In this issue of Blood, Deaglio and colleagues demonstrate that CD38 acti-vates ZAP-70 and facilitates chronic lymphocytic leukemia (CLL)–cell migration toward the chemokine SDF-1 (CXCL12), suggesting that CD38+/ZAP-70+ CLL cells have a higher propensity for homing to a supportive microenvironment.
CLL patients have a remarkably heterogenous clinical course and prognosis, and therefore numerous studies have investigated prognostic markers to assess individual risk at an early stage of the disease. The mutation status of the immunoglobulin variable (V) gene segments allows us to distinguish “mutated” from “unmutated” CLL, with low or high risk for disease progression, respectively. Subsequently, the preferential expression of CD38 and zeta-associated protein of 70 kDa (ZAP-70) in cases of unmutated CLL was discovered, and CD38 and ZAP-70 were reported as prognostic markers and surrogates for mutational status.1 Current clinical efforts regarding these biological markers are focused on standardizing the methodology for measuring CD38 and ZAP-70 expression, and on validating their prognostic impact in large, prospective trials before moving CD38 and ZAP-70 into routine practice. In parallel, a series of recent laboratory studies, including the paper by Deaglio and colleagues in this issue of Blood, addressed the function of CD38 and ZAP-70 in CLL, moving from a rather descriptive use of these markers for prognostication toward novel insights into the biology of this leukemia.
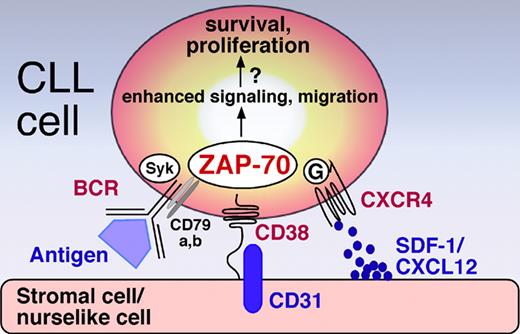
ZAP-70 initially was found to enhance IgM signaling in CLL cells, indicating that its expression enhances the capacity of CLL cells to respond to self and/or environmental antigens.2 ZAP-70 and spleen tyrosine kinase (Syk) are cytoplasmatic protein tyrosine kinases involved in immunoreceptor signaling in T cells (T-cell receptor [TCR]) and B cells (B-cell receptor [BCR]). While normal B cells generally lack ZAP-70, and rather utilize Syk for signaling via the BCR complex, both ZAP-70 and Syk become phosphorylated upon BCR activation in ZAP-70+ CLL cells. Because antigen stimulation is increasingly implicated in the pathogenesis of CLL, augmented BCR signaling through ZAP-70 could explain the more aggressive clinical course in ZAP-70+ patients. CD38 is a surface molecule expressed on lymphocytes and nonhematopoietic cells that plays a role in lymphocyte activation, proliferation, protection from apoptosis, and B-cell isotype switching. In CLL cells, CD38 ligation induces survival and proliferation signals, and allows the CLL cells to interact with CD31, the ligand for CD38, expressed by stromal and nurselike cells in the microenvironment of the marrow and secondary lymphoid tissues. Moreover, CD38+ cells within the individual clones of CLL cells are enriched for expression of Ki-67 and ZAP-70, suggesting that CD38 labels activated, proliferating CLL subclones.3 CXCR4, a chemokine receptor expressed at high levels on the surface of CLL cells,4 mediates migration of CLL cells to stromal and nurselike cells that constitutively secrete the CXCR4 ligand, called stromal-cell–derived factor-1 (SDF-1 or CXCL12).5 Richardson et al reported enhanced CLL-cell migration and survival in response to CXCL12 in ZAP-70+ cases despite similar CXCR4 surface expression, suggesting that ZAP-70 facilitates CXCR4 signaling, thereby allowing ZAP+ cells to traffic and home to a favorable environment in a CXCR4/SDF-1–dependent fashion.6 Deaglio and colleagues now close the circle by demonstrating a direct link between CD38 ligation, ZAP-70 activation, and CLL-cell migration to SDF-1, as outlined in their paper in this issue of Blood. Our current model, as displayed in the figure, therefore suggests that CD38+/ZAP-70+ CLL cells have a higher potential for migrating to stromal and nurselike cells in the marrow and secondary lymphoid tissues in response to SDF-1. There, CD38 and other critical surface molecules such as BCR and CD40 encounter the respective ligands, allowing for CLL-cell survival and clonal propagation. Collectively, these studies emphasize that prognostic markers are not an end in itself, but rather provide keys to a better understanding of the disease.
ZAP-70 integrates BCR, CD38, and CXCR4 signaling, allowing ZAP-70+/CD38+ CLL cells to home to a stromal microenvironment where they receive growth and survival signals. Ligation of CD38 by its ligand CD31 expressed on stromal and nurselike cells induces ZAP-70 phosphorylation. ZAP-70+ enhances CLL cell migration to SDF-1/CXCL12 secreted by stromal and nurselike cells by increasing CXCR4 chemokine receptor signaling downstream of CXCR4-associated G proteins. Moreover, ZAP-70 enhances the capacity of CLL cells to respond to antigen by recruitment of ZAP-70 and Syk to the cytoplasmatic portion of activated BCR complex (CD79 a/b).
ZAP-70 integrates BCR, CD38, and CXCR4 signaling, allowing ZAP-70+/CD38+ CLL cells to home to a stromal microenvironment where they receive growth and survival signals. Ligation of CD38 by its ligand CD31 expressed on stromal and nurselike cells induces ZAP-70 phosphorylation. ZAP-70+ enhances CLL cell migration to SDF-1/CXCL12 secreted by stromal and nurselike cells by increasing CXCR4 chemokine receptor signaling downstream of CXCR4-associated G proteins. Moreover, ZAP-70 enhances the capacity of CLL cells to respond to antigen by recruitment of ZAP-70 and Syk to the cytoplasmatic portion of activated BCR complex (CD79 a/b).
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal