Abstract
Trisomy 8 CD34 cells persist and even expand in patients with bone marrow failure despite a potent specific immune response against them(
Sloand EM et al; Blood 2005; 106(3):841
). We previously demonstrated dramatic increases in c-myc, survivin, and CD1 in trisomy 8 CD34 cells by microarray analysis, realtime PCR, and immunoblot (Sloand et al; Blood 2007; 109:2399
) and postulated that upregulation of c-myc located on chromosome 8 was responsible for increases in CD1 and survivin, an anti-apoptotic protein. Knock-down of either survivin or c-myc resulted in selective trisomy 8 apoptosis and death. The styryl sulfones (Reddy, M et al Acta Chim Hungarica 1984;115:269
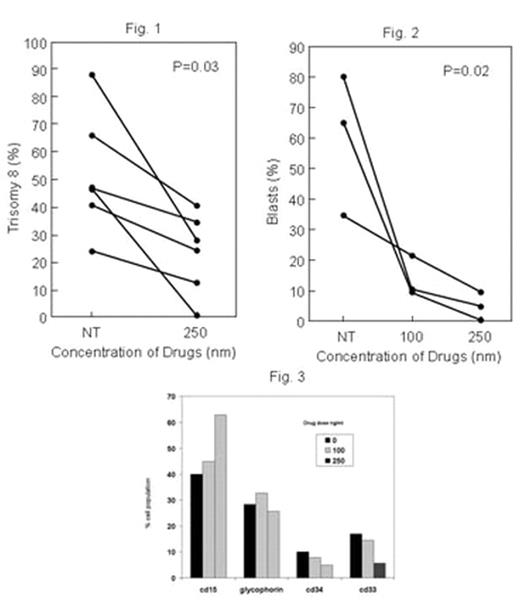
), are novel small molecule anticancer agents that inhibit cell cycle progression in cancer cells, and phase I studies for solid tumors have demonstrated little toxicity in humans (Donehower, R et al, J. Clin. Oncol. 24. 2006 abstract #13026). Initial studies in our laboratory demonstrated significant decreases in CD1 measured after trisomy 8 cells were co-cultured with increasing concentrations (10–30 nM). In this study, we examined the effect 01910.Na, a styryl sulfone, on trisomy 8 cell growth and survival. Short-term culture of mononuclear cells from four normal bone marrows with increasing concentrations of the drug (10–200nM) showed no adverse effect on hematopoietic colony formation (N=4). MDS bone marrow cells from ten trisomy 8 patients; 4 with RAEB and 6 with RA and two monosomy 7 patients with RAEB were studied. All patients with cytogenetics demonstrating trisomy 8 either had it as the sole karyotypic abnormality or with monosomy 7 in the same clone. Bone marrow was cultured with drug for 2 weeks at four different drug concentrations and fluorescent in situ hybridization performed by three blinded investigators counting 300 cells each. There was a substantial decline in the number and percent of aneuploid cells containing trisomy 8 (p=0.03; N=6) Fig 1. Other cells, including those with monosomy 7 as the sole abnormality and diploid cells were relatively unaffected. Continued treatment of the cells with a 100–250nM concentration for a 14 day period resulted in optimal killing of aneuploid cells when compared to one treatment at the beginning of the two week period. The number of blasts also decreased over the two week period when slides were prepared, stained with H&E, and read blindly by a pathologist; this effect was dose-dependent (N=3; p=0.02; Fig 2). Flow cytometric examination of a fourth patient confirmed these findings, demonstrating increased proportions of mature CD15 positive myeloid cells and decreased number of immature CD33 cells or blasts staining with CD34 concomitant with decreased numbers of trisomy 8 cells (Fig3). ON 01910.Na may prove a targeted therapy for patients with MDS and a trisomy 8 clone. A phase I/II trial is set to commence shortly.Author notes
Disclosure: No relevant conflicts of interest to declare.
2007, The American Society of Hematology
2007

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal