To the editor:
Rituximab is active as initial treatment for HIV-associated multicentric Castleman disease (HMCD). Its efficacy and safety in rituximab pretreated, relapsed patients has not been previously described. We retreated a series of patients with rituximab at histologically confirmed relapse, after they had initially responded with a sustained clinical and radiologic response including normalization of Kaposi sarcoma-associated herpesvirus (KSHV) levels, C-reactive protein (CRP), albumin, hemoglobin, and lactate dehydrogenase (LDH) within 1 month of completing 4 infusions at weekly intervals. At retreatment we observed further clinical, radiologic, biochemical, haematologic, and virologic responses with rituximab. HMCD retains sensitivity to rituximab, suggesting that relapse may not be due to progression of resistant multicentric Castleman disease (MCD) but due to ongoing lytic KSHV infection of plasmablasts. Rituximab is safe and effective for patients with relapsed HMCD.
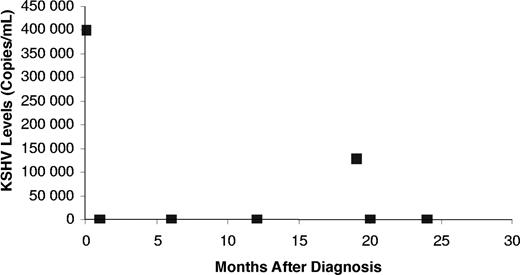
There are no established treatments for patients with relapsed HIV-associated multicentric Castleman disease. Because data from other cancers such as follicular lymphoma suggest that retreatment with rituximab may be beneficial, we present the findings of rituximab retreatment of HMCD in 3 patients who previously received this monotherapy (4 cycles of once per week rituximab therapy at a standard dose of 375mg/m2). The patient characteristics at relapse and before and after retreatment with rituxmab presentation are shown in Tables 1 and 2. All 3 patients had decreases in KSHV viral load (Figure 1), consistent with their clinical, radiologic, and biochemical responses.
Patient characteristics at relapse
| Characteristics . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Age, y | 44 | 48 | 52 |
| Duration of HIV prior to diagnosis, mo | 0 | 15 | 17 |
| Prior AIDS defining illness | Yes (TB) | Yes (KS) | Yes (KS) |
| Duration of first remission, mo | 26 | 19 | 26 |
| Duration of follow up after second remission, mo | 6 | 5 | 10 |
| Previous KS | No | Yes | Yes |
| Splenectomy after initial treatment | No | No | Yes |
| Characteristics . | Patient 1 . | Patient 2 . | Patient 3 . |
|---|---|---|---|
| Age, y | 44 | 48 | 52 |
| Duration of HIV prior to diagnosis, mo | 0 | 15 | 17 |
| Prior AIDS defining illness | Yes (TB) | Yes (KS) | Yes (KS) |
| Duration of first remission, mo | 26 | 19 | 26 |
| Duration of follow up after second remission, mo | 6 | 5 | 10 |
| Previous KS | No | Yes | Yes |
| Splenectomy after initial treatment | No | No | Yes |
TB indicates tuberculosis; and KS, Kaposi sarcoma.
Patient characteristics before and 3 months after retreatment
| Retreatment . | Patient 1* . | Patient 2* . | Patient 3* . | |||
|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | |
| Clinical symptoms | Weight loss, sweats | None | Weight loss, lethargy, sweats | None | Fevers, lethargy, weight loss | None |
| Histologic features | MCD KSHV-positive | — | MCD KSHV-positive | — | MCD KSHV-positive | — |
| Radiologic features | LNs | Partial response | HSM and LNs | Partial response | Widespread LNs | Partial response |
| CD4 count | 375 | 336 | 196 | 192 | 634 | 540 |
| LDH, U/L | 499 | 295 | 616 | 417 | 322 | 306 |
| Albumin, g/L | 29 | 42 | 26 | 34 | 31 | 42 |
| CRP | 152 | 4 | 31 | 4 | 12 | 3 |
| Hemoglobin, mmol/L | 8.8 | 14.5 | 10.1 | 14.3 | 8.1 | 13 |
| KSHV levels | 15 000 | ND | 150 000 | ND | 400 000 | ND |
| Retreatment . | Patient 1* . | Patient 2* . | Patient 3* . | |||
|---|---|---|---|---|---|---|
| Before . | After . | Before . | After . | Before . | After . | |
| Clinical symptoms | Weight loss, sweats | None | Weight loss, lethargy, sweats | None | Fevers, lethargy, weight loss | None |
| Histologic features | MCD KSHV-positive | — | MCD KSHV-positive | — | MCD KSHV-positive | — |
| Radiologic features | LNs | Partial response | HSM and LNs | Partial response | Widespread LNs | Partial response |
| CD4 count | 375 | 336 | 196 | 192 | 634 | 540 |
| LDH, U/L | 499 | 295 | 616 | 417 | 322 | 306 |
| Albumin, g/L | 29 | 42 | 26 | 34 | 31 | 42 |
| CRP | 152 | 4 | 31 | 4 | 12 | 3 |
| Hemoglobin, mmol/L | 8.8 | 14.5 | 10.1 | 14.3 | 8.1 | 13 |
| KSHV levels | 15 000 | ND | 150 000 | ND | 400 000 | ND |
— indicates not applicable; LNs, lymph nodes; HSM, hepatosplenomegaly; and ND, not detectable.
This patient is in remission for a second time with a follow-up of 24 to 36 months after initial diagnosis.
KSHV levels in patient 2. The KSHV levels in this patient demonstrated an elevated viral load at initial presentation (time 0), which declined on 4 cycles of once weekly rituximab, followed by a return of his symptoms and an increase in KSHV viral load (19 months). Once again this elevated viral load declined with rituximab therapy.
KSHV levels in patient 2. The KSHV levels in this patient demonstrated an elevated viral load at initial presentation (time 0), which declined on 4 cycles of once weekly rituximab, followed by a return of his symptoms and an increase in KSHV viral load (19 months). Once again this elevated viral load declined with rituximab therapy.
We describe, for the first time, the efficacy of retreatment with rituximab for patients with HMCD previously treated with rituximab. All 3 individuals remain in remission for a second time, after being rechallenged with this therapy. The results are encouraging; although follow up is relatively short (5-10 months).
The clinical features at relapse were similar to those at initial presentation. We recommend that after initial treatment for MCD, patients are seen on a regular basis with blood tests, as it is apparent that late presentation of MCD carries a poorer prognosis.1 In addition, KSHV appears to be a useful surrogate marker of disease activity: declining levels indicate a response and rising levels herald relapse. The response to rituximab at relapse is similar to that at initial diagnosis, in that patients respond rapidly, with resolution of their symptoms and relevant blood markers within 1 month of completing treatment.2-8 Four weekly cycles of treatment appear adequate and clinicians should not necessarily give extended courses of rituximab due to a lack of complete radiologic response.
Although retreatment with single agent rituximab has not been described previously in HMCD, the strategy has been used successfully in other tumors, such as follicular lymphoma.9,10 Indeed, the duration of response at retreatment compares favorably with the initial treatment response, suggesting that the development of acquired resistance to rituximab in follicular lymphoma is infrequent. Despite this, much preclinical work has focussed on the acquisition of resistance to rituximab, and recently a number of mechanisms has been postulated, including a reduction in the number and function of CD20 receptors on the cell surface,11 interference with signal transduction pathways involved in cell survival and disruption of apoptotic proteins such as Bcl-xL.12 The KSHV viral load data we have obtained lend credence to the hypothesis that relapsed HMCD is not the result of regrowth of resistant clones but a failure to completely eradicate KSHV harbored in B cells, and subsequent lytic infection of plasmablasts.
Large-scale studies are required to establish the role of rituximab in HMCD, including maintenance therapy after initial response to rituximab. The work presented here suggests that retreatment with rituximab is safe and effective and not associated with acquired resistance to treatment. As a randomized trial in HIV-associated non-Hodgkin lymphoma showed that the addition of rituximab to CHOP did not improve clinical outcome and was associated with significant toxicity,13 these data we present should also provide reassurance to clinicians.
Authorship
Correspondence: Mark Bower, Imperial College School of Medicine, Department of Oncology, Chelsea and Westminster Hospital, 369 Fulham Rd, London SW10 9NH, United Kingdom; e-mail: m.bower@imperial.ac.uk.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Contribution: All authors contributed to the patient care, and analyzed and provided data after they had decided on this treatment strategy together. Thus all authors conceptualized and approved the study. T.P., J.S., and M.B. wrote the final paper, which was reviewed by all the authors.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal