Latent HIV-1 infection of resting memory CD4+ T cells represents the major barrier to HIV-1 eradication. To determine whether the CCR7 ligands involved in lymphocyte migration can alter HIV-1 infection of resting CD4+ T cells, we infected purified resting CD4+ T cells after incubation with the chemokines CCL19 and CCL21. Incubation with CCL19 or CCL21 did not alter markers of T-cell activation or proliferation. However, after HIV-1 infection of CCL19- or CCL21-treated CD4+ T-cells, we observed low-level HIV-1 production but high concentrations of integrated HIV-1 DNA, approaching that seen in mitogen-stimulated T-cell blasts. Restimulation of CCL19-treated infected CD4+ T cells resulted in virus production consistent with establishment of postintegration latency. CCR7 ligands facilitate efficient entry of HIV-1 into resting CD4+ T cells. These studies demonstrate a unique action of the chemokines CCL19 and CCL21 and provide a novel model with which to study HIV-1 latency in vitro.
Introduction
The major barrier to eradication of HIV-1 is persistent long-lived and latently infected resting CD4+ T cells.1,2 Preintegration latency refers to unintegrated HIV-1 DNA that is unstable and will either degrade or will integrate into the host cell genome, usually after cell activation.3,,–6 Postintegration latency refers to the presence of integrated HIV-1 DNA in cells that are not actively producing viral particles. One of the paradoxes of postintegration latency is the inefficiency of reverse transcription and integration of HIV-1 into resting CD4+ T cells from the peripheral blood,7 whereas there is efficient infection of resting CD4+ T cells in lymphoid organ cultures in vitro or in the tissues of HIV-1-infected persons or SIV-infected macaques.8,,–11
The 2 known CCR7 ligands, CCL19 and CCL21, are constitutively expressed in lymphoid organs, particularly by resident stromal cells in the T-zone, and are critical for T-cell and dendritic cell (DC) trafficking within secondary lymphoid organs.12,–14 Given that latent HIV-1 infection predominantly occurs in CCR7 expressing resting CD4+ T cells15,,–18 and that infection of resting CD4+ T cells occurs with greater efficiency in lymphoid tissue compared with blood, we hypothesized that CCL19 and CCL21 may be critical factors that condition resting CD4+ T cells to HIV-1 infection, integration and latency.
Materials and methods
Isolation of CD4+ T cells
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll-Paque (GE Healthcare, Chalfont St. Giles, United Kingdom) density centrifugation. Resting CD4+ T cells were obtained by negative selection and magnetic beads (Figure 1A).
High levels of integrated HIV-1 in resting CD4+ T cells after incubation with CCL19 and CCL21. (A) Method used to purify resting CD4+ T cells. Resting CD4+ T cells were purified from peripheral blood mononuclear cells (PBMCs) by negative selection using mouse antibodies to human CD8 (American Type Culture Collection [ATCC], Manassas, VA), CD14 (ATCC), CD16 (ATCC), CD19 (Hedi Zola, Flinders Medical Center, Adelaide, Australia), HLA-DR (Tony D'Apice, St Vincents Hospital, Melbourne, Australia), CD69 (BD Biosciences), and CD11b (ATCC); magnetic beads conjugated with antibodies to mouse immunoglobulin G (Miltenyi Biotec, Bergisch Gladbach, Germany) and magnetic-activated cell sorting. The mean purity was 97% (range, 95%-98%). In some experiments, resting CD4+ T cells were further purified into naive (CD45RO−) and memory (CD45RO+) CD4+ T cells. (B) Method used to infect resting CD4+ T cells. Purified resting CD4+ T cells were activated for 3 days with CCL19/CCL21 or IL2/PHA or were left unactivated before infection with HIV-1. The cells were then maintained in IL-2 and supernatant and cells were collected after 4 and 7 days. (C) Low-level productive infection after infection of resting CD4+ T cells incubated with CCL19. Resting CD4+ T cells were cultured for 3 days with CCL19 (○) or IL-2/PHA (□) or were unactivated (◇) and infected with pNL4.3 HIV-1 or mock (△) as described previously in this paragraph. RT activity in the culture supernatants was determined at the indicated time points. Mean plus SD (error bar) for 6 separate experiments is shown. (D) Experiments similar to those in panel C showing productive infection in CD4+ T cells infected with AD8. (E) Quantification of integrated HIV-1 DNA. Integrated HIV-1 DNA was quantified by Alu-LTR real-time PCR. Resting CD4+ T cells were activated with CCL19 (■), IL-2/PHA (▒), or unactivated (○) and were infected with HIV-1 pNL4.3 (n = 5; median + IQR [error bar]). Infection of CCL19-treated resting CD4+ T cells with HIV-1 pNL4.3 D116N (integrase-; □) and HIV-1 pNL4.3 after incubation with CCR7 antibody 3D12 (10 μg/mL; ▩) did not show evidence of integrated HIV-1 DNA. The detection limit of the assay was 330 copies/106 cells and is shown by ----. (F) Phenotype of purified CD4+ resting T cells after activation with different stimuli. CCL19 or CCL21 (10 nM), IL-2/PHA, or unactivated cells were labeled after 3, 24, 48, and 72 hours for CD25, CD69, HLA-DR, and CCR7 and examined by flow cytometry (FCM). The percentage of CD4+ T cells that express the particular protein after 72 hours in culture is shown for all markers.
High levels of integrated HIV-1 in resting CD4+ T cells after incubation with CCL19 and CCL21. (A) Method used to purify resting CD4+ T cells. Resting CD4+ T cells were purified from peripheral blood mononuclear cells (PBMCs) by negative selection using mouse antibodies to human CD8 (American Type Culture Collection [ATCC], Manassas, VA), CD14 (ATCC), CD16 (ATCC), CD19 (Hedi Zola, Flinders Medical Center, Adelaide, Australia), HLA-DR (Tony D'Apice, St Vincents Hospital, Melbourne, Australia), CD69 (BD Biosciences), and CD11b (ATCC); magnetic beads conjugated with antibodies to mouse immunoglobulin G (Miltenyi Biotec, Bergisch Gladbach, Germany) and magnetic-activated cell sorting. The mean purity was 97% (range, 95%-98%). In some experiments, resting CD4+ T cells were further purified into naive (CD45RO−) and memory (CD45RO+) CD4+ T cells. (B) Method used to infect resting CD4+ T cells. Purified resting CD4+ T cells were activated for 3 days with CCL19/CCL21 or IL2/PHA or were left unactivated before infection with HIV-1. The cells were then maintained in IL-2 and supernatant and cells were collected after 4 and 7 days. (C) Low-level productive infection after infection of resting CD4+ T cells incubated with CCL19. Resting CD4+ T cells were cultured for 3 days with CCL19 (○) or IL-2/PHA (□) or were unactivated (◇) and infected with pNL4.3 HIV-1 or mock (△) as described previously in this paragraph. RT activity in the culture supernatants was determined at the indicated time points. Mean plus SD (error bar) for 6 separate experiments is shown. (D) Experiments similar to those in panel C showing productive infection in CD4+ T cells infected with AD8. (E) Quantification of integrated HIV-1 DNA. Integrated HIV-1 DNA was quantified by Alu-LTR real-time PCR. Resting CD4+ T cells were activated with CCL19 (■), IL-2/PHA (▒), or unactivated (○) and were infected with HIV-1 pNL4.3 (n = 5; median + IQR [error bar]). Infection of CCL19-treated resting CD4+ T cells with HIV-1 pNL4.3 D116N (integrase-; □) and HIV-1 pNL4.3 after incubation with CCR7 antibody 3D12 (10 μg/mL; ▩) did not show evidence of integrated HIV-1 DNA. The detection limit of the assay was 330 copies/106 cells and is shown by ----. (F) Phenotype of purified CD4+ resting T cells after activation with different stimuli. CCL19 or CCL21 (10 nM), IL-2/PHA, or unactivated cells were labeled after 3, 24, 48, and 72 hours for CD25, CD69, HLA-DR, and CCR7 and examined by flow cytometry (FCM). The percentage of CD4+ T cells that express the particular protein after 72 hours in culture is shown for all markers.
HIV-1 infection
Purified resting CD4+ T cells (either total or purified CD45RO− (naive) and CD45RO+ (memory) T cells) were cultured for 3 days in the presence of CCL19 (10-100 nM) or CCL21 (10-100 nM; R&D Systems, Minneapolis, MN), phytohemagglutinin (PHA; 10 μg/mL)/interleukin-2 (IL-2) (10 U/mL; Boehringer Mannheim, Mannheim, Germany) or left unactivated. In some experiments, an antibody to CCR7, 3D12 (10 μg/mL; BD Biosciences, Franklin Lakes, NJ) or IgG2a isotype control was added to resting CD4+ T cells 24 hours before addition of CCL19. We then infected the cells with either pNL4.3 (X4 using) or AD8 (R5 using) HIV-1 or HIV-1 containing a deletion in the nef gene (Δnef) and replaced with enhanced green fluorescent protein (EGFP) [pNL4.3 Δnef EGFP and AD8 Δnef EGFP] or pNL4.3 with a mutation in integrase D116N19 (Figure 1B). All infections were performed at a multiplicity of infection of 1 count per minute reverse transcriptase (RT) per cell. RT concentration was determined as described previously.20 In some experiments, integrated HIV-1 DNA was quantified using Alu-long terminal repeat (LTR) real-time polymerase chain reaction (PCR) as described previously.7,21
Flow cytometry
Cells were washed and stained with anti-CD25-phycoerythrin (PE), anti-CD69-fluorescein isothiocyanate (FITC), human leukocyte antigen (HLA)-DR-PE, anti-CCR7-PE, anti-CCR5-PE, and anti-CXCR4-PE (BD Biosciences). Intracellular staining for expression of Ki-67 was performed as described previously.22 Analysis was performed after 3, 24, 48, and 72 hours in culture using a FACSCalibur flow cytometer (BD Biosciences).
Identification of integrated virus
To determine whether integrated HIV-1 DNA was replication-competent, purified resting CD4+ T cells were incubated with CCL19 or IL-2/PHA or were left unactivated (as described above) and infected with HIV-1. The cells were then restimulated in the presence or absence of L8 (Merck, White House Station, NJ) using a modified version of a method described previously (Figure 2A).3
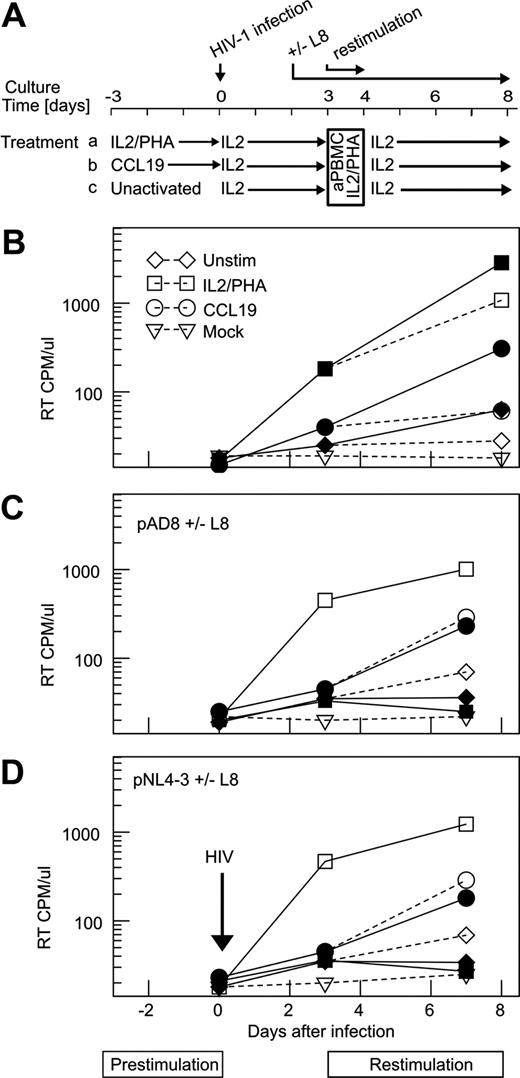
Detection of virus after restimulation of infected cells. (A) Method used for infection and restimulation. Resting CD4+ T cells cultured for 3 days with CCL19 or PHA/IL-2 or left unactivated were subjected to a second round of activation by the addition of activated PBMCs (aPBMC) at a ratio of 1:1 together with PHA and IL-2. The PHA was removed the next day, and cultures were maintained in IL-2 alone. In some experiments, the integrase inhibitor L8 was added before the second stimulation. As a control for the activity of L8, L8 was added 24 hours before the initial infection of PHA-stimulated PBMCs. Supernatants and cells were collected for quantification of RT and integrated HIV-1 DNA, respectively. (B) RT production after restimulation of resting CD4+ T cells. Resting CD4+ T cells were cultured for 3 days with CCL19 (●), or IL2/PHA (■) or unactivated (△) before infection with HIV-1 AD8 or mock (△). Cultures were restimulated (closed symbols, solid lines) or not restimulated (open symbols, dashed lines) as described above in panel A. RT activity in culture supernatant from a representative experiment is shown (from 3 replicate experiments). (C) RT production after restimulation in the presence and absence of an integrase inhibitor. Resting CD4+ T cells were cultured for 3 days with CCL19 (●) or unactivated (◇) before infection with HIV-1 AD8 or mock (△). Integration competent virus in CCL19-activated or unactivated CD4+ T cells was identified by culture in the presence (closed symbols, solid lines) or absence (open symbols, dashed lines) of an HIV-1 integrase inhibitor (L-8; Merck) for 24 hours before the second round of activation with PBMC, PHA and IL-2. The PHA was removed the next day and the cultures were kept in IL-2 with or without L-8. As a control for activity of L8, PBMCs were stimulated with PHA/IL-2 and then infected in the presence (■) or absence (□) of L8 (added 24 hours before infection). RT activity in culture supernatant from a representative experiment is shown (from a total of 4 replicate experiments). (D) The identical experiment to panel C but infection was with pNL4.3. RT activity in culture supernatant from a representative experiment is shown (from a total of 2 replicate experiments).
Detection of virus after restimulation of infected cells. (A) Method used for infection and restimulation. Resting CD4+ T cells cultured for 3 days with CCL19 or PHA/IL-2 or left unactivated were subjected to a second round of activation by the addition of activated PBMCs (aPBMC) at a ratio of 1:1 together with PHA and IL-2. The PHA was removed the next day, and cultures were maintained in IL-2 alone. In some experiments, the integrase inhibitor L8 was added before the second stimulation. As a control for the activity of L8, L8 was added 24 hours before the initial infection of PHA-stimulated PBMCs. Supernatants and cells were collected for quantification of RT and integrated HIV-1 DNA, respectively. (B) RT production after restimulation of resting CD4+ T cells. Resting CD4+ T cells were cultured for 3 days with CCL19 (●), or IL2/PHA (■) or unactivated (△) before infection with HIV-1 AD8 or mock (△). Cultures were restimulated (closed symbols, solid lines) or not restimulated (open symbols, dashed lines) as described above in panel A. RT activity in culture supernatant from a representative experiment is shown (from 3 replicate experiments). (C) RT production after restimulation in the presence and absence of an integrase inhibitor. Resting CD4+ T cells were cultured for 3 days with CCL19 (●) or unactivated (◇) before infection with HIV-1 AD8 or mock (△). Integration competent virus in CCL19-activated or unactivated CD4+ T cells was identified by culture in the presence (closed symbols, solid lines) or absence (open symbols, dashed lines) of an HIV-1 integrase inhibitor (L-8; Merck) for 24 hours before the second round of activation with PBMC, PHA and IL-2. The PHA was removed the next day and the cultures were kept in IL-2 with or without L-8. As a control for activity of L8, PBMCs were stimulated with PHA/IL-2 and then infected in the presence (■) or absence (□) of L8 (added 24 hours before infection). RT activity in culture supernatant from a representative experiment is shown (from a total of 4 replicate experiments). (D) The identical experiment to panel C but infection was with pNL4.3. RT activity in culture supernatant from a representative experiment is shown (from a total of 2 replicate experiments).
Statistical analysis
The Mann-Whitney nonparametric U test was used to determine any significant differences between culture conditions. A P value less than .05 was considered significant.
Results and discussion
Incubation with CCL19 increased the permissiveness of resting CD4+ T cells to HIV-1 infection
Highly purified total resting CD4+ T cells were incubated with CCL19 or PHA/IL-2 or were left unactivated for 3 days before infection with HIV-1. After infection with pNL4.3, there was low-level production of RT in cells stimulated with CCL19 (n = 6; mean ± SD = 73 ± 26 cpm/mL at day 7 after infection) compared with no RT production in unactivated cells (n = 6; 30 ± 14 cpm/mL; P = .014), and an early peak of productive infection after stimulation with IL2/PHA (n = 6; 1625 ± 461 cpm/mL, P = .025) (Figure 1C). Similar findings were obtained after infection with HIV-1 AD8 (n = 3; Figure 1D). The low-level production of RT was similar whether the cells were incubated with CCL19 or CCL21 (10 nM) alone or in combination (data not shown).
We identified a high concentration of integrated HIV-1 DNA 4 days after infection with pNL4.3 of resting CD4+ T cells pretreated with 10 nM CCL19 (n = 5; median [interquartile range, IQR]: 29 000 [3860-78 000] copies per million cells; Figure 1E) compared with infection of unactivated resting CD4+ T cells (< 330 copies per million cells; P < .001). It is noteworthy that the frequency of integrated DNA in the CCL19 treated resting CD4+ T cells was on average only 6-fold less than in cells pre-treated with PHA/IL2 (n = 5; 200 000 [31 000-250 000] copies per million cells; P = .05). We had similar results after infection with HIV-1 AD8.
We separated resting CD4+ T cells into CD45RO+- and CD45RO−-enriched fractions, and only identified HIV-1 entry and integration in the CD45RO+ fraction (n = 2; data not shown). After infection with EGFP-expressing X4 and R5 viruses, we were unable to show any EGFP expression in either the resting or CCL19 conditioned resting CD4+ T cells (data not shown), suggesting that the low level RT production was unlikely to be secondary to a small population of productively infected cells. In summary, these data demonstrate high levels of HIV-1 integration and low level RT production in resting CD4+ memory T cells after incubation with CCL19 or CCL21
Incubation of resting CD4+ T cells with CCL19 and CCL21 led to no change in T-cell activation and proliferation
Flow cytometry demonstrated that stimulation with CCL19, CCL21, or both did not alter the expression of any activation markers (HLA-DR, CD25, or CD69), (Figure 1F), and there was no change in intracellular expression of Ki-67 or surface expression of CXCR4 (data not shown). However, in cells incubated with CCL19 or CCL21 compared with unactivated control cells, there was a modest down-regulation of expression of CCR7 (Figure 1F) and a slight increase in CCR5 expression. A decrease in the mean fluorescence intensity (MFI) of CCR7 was identified as early as 3 hours after CCL19 stimulation (data not shown).
Integrated virus was identified after incubation with CCL19
Virus replication was induced after stimulation of the HIV-1-infected, CCL19-conditioned resting CD4+ T cells (Figure 2A). This was observed in both the presence and absence of an integrase inhibitor (L8) after infection with either AD8 (Figure 2B,C) or pNL4.3 (Figure 2D). We quantified the relative increase in RT 7 days after restimulation compared with cells cultured with the chemokine alone or mitogen, but without a second restimulation. In the CCL19-conditioned cells, after restimulation we observed an increase in RT both in the presence and absence of L8. In contrast, in the unactivated cells, we observed no increase in RT in the presence of L8. There was only an increase in RT in the absence L8 (Figure 2C,D). These findings are consistent with the presence of integrated virus in CCL19 conditioned CD4+ T cells but not in unactivated CD4+ T cells.
Pretreatment of resting CD4+ T cells from blood with CCL19 or CCL21 allows for efficient HIV-1 entry and viral integration and is associated with restricted viral expression consistent with a robust in vitro model for postintegration HIV-1 latency. These studies demonstrate a novel action of the CCR7 ligands to facilitate infection of resting CD4+ T cells and establish latency.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This study was supported by National Health and Medical Research Council program grant 358399 (S.R.L.) and American Foundation for AIDS Research grant 106715-40 with support from Concerned Parents for AIDS Research (S.R.L., P.U.C., and S.S.).
Authorship
Contribution: S.S. carried out the cellular and virologic assays and drafted the manuscript. A.S., F.W., and M.X. participated in coordination of the experiments, developed the real-time assays, and performed initial real-time assays for these experiments. P.U.C. participated in the conception and design of the study, provided supervision for experimental work, provided statistical support, and helped write the manuscript. S.R.L. conceived of the study, participated in the design and coordination, and helped write the manuscript. All authors read and approved of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Prof Sharon R. Lewin, Infectious Diseases Unit, Alfred Hospital, Level 2, Burnet Building, 85 Commercial Rd, Melbourne, Victoria, 3004, Australia; e-mail:s.lewin@alfred.org.au.

![Figure 1. High levels of integrated HIV-1 in resting CD4+ T cells after incubation with CCL19 and CCL21. (A) Method used to purify resting CD4+ T cells. Resting CD4+ T cells were purified from peripheral blood mononuclear cells (PBMCs) by negative selection using mouse antibodies to human CD8 (American Type Culture Collection [ATCC], Manassas, VA), CD14 (ATCC), CD16 (ATCC), CD19 (Hedi Zola, Flinders Medical Center, Adelaide, Australia), HLA-DR (Tony D'Apice, St Vincents Hospital, Melbourne, Australia), CD69 (BD Biosciences), and CD11b (ATCC); magnetic beads conjugated with antibodies to mouse immunoglobulin G (Miltenyi Biotec, Bergisch Gladbach, Germany) and magnetic-activated cell sorting. The mean purity was 97% (range, 95%-98%). In some experiments, resting CD4+ T cells were further purified into naive (CD45RO−) and memory (CD45RO+) CD4+ T cells. (B) Method used to infect resting CD4+ T cells. Purified resting CD4+ T cells were activated for 3 days with CCL19/CCL21 or IL2/PHA or were left unactivated before infection with HIV-1. The cells were then maintained in IL-2 and supernatant and cells were collected after 4 and 7 days. (C) Low-level productive infection after infection of resting CD4+ T cells incubated with CCL19. Resting CD4+ T cells were cultured for 3 days with CCL19 (○) or IL-2/PHA (□) or were unactivated (◇) and infected with pNL4.3 HIV-1 or mock (△) as described previously in this paragraph. RT activity in the culture supernatants was determined at the indicated time points. Mean plus SD (error bar) for 6 separate experiments is shown. (D) Experiments similar to those in panel C showing productive infection in CD4+ T cells infected with AD8. (E) Quantification of integrated HIV-1 DNA. Integrated HIV-1 DNA was quantified by Alu-LTR real-time PCR. Resting CD4+ T cells were activated with CCL19 (■), IL-2/PHA (▒), or unactivated (○) and were infected with HIV-1 pNL4.3 (n = 5; median + IQR [error bar]). Infection of CCL19-treated resting CD4+ T cells with HIV-1 pNL4.3 D116N (integrase-; □) and HIV-1 pNL4.3 after incubation with CCR7 antibody 3D12 (10 μg/mL; ▩) did not show evidence of integrated HIV-1 DNA. The detection limit of the assay was 330 copies/106 cells and is shown by ----. (F) Phenotype of purified CD4+ resting T cells after activation with different stimuli. CCL19 or CCL21 (10 nM), IL-2/PHA, or unactivated cells were labeled after 3, 24, 48, and 72 hours for CD25, CD69, HLA-DR, and CCR7 and examined by flow cytometry (FCM). The percentage of CD4+ T cells that express the particular protein after 72 hours in culture is shown for all markers.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-06-097907/3/m_zh80020811440001.jpeg?Expires=1767743725&Signature=X1yRiPv7N1Dp3LwNsvJT8FIPaZupJ1sP4XNEC48seXXRZN1eDojl0gSn4vnPNYXaYjv5S-0ahkM5PcIdcA6IBuy~fTMc5X67Wa-edxsPMxwoM1cfcAw5AU8VWBszMOntckCgvRCEYrgsdRZiAaLesRpWQjrcDIbrAbFduqho~JX692Fozip3N8BRw2NArq~l5iOTvdnje2oSGtrMDYdGww3Tpjgo61a7eEnXMBGAKuPE7g-b54zKsemX3bDwqvoLM8DCtcp34i7MiIDGF5QMtqwI02dHs6siadn79iSol1VtfA94BePFgpkyEjPXQzQoY3ihiemqv59w1~mk1xpgOQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal