytotoxic responses, in response to stimulation with several human herpes viruses. We have also demonstrated that frequencies of WT1-specific IFN-g+ CD4 and CD8 T cells are not diminished after the process of alloanergization, showing that a T cell mediated GvL effect may be retained. Importantly we demonstrated retention of pathogen and leukemia antigen-specific responses to both MHC Class I- and II-restricted antigens and in both HLA-A2+ and non-HLA-A2+ responders. These data confirm that the technique of alloanergization can be used to provide non-alloreactive donor T cells without loss of beneficial CD4 and CD8 donor immunity. The optimal dose of HLA-mismatched alloanergized donor T cells that will improve immune reconstitution whilst controlling acute GvHD after HLA-mismatched HSCT remains to be defined. To answer this question, we have embarked on a dose-escalating clinical study of delayed alloanergized donor T cell infusion to improve immune reconstitution after haploidentical HSCT.
Author notes
Disclosure: No relevant conflicts of interest to declare.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal