Heparin cofactor II (HCII) is a plasma protein that inhibits thrombin when bound to dermatan sulfate or heparin. HCII-deficient mice are viable and fertile but rapidly develop thrombosis of the carotid artery after endothelial injury. We now report the effects of HCII deficiency on atherogenesis and neointima formation. HCII-null or wild-type mice, both on an apolipoprotein E–null background, were fed an atherogenic diet for 12 weeks. HCII-null mice developed plaque areas in the aortic arch approximately 64% larger than wild-type mice despite having similar plasma lipid and glucose levels. Neointima formation was induced by mechanical dilation of the common carotid artery. Thrombin activity, determined by hirudin binding or chromogenic substrate hydrolysis within 1 hour after injury, was higher in the arterial walls of HCII-null mice than in wild-type mice. After 3 weeks, the median neointimal area was 2- to 3-fold greater in HCII-null than in wild-type mice. Dermatan sulfate administered intravenously within 48 hours after injury inhibited neointima formation in wild-type mice but had no effect in HCII-null mice. Heparin did not inhibit neointima formation. We conclude that HCII deficiency promotes atherogenesis and neointima formation and that treatment with dermatan sulfate reduces neointima formation in an HCII-dependent manner.
Introduction
Thrombin may participate in formation of atherosclerotic plaques and stimulate proliferation of arterial smooth muscle cells following angioplasty and stent placement. Disruption of the endothelium or the fibrous cap of an atherosclerotic plaque during angioplasty exposes plasma factor VIIa to tissue factor in the arterial wall.1,2 The factor VIIa/tissue factor complex then converts factor X to factor Xa, which in combination with factor Va converts prothrombin to thrombin. Thrombin converts fibrinogen to fibrin monomers, which polymerize to form a clot, and stimulates platelet aggregation and degranulation by cleaving G-protein–coupled protease activated receptors (specifically, PAR1 and PAR4) on the platelet membrane.3 The earliest histologic response to stent placement includes local deposition of fibrin and platelets, providing good evidence for thrombin generation in this setting.4 Thrombin can also activate PAR1 on nearby endothelial cells.3 In response, the endothelial cells express adhesion molecules and release a variety of mediators that recruit platelets and leukocytes. Therefore, thrombin could play a role in the infiltration of neutrophils, lymphocytes, and macrophages that occurs during the first few days after stent placement. Over the next 2 to 4 weeks, the fibrin and platelets disappear, and restenosis may occur as a result of proliferation of smooth muscle cells and deposition of extracellular matrix in the neointima.4 Thrombin may induce smooth muscle cell proliferation directly, by activation of PAR1 on these cells, or indirectly, by causing platelets to secrete platelet-derived growth factor
Several of these thrombin-dependent events, including stimulation of platelets, endothelial cells, and smooth muscle cells, may also occur during development of the atherosclerotic plaque.1,2 Tissue factor is abundant in atherosclerotic plaques and probably triggers thrombin generation during episodes of limited endothelial desquamation or disruption of microvessels within the plaque.5 Such episodes are thought to initiate rapid expansion of the atheroma.1 Thrombin activity can be detected in atherosclerotic lesions with probes such as hirudin or chromogenic substrates.6
Recent clinical studies have shown an inverse correlation between plasma concentrations of an endogenous thrombin inhibitor, heparin cofactor II (HCII), and the prevalence of both carotid atherosclerosis7 and in-stent restenosis.8,9 Binding of HCII to dermatan sulfate or heparin increases the rate of thrombin inhibition approximately 10 000-fold.10 Dermatan sulfate is present in the arterial wall, where it may interact with HCII after disruption of the endothelium.11
We previously generated HCII-deficient mice by deletion of an exon encoding the N-terminal half of the protein.12 HCII−/− mice are born at the expected Mendelian frequency; have normal growth and survival; have normal hematopoietic, hepatic, and renal function; and produce litters of normal size in the C57BL/6 genetic background. HCII−/− mice show no evidence of spontaneous thrombosis or disseminated intravascular coagulation, which occur in mice with deficiencies of other anticoagulant proteins, including antithrombin,13 protein C,14 and tissue factor pathway inhibitor.15 Plasma from HCII−/− mice contains no HCII antigen detectable by Western blotting or enzyme-linked immunosorbent assay (ELISA) using a polyclonal IgG and no HCII activity assayed by the ability to inhibit thrombin in the presence of dermatan sulfate. Thus, unchallenged HCII-deficient mice appear to have a normal phenotype.
We found, however, that HCII-deficient mice develop carotid arterial thrombi more rapidly than do wild-type mice following oxidative damage to the endothelium.12 We also demonstrated that dermatan sulfate inhibits thrombosis when administered intravenously to wild-type mice but has no effect in HCII-deficient mice.16 These results established that HCII has antithrombotic activity in vivo and that its activity can be augmented by infusion of dermatan sulfate. In the current study, we compared atherogenesis and neointima formation in wild-type and HCII-deficient mice and investigated the effect of intravenous dermatan sulfate administration on neointima formation.
Materials and methods
Mice
All experimental protocols were approved by the Animal Studies Committee of Washington University School of Medicine. HCII-deficient mice generated previously12 were backcrossed 15 or more times with inbred C57BL/6 mice purchased from Taconic (Germantown, NY). HCII+/+ mice from double heterozygous matings or wild-type C57BL/6 mice from Taconic served as controls. ApoE-deficient mice in the C57BL/6 background (strain B6.129P2-Apoetm1Unc/J; stock no. 002052) were purchased from The Jackson Laboratory (Bar Harbor, ME). HCII−/− mice were crossed with apoE−/− mice to generate double heterozygotes (apoE+/−HCII+/−). The double heterozygous mice were bred to obtain littermates of the desired genotypes.
Atherogenesis
Mice were fed a Western diet (0.15% cholesterol/42% fat; TD 88137; Harlan Teklad, Madison, WI) beginning 8 weeks after birth. At 20 weeks, they were fasted for 4 hours and then killed. Fasting serum was assayed for glucose, cholesterol, triglycerides, and free fatty acids.17,18 The aortas were removed, opened longitudinally, and pinned as previously described.17 Images were acquired with an SMZ800 stereomicroscope (Nikon, Melville, NY) equipped with a Nikon Coolpix 990 digital camera and analyzed with ImageJ 1.36 software (National Institutes of Health, http://rsb.info.nih.gov/ij/). Lesion areas are reported as the percentage of involvement of the intimal surface for the arch (extending from the aortic valve to a point just distal to the left subclavian artery), the thoracic aorta (extending to the last intercostal artery), and the abdominal aorta (extending to the ileal bifurcation). Lesion areas were determined without knowledge of the animal's genotype.
Carotid artery injury
Mice weighing 25 plus or minus 2 g were anesthetized with 80 mg/kg intraperitoneal pentobarbital. The left proximal common and internal carotid arteries were clamped, and a beaded probe 0.63 mm in diameter was introduced into the left external carotid artery through a transverse arteriotomy as previously described.19 The probe was advanced toward the aortic arch and withdrawn 3 times, dilating the common carotid artery to approximately twice its normal diameter. The external carotid artery was then ligated on both sides of the arteriotomy, the clamps were removed, and the skin was closed with a 6-0 suture.
Some HCII−/− or wild-type mice were given a series of 4 tail vein injections of dermatan sulfate or heparin 10 minutes, 12 hours, 24 hours, and 48 hours after carotid injury. Dermatan sulfate from porcine intestinal mucosa (Sigma, St Louis, MO) was treated with nitrous acid20 to remove contaminating heparin or heparan sulfate and was administered at a dose of 20 μg/g body weight from a 20 μg/μL filter-sterilized stock solution in PBS. Heparin from porcine intestinal mucosa (Sigma) was administered at a dose of 0.125 μg/g body weight from a 0.125 μg/μL filter-sterilized stock solution in PBS.
Histologic analysis
Twenty-one days after the surgery, mice were anesthetized by intraperitoneal injection of 80 mg/kg pentobarbital. An incision was made in the right atrium, and the animal was perfused with 10% buffered formalin (pH 6.8) for 5 minutes at a constant pressure of 100 mmHg through a catheter placed in the left ventricle. The carotid artery was harvested and embedded in paraffin, and 5-μm cross sections were obtained at 100-μm intervals across the injured segment (2 mm). The sections were stained for elastin with Verhoeff van Gieson stain, mounted in mounting medium (Vector Laboratories, Burlingame, CA), and examined with a Leica DMLS microscope (Leica Microsystems, Bannockburn, IL) equipped with 10× ocular and 40× objective lenses. Images were acquired with a QImaging MicroPublisher digital camera and QCapture version 1.1.4 software (QImaging, Burnaby, BC). Cross-sectional areas of the external elastic lamina, internal elastic lamina, and lumen were determined with ImageJ 1.36 software after traces were obtained with a Graphire model CTE-430 pen tablet (Wacom, Vancouver, WA). The intimal area was calculated by subtraction of the lumen area from the total area encompassed by the internal elastic lamina. The medial area was calculated by subtraction of the area encompassed by the internal elastic lamina from that of the external elastic lamina. Measurements were made without knowledge of the animal's genotype.
Paraffin-embedded sections were stained for smooth muscle cells with mouse monoclonal anti–α-smooth muscle actin IgG (no. A2547; Sigma) at a 1:1000 dilution or for macrophages with purified rat anti–mouse Mac-3 IgG (no. 550292; BD PharMingen, San Jose, CA) at a 1:500 dilution. Binding of mouse IgG to the tissue was detected with the Mouse on Mouse Peroxidase Kit (no. PK-2200; Vector Laboratories). Binding of rat IgG was detected with the Vectastain Elite ABC Kit (Vector Laboratories). 3,3′-Diaminobenzidine (no. SK-4100; Vector Laboratories) was used as the peroxidase substrate.
Detection of thrombin in the carotid artery
Thrombin activity was detected essentially as described by Stoop et al.6 Wild-type or HCII−/− mice were killed 30 minutes after carotid injury by intracardiac injection of 26% pentobarbital (Sleepaway; Fort Dodge Animal Health, Fort Dodge, IA). Segments (3-mm) of the injured and contralateral uninjured vessels were quickly removed, opened longitudinally with scissors, examined for the presence of thrombi, and rinsed in ice-cold PBS. The tissue samples were then placed into cuvettes with 0.5 mL 0.3 mM tosyl-Gly-Pro-Arg-p-nitroanilide (Chromozym TH; Roche Diagnostics, Indianapolis, IN) in 50 mM Tris-HCl, 150 mM NaCl, 1 mg/mL poly(ethylene glycol) 8000, pH 7.4, and the absorbance at 405 nm was recorded continuously for 2 to 4 hours. In some experiments, 10 nM Phe-Pro-Arg-chloromethylketone (no. FPRCK-01; Haematologic Technologies, Essex Junction, VT) or 10 units/mL recombinant hirudin (no. 377853; Calbiochem, San Diego, CA) was added to inhibit the amidolytic activity.
Carotid arteries were also collected 60 minutes after injury, embedded in Tissue-Tek Optimal Cutting Temperature compound (Sakura, Torrance, CA), and frozen on dry ice. Frozen cross sections 8-μm thick were mounted on glass slides, air-dried, and covered with 100 μL 0.2 unit/mL recombinant hirudin in PBS at room temperature. After 1 hour, the slides were rinsed with PBS, fixed with acetone for 20 minutes at 4°C, and then incubated for 1 hour with sheep anti–hirudin IgG (Affinity Biologicals, Hamilton, ON) diluted 1:250 in PBS. Antibody binding was detected with the Vectastain Elite ABC Kit and 3,3′-diaminobenzidine. The slides were counterstained with Gill hematoxylin.
Statistical analysis
Serum lipid and glucose levels, atherosclerotic lesion areas, and amidolytic activities are expressed as the mean value plus or minus 1 SD; statistical significance was determined with the Student 2-tailed t test for independent samples. Median values are reported for measurements of intimal and medial areas, since they did not fit a normal distribution; statistical significance was determined with the Mann-Whitney U test (Wilcoxon rank sum test) as described at http://elegans.swmed.edu/∼leon/stats/utest.html. P values less than .05 were considered significant for both tests.
Results
Effect of HCII deficiency on atherogenesis
To study the effect of HCII deficiency on atherogenesis, we generated mice that were deficient in both HCII and apolipoprotein E (apoE). The progenies of double heterozygous (apoE+/−HCII+/−) breeding pairs were genotyped at 3 weeks of age (Table 1). The ratio of apoE+/+/apoE+/−/apoE−/− mice deviated from the expected Mendelian frequency regardless of the HCII genotype (overall, 236:212:89 observed vs 134:269:134 expected). This finding suggests that there is an early survival disadvantage associated with the apoE-null allele, which to our knowledge has not been reported previously. In groups of mice with each of the 3 apoE genotypes, however, the observed ratios of HCII+/+/HCII+/−/HCII−/− were approximately 1:2:1. Thus, HCII deficiency does not appear to affect survival of apoE-null mice.
Frequency of progeny from double heterozygous HCII+/−apoE+/− breeding pairs
| . | apoE+/+ . | apoE+/− . | apoE−/− . | Total . |
|---|---|---|---|---|
| HCII+/+ | 56 (34) | 52 (67) | 18 (34) | 126 (134) |
| HCII+/− | 111 (67) | 94 (134) | 43 (67) | 248 (269) |
| HCII−/− | 69 (34) | 66 (67) | 28 (34) | 163 (134) |
| Total | 236 (134) | 212 (269) | 89 (134) | 537 |
| . | apoE+/+ . | apoE+/− . | apoE−/− . | Total . |
|---|---|---|---|---|
| HCII+/+ | 56 (34) | 52 (67) | 18 (34) | 126 (134) |
| HCII+/− | 111 (67) | 94 (134) | 43 (67) | 248 (269) |
| HCII−/− | 69 (34) | 66 (67) | 28 (34) | 163 (134) |
| Total | 236 (134) | 212 (269) | 89 (134) | 537 |
Genotypes were determined for surviving pups at 3 weeks of age. Data are number of progeny with the expected Mendelian frequencies of each genotype (rounded to the nearest whole number) in parentheses.
Littermates of various genotypes (apoE+/+HCII+/+, apoE−/−HCII+/+, apoE+/+HCII−/−, and apoE−/−HCII−/−) were fed a Western diet between the ages of 8 and 20 weeks and were then killed. Figure 1 shows typical images of pinned aortas obtained from apoE+/+HCII−/− and apoE−/−HCII−/− mice. Atherosclerotic lesions in apoE−/− mice were clearly demarcated from the surrounding normal intimal surface. No lesions were observed in apoE+/+ mice regardless of the HCII genotype. In the apoE-null background, however, atherosclerotic plaque areas were significantly greater in HCII−/− mice in comparison with HCII+/+ mice in the aortic arch (18.0% ± 4.7% vs 11.1% ± 3.0%, P = .001) but not in the thoracic aorta (4.4% ± 2.2% vs 2.8% ± 2.3%, P = .117) (Figure 2). Although the lesions in apoE−/−HCII+/+ and apoE−/−HCII−/− mice differed in total area, they were similar in gross appearance. No lesions were present in the abdominal aorta. Serum cholesterol, triglycerides, free fatty acids, and glucose were elevated in apoE-null mice as previously reported,21 but these parameters were unaffected by the HCII genotype (Table 2). In apoE+/+ mice, the HCII genotype did not affect the cholesterol, triglyceride, or glucose concentrations, but HCII−/− mice had approximately 30% lower levels of free fatty acids (P = .04).
Atherosclerotic lesions. Typical appearance of aortas from apoE+/+HCII−/− (A) and apoE−/−HCII−/− (B) mice. Mice were fed a Western diet beginning at 8 weeks of age and were killed at 20 weeks of age. To show the entire aorta in a single image, each panel is a composite of 3 adjacent microscopic fields. Atherosclerotic plaques are present in the aortic arch and to a lesser extent in the thoracic aorta of the apoE−/−HCII−/− mouse (B).
Atherosclerotic lesions. Typical appearance of aortas from apoE+/+HCII−/− (A) and apoE−/−HCII−/− (B) mice. Mice were fed a Western diet beginning at 8 weeks of age and were killed at 20 weeks of age. To show the entire aorta in a single image, each panel is a composite of 3 adjacent microscopic fields. Atherosclerotic plaques are present in the aortic arch and to a lesser extent in the thoracic aorta of the apoE−/−HCII−/− mouse (B).
Atherosclerotic plaque formation in aortas of HCII+/+ and HCII−/− mice in the apoE-null background. Lesion areas are expressed as percentages of the total intimal surface area of the aortic arch, the thoracic aorta, and the abdominal aorta. No lesions were observed in HCII+/+ or HCII−/− mice in the wild-type apoE background. Mean lesion areas are indicated. Error bars equal 1 SD.
Atherosclerotic plaque formation in aortas of HCII+/+ and HCII−/− mice in the apoE-null background. Lesion areas are expressed as percentages of the total intimal surface area of the aortic arch, the thoracic aorta, and the abdominal aorta. No lesions were observed in HCII+/+ or HCII−/− mice in the wild-type apoE background. Mean lesion areas are indicated. Error bars equal 1 SD.
Serum lipid and glucose concentrations in Western diet–fed mice at 20 weeks of age
| . | Cholesterol, mg/dL . | Triglycerides, mM . | Free fatty acids, mM . | Glucose, mg/dL . |
|---|---|---|---|---|
| apoE+/+HCII+/+, n = 17 | 168 ± 55 | 71 ± 37 | 0.81 ± 0.33 | 236 ± 67 |
| apoE+/+HCII−/−, n = 17 | 173 ± 62 | 57 ± 22 | 0.56 ± 0.35 | 231 ± 60 |
| P* | .371 | .162 | .040 | .816 |
| apoE−/−HCII+/+, n = 13 | 1565 ± 230 | 246 ± 100 | 2.33 ± 0.86 | 422 ± 150 |
| apoE−/−HCII−/−, n = 18 | 1528 ± 235 | 217 ± 81 | 2.05 ± 0.60 | 456 ± 166 |
| P† | .670 | .386 | .285 | .558 |
| . | Cholesterol, mg/dL . | Triglycerides, mM . | Free fatty acids, mM . | Glucose, mg/dL . |
|---|---|---|---|---|
| apoE+/+HCII+/+, n = 17 | 168 ± 55 | 71 ± 37 | 0.81 ± 0.33 | 236 ± 67 |
| apoE+/+HCII−/−, n = 17 | 173 ± 62 | 57 ± 22 | 0.56 ± 0.35 | 231 ± 60 |
| P* | .371 | .162 | .040 | .816 |
| apoE−/−HCII+/+, n = 13 | 1565 ± 230 | 246 ± 100 | 2.33 ± 0.86 | 422 ± 150 |
| apoE−/−HCII−/−, n = 18 | 1528 ± 235 | 217 ± 81 | 2.05 ± 0.60 | 456 ± 166 |
| P† | .670 | .386 | .285 | .558 |
Data are expressed as mean plus or minus 1 SD.
apoE+/+HCII+/+ versus apoE+/+HCII−/−.
apoE−/−HCII+/+ versus apoE−/−HCII−/−.
Effect of HCII deficiency on neointima formation
Neointima formation was assessed in HCII+/+ and HCII−/− mice (both on an apoE+/+ background) 3 weeks after mechanical dilation of the common carotid artery (Figure 3). The neointima was composed predominantly of smooth muscle cells, which were also easily identified in the media of uninjured arteries. Macrophages were not detectable in the neointima or media of either genotype (data not shown). Twenty cross sections of each injured artery were examined at 100-μm intervals, the section with the greatest intimal area was identified, and the intimal and medial areas in this section were determined. The median intimal areas were significantly greater in HCII−/− mice than in HCII+/+ mice (77 000 μm2 vs 30 000 μm2, P = .009) (Figure 4). Medial areas did not differ between the 2 genotypes (40 000 μm2 vs 38 000 μm2; P = .84).
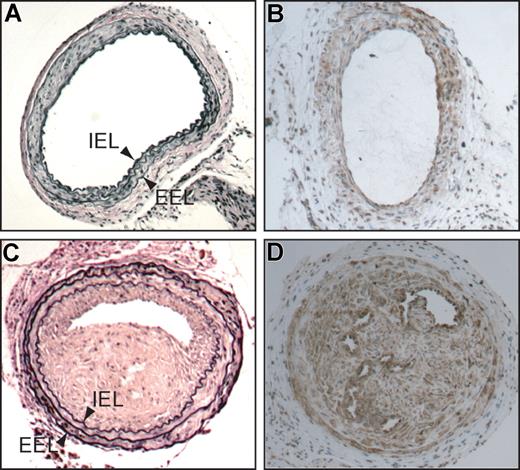
Common carotid arteries of HCII−/− mice 3 weeks after mechanical dilation with a beaded wire probe. (A,C) Cross sections stained with Verhoeff van Gieson stain for elastin. (B,D) Cross sections stained with a monoclonal anti–α-smooth muscle actin IgG. (C,D) Injured (left) arteries. (A,B) Contralateral uninjured (right) arteries. IEL indicates internal elastic lamina; EEL, external elastic lamina.
Common carotid arteries of HCII−/− mice 3 weeks after mechanical dilation with a beaded wire probe. (A,C) Cross sections stained with Verhoeff van Gieson stain for elastin. (B,D) Cross sections stained with a monoclonal anti–α-smooth muscle actin IgG. (C,D) Injured (left) arteries. (A,B) Contralateral uninjured (right) arteries. IEL indicates internal elastic lamina; EEL, external elastic lamina.
Neointimal and medial areas of carotid arteries from HCII+/+ and HCII−/− mice 3 weeks after injury. Some mice were given 4 equal doses of dermatan sulfate (+ DS, 20 μg/g body weight) or heparin (+ Hep, 0.125 μg/g body weight) 10 minutes, 12 hours, 24 hours, and 48 hours after injury. The columns indicate the median values for each group.
Neointimal and medial areas of carotid arteries from HCII+/+ and HCII−/− mice 3 weeks after injury. Some mice were given 4 equal doses of dermatan sulfate (+ DS, 20 μg/g body weight) or heparin (+ Hep, 0.125 μg/g body weight) 10 minutes, 12 hours, 24 hours, and 48 hours after injury. The columns indicate the median values for each group.
Dermatan sulfate inhibits neointima formation in wild-type mice
Four equal doses of dermatan sulfate (20 μg/g) were administered intravenously to wild-type mice 10 minutes, 12 hours, 24 hours, and 48 hours after carotid injury. This regimen decreased neointima formation significantly below the level observed in untreated wild-type mice (0 μm2 vs 30 000 μm2, P = .003) (Figure 4). The inhibitory effect was HCII dependent, since treatment with dermatan sulfate had no effect on neointima formation in HCII−/− mice in comparison with untreated HCII−/− mice (111 000 μm2 vs 77 000 μm2, P = .37). Administration of heparin (0.125 μg/g) to wild-type mice at the same dosage intervals had no effect on neointima formation in comparison with untreated wild-type mice (57 000 μm2 vs 30 000 μm2, P = .51). Neither dermatan sulfate nor heparin affected the medial area (P ≥ .36 for both experimental groups). The doses of dermatan sulfate and heparin chosen for these experiments were previously shown to have equivalent antithrombotic effects in wild-type mice, prolonging the thrombosis time of the carotid artery after photochemical injury from approximately 60 minutes to 100 minutes.16
Increased thrombin activity in injured carotid arteries of HCII-deficient mice
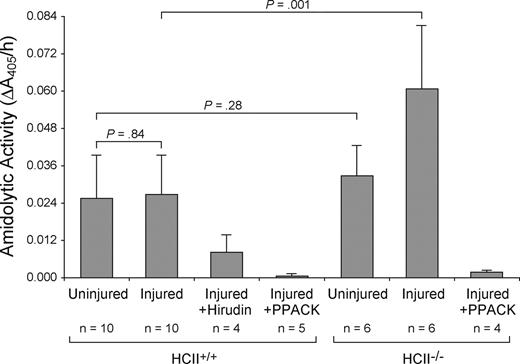
Amidolytic activity was measured 30 minutes after mechanical injury by incubation of a 3-mm segment of the carotid artery with the chromogenic substrate tosyl-Gly-Pro-Arg-p-nitroanilide. The contralateral uninjured carotid artery of each mouse served as a control. Similar amounts of amidolytic activity were detected in the injured and uninjured carotid arteries of wild-type mice (0.027 ± 0.013 AU/h vs 0.026 ± 0.014 AU/h, P = .84) (Figure 5). This rate of substrate hydrolysis was approximately equal to that observed with a solution containing 40 pM purified thrombin. The amidolytic activity was 70% inhibited by the thrombin-specific inhibitor hirudin and almost completely inhibited by Phe-Pro-Arg-chloromethylketone, which preferentially inactivates thrombin under the conditions used in this experiment.22 While the amidolytic activity of uninjured carotid arteries from HCII−/− mice was similar to that of wild-type mice (0.033 ± 0.010 AU/h vs 0.026 ± 0.014 AU/h, P = .28), the activity of injured carotid arteries from HCII−/− mice was approximately 2-fold greater in comparison with that of wild-type mice (0.061 ± 0.020 AU/h vs 0.027 ± 0.013 AU/h, P = .001) and was also substantially inhibited by Phe-Pro-Arg-chloromethylketone. Thrombi were observed more frequently in the injured arteries of HCII−/− mice (5 of 6) than of wild-type mice (5 of 10).
Amidolytic activity associated with carotid arteries. The left (injured) and right (uninjured) carotid arteries were harvested 30 minutes after injury, opened longitudinally, rinsed, and incubated with the chromogenic substrate tosyl-Gly-Pro-Arg-p-nitroanilide. The rate of substrate hydrolysis was determined by the change in absorbance at 405 nm. Changes in A405 were linear over the time course of the experiment. Phe-Pro-Arg-chloromethylketone (PPACK, 10 nM) or recombinant hirudin (10 units/mL) was added along with the substrate as indicated. Mean values are indicated. Error bars equal 1 SD.
Amidolytic activity associated with carotid arteries. The left (injured) and right (uninjured) carotid arteries were harvested 30 minutes after injury, opened longitudinally, rinsed, and incubated with the chromogenic substrate tosyl-Gly-Pro-Arg-p-nitroanilide. The rate of substrate hydrolysis was determined by the change in absorbance at 405 nm. Changes in A405 were linear over the time course of the experiment. Phe-Pro-Arg-chloromethylketone (PPACK, 10 nM) or recombinant hirudin (10 units/mL) was added along with the substrate as indicated. Mean values are indicated. Error bars equal 1 SD.
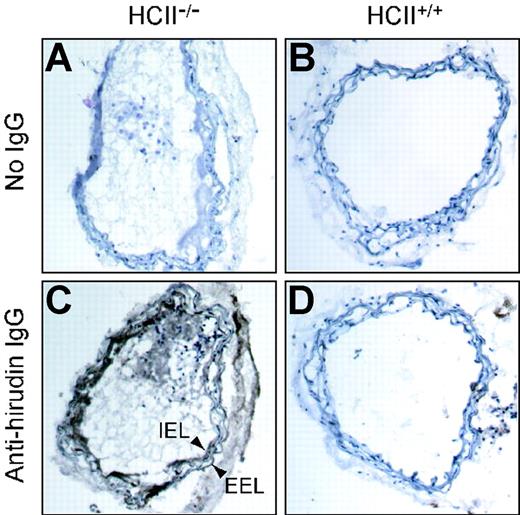
Experiments in which injured arterial segments from HCII−/− mice were incubated with tosyl-Gly-Pro-Arg-p-nitroanilide for 2 hours, then rinsed with buffer and incubated with fresh substrate, revealed that the rate of substrate hydrolysis was unchanged after rinsing (data not shown). This observation suggested that the thrombin remained active and stably associated with the vessel. To determine the location of thrombin activity in the vessel wall, we prepared frozen sections of carotid arteries harvested 1 hour after injury, incubated them with hirudin, and then detected the bound hirudin with an antibody. Hirudin binding was observed only in the injured vessels from HCII−/− mice (Figure 6), where it was located predominantly in the intima and media. Staining was less intense in the adventitia and in thrombi present in the lumen of the vessel.
Binding of hirudin to arterial sections. Frozen sections were prepared from carotid arteries of HCII−/− (A,C) or HCII+/+ (B,D) mice harvested 1 hour after injury. The sections were then incubated with hirudin in vitro, and bound hirudin was detected with sheep anti–hirudin IgG and a secondary antibody linked to peroxidase (C,D). Controls in which the primary antibody was omitted are shown (A,B). A thrombus is visible in the arterial lumen of the HCII−/− mouse. IEL indicates internal elastic lamina; EEL, external elastic lamina.
Binding of hirudin to arterial sections. Frozen sections were prepared from carotid arteries of HCII−/− (A,C) or HCII+/+ (B,D) mice harvested 1 hour after injury. The sections were then incubated with hirudin in vitro, and bound hirudin was detected with sheep anti–hirudin IgG and a secondary antibody linked to peroxidase (C,D). Controls in which the primary antibody was omitted are shown (A,B). A thrombus is visible in the arterial lumen of the HCII−/− mouse. IEL indicates internal elastic lamina; EEL, external elastic lamina.
Dermatan sulfate decreases arterial thrombin activity in wild-type mice
Dermatan sulfate (20 μg/g) or an equal volume of normal saline was administered intravenously to wild-type mice 5 minutes after carotid injury. The injured arterial segments were harvested 30 minutes later and assayed for thrombin activity with tosyl-Gly-Pro-Arg-p-nitroanilide. As shown in Figure 7, thrombin activity was significantly lower in arterial segments from dermatan sulfate–treated mice in comparison with saline-treated controls (0.020 ± 0.004 AU/h vs 0.036 ± 0.008 AU/h, P = .014).
Decreased amidolytic activity in arteries of mice treated with dermatan sulfate. Dermatan sulfate (20 μg/g) or an equal volume of normal saline was administered intravenously to wild-type mice 5 minutes after carotid injury. The injured arterial segments were harvested 30 minutes later and assayed for thrombin activity with tosyl-Gly-Pro-Arg-p-nitroanilide. Mean values are indicated. Error bars equal 1 SD.
Decreased amidolytic activity in arteries of mice treated with dermatan sulfate. Dermatan sulfate (20 μg/g) or an equal volume of normal saline was administered intravenously to wild-type mice 5 minutes after carotid injury. The injured arterial segments were harvested 30 minutes later and assayed for thrombin activity with tosyl-Gly-Pro-Arg-p-nitroanilide. Mean values are indicated. Error bars equal 1 SD.
Discussion
Previous studies support the hypothesis that thrombin generation and atherogenesis are intimately linked. For example, heterozygous deficiency of tissue factor pathway inhibitor, which blocks the procoagulant activity of factor VIIa bound to tissue factor, promotes atherosclerosis in apoE-null mice.23 Decreased inhibition of factor VIIa/tissue factor is likely to enhance the production of active thrombin, which can be detected in human atherosclerotic lesions.6 Thrombin may promote atherogenesis by stimulation of smooth muscle cell proliferation, by recruitment of macrophages to the lesion, or by some other mechanism.
HCII has been found in the intima of normal human arteries where it might inhibit thrombin.24 Arterial smooth muscle cells normally synthesize proteoglycans that stimulate the thrombin-HCII reaction and that may serve as part of an autoregulatory mechanism to prevent proliferation of these cells in the intima.25 Although dermatan sulfate is more abundant in atherosclerotic plaques than in normal arteries, its structure appears to be altered such that its ability to stimulate HCII is reduced.26 Therefore, an HCII-dependent mechanism to limit smooth muscle cell proliferation might be lost during atheroma formation.
Two clinical studies have explored the relationship between plasma HCII levels and atherosclerotic disease. Using ultrasonography to detect atherosclerotic lesions in the carotid artery, Aihara et al7 found a negative correlation between plasma HCII activity and plaque thickness in 306 elderly Japanese patients and suggested that HCII inhibits atherogenesis. By contrast, Giri et al27 found no correlation between baseline HCII antigen levels and development of symptomatic coronary heart disease in 378 middle-aged subjects followed for an average of 11.7 years in the Atherosclerosis Risk in Communities Study. The apparent discrepancy between the results of these 2 studies may reflect differences in study design (prospective vs cross-sectional), the age or ethnic background of the subjects, the clinical end point (symptomatic coronary heart disease vs carotid atherosclerosis), or the assay method (activity vs antigen). Furthermore, studies on the distribution of radiolabeled HCII in humans28 or rabbits29 suggest that 40% to 60% of the HCII equilibrates into compartments outside the bloodstream. Thus, the plasma HCII concentration may not reflect the total amount of HCII present in specific vascular beds.
Our current finding that the total area of atherosclerotic plaque in the aortic arch was approximately 64% greater in apoE−/−HCII−/− mice than in apoE−/−HCII+/+ mice provides direct evidence for involvement of HCII in atherogenesis and is consistent with Aihara et al's clinical report.7 In our model, HCII deficiency promoted atherogenesis only in apoE-null mice, which develop hyperlipidemia and hyperglycemia when fed a Western diet. Therefore, HCII deficiency by itself is insufficient to trigger atherogenesis in this model.
Several lines of experimental evidence suggest that thrombin influences smooth muscle cell proliferation in the intima, which may lead to restenosis following angioplasty. For example, neointima formation in response to mechanical injury of the carotid artery is less intense in PAR1-null mice than in wild-type mice.30 This difference appears to reflect defective thrombin signaling in smooth muscle cells or perhaps endothelial cells, since the platelets of PAR1-null mice remain responsive to thrombin (in contrast to human platelets, mouse platelets express PAR3 and PAR4, but not PAR1). In addition, a synthetic peptide analog that selectively antagonizes PAR1 reduces neointima formation in rats.31 Various thrombin-specific inhibitors (eg, hirudin and its derivatives) also diminish neointima formation in experimental animals.32
Two clinical reports suggest that levels of HCII activity in the lower range of normal predispose patients to in-stent restenosis. In a series of 134 patients who were studied 6 months after coronary angioplasty and stent placement, Takamori et al9 found restenosis rates that varied from 6.7% in patients with plasma HCII levels more than 110% of the mean to 30% in patients with HCII levels less than 80%. In the second study, Schillinger et al8 measured HCII activity in 63 patients 24 hours before femoropopliteal stent implantation. In agreement with the first study, restenosis after 12 months of follow-up occurred at a significantly higher rate in patients with HCII levels 100% or less (65% restenosis) than in patients with HCII levels more than 100% (28% restenosis).
Our experiments provide direct evidence that HCII inhibits neointima formation and are consistent with the clinical observations summarized in the preceding paragraph. Three weeks after mechanical dilation of the common carotid artery, intimal areas were significantly greater in HCII-null mice than in wild-type mice. HCII most likely functions in this model by inhibiting thrombin. In support of this hypothesis, we found that thrombin activity 30 minutes after mechanical dilation was significantly higher in injured carotid arterial segments of HCII−/− mice in comparison with those of HCII+/+ mice (Figure 5). Unexpectedly, thrombin activity was detectable at similar levels in the uninjured (contralateral) arterial segments from both HCII−/− and HCII+/+ mice; this activity could represent thrombin associated with the vessel wall in vivo and/or thrombin generated during manipulation of the vessel ex vivo. In HCII−/− mice, the activity present in the injured arteries was approximately twice that of the uninjured arteries. By contrast, in HCII+/+ mice, there was no apparent increase in arterial thrombin activity with injury. Hirudin staining of frozen sections of injured carotid arteries from HCII+/+ mice was negative, suggesting that thrombin was either absent or present at levels below the threshold for detection by this method. In comparison, thrombin was readily detected by hirudin staining of injured carotid arteries from HCII−/− mice.
Administration of dermatan sulfate during the first 48 hours after injury markedly inhibited neointima formation in HCII+/+ mice but not in HCII−/− mice. Since more than 90% of an intravenous dose of dermatan sulfate is cleared from the circulation within one hour,16 it appears to exert its effect early in the process in neointima formation. These results indicate that the antiproliferative effect of dermatan sulfate is HCII dependent and are consistent with a previous report that dermatan sulfate, but not heparin, attenuates smooth muscle cell proliferation after carotid arterial injury in rabbits.33 Some of the thrombin generated after vascular injury may remain bound to fibrin or to components of the vessel wall in an active form that is protected from inhibition by circulating antithrombin-heparin complexes but is susceptible to inhibition by HCII–dermatan sulfate.34 Indeed, dermatan sulfate administered to HCII+/+ mice 5 minutes after the onset of mechanical injury reduced the amount of thrombin activity present in the injured vessel by approximately 50% (Figure 7). The striking inhibitory effect of dermatan sulfate on neointima formation may have therapeutic implications in the setting of vascular surgery, angioplasty, or stent placement.
Our findings are similar to those reported by Aihara et al,35 who observed increased intimal hyperplasia and atherogenesis in heterozygous HCII-deficient mice. While our HCII−/− mice have a normal phenotype unless challenged by vascular injury, their HCII-null construct resulted in embryonic lethality in the homozygous state. The more severe phenotype of Aihara et al's HCII−/− mice35 is currently unexplained, but it may be due to genetic differences in the background strain or to unrecognized effects of the targeting construct on neighboring genetic loci.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by grants from the National Institutes of Health (R01 HL55520) and the Edward Mallinckrodt Jr Foundation (D.M.T.). Lipid and glucose assays were performed in the Washington University Clinical Nutrition Research Unit supported by NIH grant P30 DK56341.
National Institutes of Health
Authorship
Contribution: C.P.V. designed and performed the neointima experiments, analyzed data, and wrote the paper; L.H. designed and performed the atherogenesis experiments, analyzed data, and contributed to writing the paper; D.M.T. supervised the study and revised the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Douglas M. Tollefsen, Hematology Division, Campus Box 8125, Washington University Medical School, 660 South Euclid Avenue, St Louis, MO 63110; e-mail:tollefsen@im.wustl.edu.





This feature is available to Subscribers Only
Sign In or Create an Account Close Modal