Tumor-associated macrophages (TAMs), the most abundant immunosuppressive cells in the tumor microenvironment, originate from blood monocytes and exhibit an IL-10highIL-12low M2 profile. The factors involved in TAM generation remain unidentified. We identify here leukemia inhibitory factor (LIF) and IL-6 as tumor microenvironmental factors that can promote TAM generation. Ovarian cancer ascites switched monocyte differentiation into TAM-like cells that exhibit most ovarian TAM functional and phenotypic characteristics. Ovarian cancer ascites contained high concentrations of LIF and IL-6. Recombinant LIF and IL-6 skew monocyte differentiation into TAM-like cells by enabling monocytes to consume monocyte–colony-stimulating factor (M-CSF). Depletion of LIF, IL-6, and M-CSF in ovarian cancer ascites suppressed TAM-like cell induction. We extended these observations to different tumor-cell line supernatants. In addition to revealing a new tumor-escape mechanism associated with TAM generation via LIF and IL-6, these findings offer novel therapeutic perspectives to subvert TAM-induced immunosuppression and hence improve T-cell–based antitumor immunotherapy efficacy.
Introduction
Circulating monocytes are precursors that can differentiate into a variety of tissue-resident macrophages (MΦs) or dendritic cells (DCs), and even osteoclasts.1 MΦs exhibit a variety of activities, some of which are in opposition (ie, proinflammatory versus anti-inflammatory, immunostimulatory versus immunosuppressive, and tissue destructive versus reconstructive).1 The functional heterogeneity of MΦs depends, at least in part, on the local microenvironment.2,3 In analogy with the Th1/Th2 dichotomy of T-cell responses, MΦs exposed to IFNγ or IL-4 have been referred to as M1s or M2s (also called alternatively activated MΦs), respectively.4 M1s produce IL-12 and TNFα and are potent killers of microorganisms (especially intracellular pathogens) and tumor cells. M2s produce IL-10 but not IL-12, scavenge debris, tune inflammatory responses, and promote humoral immunity and tissue repair.5
The detection in cancer patients of tumor-specific T cells that kill ex vivo autologous tumor cells demonstrates that numerous tumor-cell types are potentially immunogenic. However, spontaneous clearance of established tumors by immune mechanisms is rare and active antitumor immunotherapy usually has poor clinical efficacy.6 It is now largely documented that established tumors propagate conditions that favor their immune escape.6 Tumor-associated macrophages (TAMs) and regulatory T cells (Tregs) accumulate at tumor sites and maintain immune tolerance that contributes to defeating tumor immunity.6,7 TAMs are far more abundant than Tregs and, in various solid tumors, constitute the major components of the leukocyte infiltrate. In most cases, especially breast, prostate, cervical, and ovarian cancers, TAM density is correlated with poor prognosis.8,–10 Strong evidence suggests that TAMs also promote cancer progression and metastasis.8,11,12 TAMs are polarized M2 cells with potent immunosuppressive functions. They have poor antigen-presenting capacity, prevent T-cell activation, and may contribute to suppressing DC functions.4,13,14 They also promote the recruitment of Tregs and Th2 cells (through CC chemokine ligand 17 [CCL17] and CCL22 secretion) and naive T cells (through CCL18). Naive T-cell activation, in an environment dominated by immature DCs and TAMs, is likely to induce anergy.10,15 In addition, TAM production of growth and angiogenic factors (ie, vascular endothelial growth factor [VEGF] and platelet-derived endothelial cell growth factor [PDGF]), proteases (ie, matrix metalloproteinase 9 [MMP9]), and chemokines (eg, CCL2) favors tumor-cell proliferation, angiogenesis, dissolution of connective tissues, and metastasis.8,12,14,16
The origin of TAMs has mostly been studied in mice in terms of precursor recruitment, survival, and proliferation. TAMs derive from circulating monocytes that are recruited into tumors by chemotactic factors, such as monocyte–colony-stimulating factor (M-CSF) and CCL2.12 IL-6,17,18 IL-10,19 and VEGF20,–22 are present in tumor microenvironment and might also contribute to TAM accumulation by preventing monocyte differentiation into DCs. M-CSF promotes TAM survival16 and induces murine TAM proliferation.23 It is thought that the tumor microenvironment programs MΦs to acquire immunosuppressive functions and to adopt trophic roles found during development and repair.8,9 The recent observations that ovarian tumor cells polarized MΦs toward TAM-like cells24,25 and that ovarian carcinoma ascites induced monocyte expression of the immunosuppressive molecule B7-H426 inferred tumor microenvironmental factor involvement in TAM generation. Currently, the tumor environmental factor(s) that programs the TAM phenotype remains unknown. The identification of this factor(s) appears crucial in the development of strategies to prevent and/or reverse the immunosuppressive TAM phenotype and thereby increase the efficacy of T-cell–based cancer vaccines.
Materials and methods
Ovarian cancer ascites and CD14+ cells
Patient samples were obtained with written informed consent in accordance with the Angers University Hospital ethics committee requirements and the Declaration of Helsinki. Ascites were collected aseptically, and cells were isolated by standard Ficoll-Paque density-gradient (Amersham Biosciences, Uppsala, Sweden). CD14+ cells (TAMs) were purified by positive selection using magnetic-activated cell sorting (MACS) technology (Miltenyi Biotec, Bergisch Gladbach, Germany). Ascites fluids were collected after centrifugation and filtration.
Cell purification and generation
Blood samples were obtained with written informed consent in accordance with the Angers University Hospital ethics committee requirements. Peripheral blood mononuclear cells (PBMCs) from healthy donor blood were isolated by standard Ficoll-Paque density-gradient (Amersham Biosciences). CD14+ monocytes and CD4+ T cells were isolated from PBMCs by positive selection (MACS technology; Miltenyi Biotec). Cells were cultured in medium consisting of RPMI 1640 medium (Biowhittaker Cambrex, Verviers, Belgium) supplemented with 10% fetal calf serum (Biowest, Nuaillé, France), 2 mM l-glutamine, 1 mM sodium pyruvate, 0.1 mM nonessential amino acids, 10 mM HEPES, 100 U/mL penicillin, and 100 μg/mL streptomycin (all from Biowhittaker Cambrex). Myeloid cells were maintained in complete medium (CM) consisting of culture medium supplemented with 20 ng/mL granulocyte-macrophage–colony-stimulating factor (GM-CSF). MΦs and immature DCs were differentiated from CD14+ monocytes cultured for 5 days in CM at 106 cells/mL without or with 20 ng/mL IL-4, respectively. In some experiments, they were incubated with ovarian ascites (diluted 1:10; TA-MΦs), cell-free supernatants (SNs) of different cell lines (SN-MΦs), LIF (25 ng/mL; LIF-MΦs), IL-6 (50 ng/mL; IL-6-MΦs), OSM (50 ng/mL), or M-CSF (50 ng/mL; M-CSF-MΦs) in CM during the differentiation process. In others, monocytes were cultured in CM in the absence or presence of 10 to 100 ng/mL IL-6 or LIF. In some experiments, day-4 MΦs and DCs were activated either with 200 (for MΦs) or 20 (for DCs) ng/mL LPS (Sigma, St Louis, MO) for 48 hours. To generate M2a-c, day-5 MΦs were exposed to 50 ng/mL IL-4 (M2a), IL-1β (M2b), or IL-10 (M2c) for 48 hours. In neutralization experiments, monocytes were maintained in CM with IL-6, LIF, or M-CSF with 4 μg/mL anti–IL-6 and/or anti–M-CSF mAbs, or controls mAbs (added on day 0 and 2). M-CSF was given by Wyeth Research (Cambridge, MA). IL-6 was from Immunotools (Friesoythe, Germany). LIF was produced and purified in-house; other cytokines and neutralizing Abs were from R&D Systems (Abingdon, United Kingdom).
Cell line culture
HepG2, 5637, and A172 tumor-cell lines were from ATCC (Manassas, VA). Supernatants were collected at 80% cell confluence.
Cytokine quantification
IL-1β, IL-4, IL-10, IL-12, IL-13, LIF, OSM, TNFα, TGFβ, M-CSF, CCL1, CCL17, CCL18, CCL22, and VEGF enzyme-linked immunosorbent assay (ELISA) were from R&D Systems. IL-6 and IFNγ ELISA were from BD Biosciences (San Jose, CA) and Mabtech AB (Stockholm, Sweden), respectively. PTX3 was quantified by ELISA as described.29
Cytokine depletion
Ascites and tumor-cell line SNs were incubated with 5 μg/mL anti–IL-6, –LIF, -OSM, and/or –M-CSF Ab or with isotype control Abs (all from R&D Systems) prior to incubation with protein A sepharose beads (Amersham Biosciences). Depletions were verified by ELISA. Results are expressed as percentages of restoration or inhibition of expression of the indicated markers, defined as follows: % = (B − A)/(B − C) × 100, where A represents the depleted SN; B, nondepleted SN, and C, CM.
Fluorescence-activated cell sorting (FACS) analysis
Cell phenotypes were analyzed using FITC-labeled anti-CD1a, -CD80, -CD86, and -ILT2 (all from BD Pharmingen, San Diego, CA); PE-labeled anti-CD14 (Dako, Glostrup, Denmark); PC5-labeled ILT3 (Immunotech, Marseille, France); unlabeled anti-CD83 (Immunotech) and anti-CD163 (BD Pharmingen) revealed by FITC-labeled anti–mouse IgG antibody (BD Pharmingen); and unlabeled anti–MCSF-R (Santa Cruz Biotechnology, Santa Cruz, CA) revealed by FITC-labeled anti–rat IgG antibody (BD Pharmingen). Isotype control mAbs were from BD Pharmingen. Results are expressed as mean fluorescence intensities (MFIs) after subtraction of the value obtained with the control mAb.
Mixed lymphocyte reaction (MLR)
MΦs were stimulated for 48 hours with LPS, washed, and cultured at 0.8 × 103, 4 × 103, or 20 × 103 cells in 96-well flat-bottomed plates with 105 purified allogenic CD4+ T cells. After 4 days, cells were pulsed for 16 hours with [3H]-thymidine (0.25 μCi [0.0093]/well; Amersham Biosciences) and its incorporation was measured by standard liquid-scintillation counting. Results are expressed in cpm (means of triplicate values).
Inhibition of T-cell proliferation
DCs and MΦs were stimulated for 48 hours with LPS, washed, and cultured in triplicate at 4 × 103 cells in 96-well flat-bottomed plates with 105 purified allogenic CD4+ T cells plus 10 μg/mL anti-CD3 mAb (clone OKT3; ATCC) and 20 U/mL IL-2 (Immunotools). After 4 days, cells were pulsed as described in “Mixed lymphocyte reaction (MLR).” T-cell proliferation was evaluated and expressed as percentages of variation defined as follows: % = (A − B)/B × 100, where A indicates proliferation with antigen-presenting cells (APCs); and B, proliferation without APCs.
In other experiments, 48-hour LPS-activated MΦs were cultured at 2 × 104 cells in 96-well flat-bottomed plates with 105 purified allogenic CD4+ T cells, previously stained with 0.5 μM fluorescent dye CFDA-SE (Molecular Probes, Carlsbad, CA), activated with 10 μg/mL anti-CD3 mAb plus 20 U/mL IL-2 in the absence or presence of 200 μM 1-d-methyl-tryptophan (1-MT; Sigma). At day 5, cells were stained with 7-AAD and APC-labeled annexin-V (BD Biosciences), and the percentage of living cells was analyzed by FACS. CD4+ T-cell proliferation was measured by CFDA-SE dilution on living cells.
PCR analysis
Total RNA was extracted using Trizol and reverse transcribed using the superscript II RNaseH− Reverse Transcriptase (both from Invitrogen, Carlsbad, CA). RNA integrity and cDNA synthesis were verified by amplifying GAPDH cDNA. For quantitative polymerase chain reaction (PCR), amplification was done using iQ SYBR Green Supermix (Bio-Rad, Hercules, CA) and specific gene expression was calculated using the 2−ΔΔCT method (using GAPDH as calibrator). The primer sequences used are available upon request.
Statistical analysis
Data are shown as means plus or minus SD and were analyzed by the Mann-Whitney test, with P values less than .05 being considered significant.
Results
Ovarian cancer ascites switch monocyte differentiation into TAM-like cells
In an attempt to determine how the tumor environment might influence TAM generation, we first compared the phenotypes of CD14+ tumor MΦs isolated from ovarian cancer ascites (TAMs) and healthy donor monocytes cultured without (MΦs) or with ovarian tumor ascites (TA-MΦs). Under these 3 conditions, differentiated cells exhibited MΦ characteristics (expressed CD14 and CD163) but not DC features (CD1a− and no CD83 neoexpression after LPS stimulation; Table 1). Compared with MΦs, TAMs and TA-MΦs expressed more CD14 and CD163 (Figure 1A), and the inhibitory receptors, Ig-like transcript 2 (ILT2) and ILT3 (Table 1). Expressions of the costimulatory molecules CD80 and CD86 (Table 1) and of B7-H1, CD54, MHC I, and MHC II (data not shown) were similar on MΦs, TAMs, and TA-MΦs.
Analysis of cell surface markers on macrophage subsets
| Markers . | MΦs . | TAMs . | TA-MΦs . | LIF-MΦs . | IL-6-MΦs . | M-CSF-MΦs . |
|---|---|---|---|---|---|---|
| CD14 | +++ | +++++ | +++++ | +++++ | +++++ | ++++ |
| CD163 | + | ++++ | ++++ | ++++ | ++++ | ++ |
| ILT2 | + | ++ | ++ | ++ | ++ | + |
| ILT2* | + | ++ | ++ | ++ | ++ | + |
| ILT3 | + | ++ | ++ | ++ | ++ | + |
| ILT3* | ++ | +++ | +++ | +++ | +++ | ++ |
| CD80* | ++ | +/− | + | + | + | + |
| CD86* | +++ | +/− | +/− | +/− | +/− | + |
| Markers . | MΦs . | TAMs . | TA-MΦs . | LIF-MΦs . | IL-6-MΦs . | M-CSF-MΦs . |
|---|---|---|---|---|---|---|
| CD14 | +++ | +++++ | +++++ | +++++ | +++++ | ++++ |
| CD163 | + | ++++ | ++++ | ++++ | ++++ | ++ |
| ILT2 | + | ++ | ++ | ++ | ++ | + |
| ILT2* | + | ++ | ++ | ++ | ++ | + |
| ILT3 | + | ++ | ++ | ++ | ++ | + |
| ILT3* | ++ | +++ | +++ | +++ | +++ | ++ |
| CD80* | ++ | +/− | + | + | + | + |
| CD86* | +++ | +/− | +/− | +/− | +/− | + |
The expression of the indicated markers was analyzed by FACS on Mϕs isolated from ovarian tumor ascites and on monocytes cultured for 5 days without (MΦs) or with ovarian tumor ascites (TA-MΦs), IL-6 (IL-6-MΦs), LIF (LIF-MΦs), or M-CSF (M-CSF-MΦs). Results are expressed as mean fluorescence intensity (MFI) and are representative of 5 separate experiments. For CD1a and CD83*, MFI was not detected in any of the subsets. For CD80 and CD86, MFI was less than 10.
+++ indicates MFI greater than 60 and less than 400; +++++, MFI greater than 1000; ++++, MFI greater than 400 and less than 1000; +, MFI greater than 10 and less than 30; ++, MFI greater than 30 and less than 60; −, MFI not detected; and +/−, MFI less than 10.
Cells were further stimulated for 48 hours with LPS before FACS analysis.
TA-MΦs exhibit ovarian TAM phenotypic characteristics. (A) Analysis of CD14 and CD163 expression on freshly isolated ovarian tumor CD14+ cells (TAMs) and on healthy donor monocytes cultured for 5 days in CM without (MΦs) or with ovarian tumor ascites (TA-MΦs), or with the SNs of the 5637, A172, or HepG2 tumor-cell lines (SN-MΦs). (B) MΦs, TAMs, TA-MΦs, and SN-MΦs were stimulated for 48 hours with LPS before FACS analysis of CD86 and ILT3 expressions. (C) TAMs, MΦs, TA-MΦs, and SN-MΦs were stimulated for 48 hours with LPS before IL-10 and IL-12 quantification. Results are expressed in MFI (A,B) or in ng/mL or pg/mL (C), as means plus or minus SD of experiments performed with TAMs from 10 patients, or experiments performed with monocytes from 4 healthy donors treated either with 4 different ascites fluids or with tumor-cell supernatants. (D) MΦs, TAMs, and TA-MΦs were activated for 48 hours with LPS and incubated, in graded doses, with allogenic CD4+ T cells. (E) MΦs, TAMs, TA-MΦs, and DCs were stimulated for 48 hours with LPS and incubated with allogenic CD4+ T cells plus anti-CD3 mAb and IL-2. (D,E) [3H]-thymidine incorporation was measured on day 4. Results are expressed in cpm (D) or in variation of T-cell proliferation (E) as means plus or minus SD of experiments realized with TAMs and ascites of 5 ovarian cancer patients. *P < .05.
TA-MΦs exhibit ovarian TAM phenotypic characteristics. (A) Analysis of CD14 and CD163 expression on freshly isolated ovarian tumor CD14+ cells (TAMs) and on healthy donor monocytes cultured for 5 days in CM without (MΦs) or with ovarian tumor ascites (TA-MΦs), or with the SNs of the 5637, A172, or HepG2 tumor-cell lines (SN-MΦs). (B) MΦs, TAMs, TA-MΦs, and SN-MΦs were stimulated for 48 hours with LPS before FACS analysis of CD86 and ILT3 expressions. (C) TAMs, MΦs, TA-MΦs, and SN-MΦs were stimulated for 48 hours with LPS before IL-10 and IL-12 quantification. Results are expressed in MFI (A,B) or in ng/mL or pg/mL (C), as means plus or minus SD of experiments performed with TAMs from 10 patients, or experiments performed with monocytes from 4 healthy donors treated either with 4 different ascites fluids or with tumor-cell supernatants. (D) MΦs, TAMs, and TA-MΦs were activated for 48 hours with LPS and incubated, in graded doses, with allogenic CD4+ T cells. (E) MΦs, TAMs, TA-MΦs, and DCs were stimulated for 48 hours with LPS and incubated with allogenic CD4+ T cells plus anti-CD3 mAb and IL-2. (D,E) [3H]-thymidine incorporation was measured on day 4. Results are expressed in cpm (D) or in variation of T-cell proliferation (E) as means plus or minus SD of experiments realized with TAMs and ascites of 5 ovarian cancer patients. *P < .05.
We then compared the phenotypes of these 3 cell types in response to LPS stimulation. TAMs and TA-MΦs maintained higher levels of CD14, CD163 (data not shown), ILT2 (Table 1), and ILT3 (Table 1; Figure 1B) expression than MΦs. Surprisingly, while LPS poorly affected TAM and TA-MΦ CD80 and CD86 expression (Table 1), it induced a potent up-regulation of CD80 (Table 1) and CD86 expression on MΦs (Figure 1B). In response to LPS, expressions of B7-H1, CD54, MHC I, and MHC II were similar on MΦs, TAMs, and TA-MΦs (data not shown). As previously described,12 TAMs had an IL-10highIL-12low M2 phenotype (Figure 1C). Pertinently, LPS-stimulated TA-MΦs produced higher levels of IL-10 than MΦs and weak or undetectable levels of IL-12, typical of M2 polarization (Figure 1C). Lastly, TA-MΦs and, as expected, TAMs10,15,26 had poorer T-cell costimulatory properties (MLR assay; Figure 1D) and suppressed TCR-dependent T-cell proliferation more effectively than MΦs (Figure 1E; as positive control, mature CD83+ DCs up-regulated T-cell proliferation). Together, these data suggest that soluble mediators present in ovarian cancer ascites fluids induce monocyte differentiation into immunosuppressive MΦs.
We then analyzed the effects of cell-free supernatants (SNs) from different tumor-cell lines (urinary bladder carcinoma 5637 [5637], glioblastoma A172 [A172], and hepatocellular carcinoma HepG2 [HepG2]) on monocyte differentiation. SNs from all 3 cell lines also skewed monocyte differentiation into CD14highCD163high (Figure 1A)–, ILT3highCD86low (Figure 1B)–, ILT2high (data not shown)–, and IL-10highIL-12low (Figure 1C)–polarized M2 TAM-like cells. As control, the SNs of primary cultured human epithelial cells (keratinocytes) had no effect on monocyte differentiation into MΦs (data not shown).
These data demonstrate strong CD14 and CD163 expression and imbalanced costimulatory CD86 versus inhibitory ILT2 and ILT3 molecule expression, as phenotypic characteristics of ovarian TAMs. They also show that soluble factors, present in ovarian cancer ascites fluids or secreted by some tumor-cell lines, skew monocyte differentiation into M2 immunosuppressive cells exhibiting several TAM phenotypic and functional characteristics.
Ovarian TAMs and TA-MΦs define a novel subset of M2 cells, distinct from M2a-c
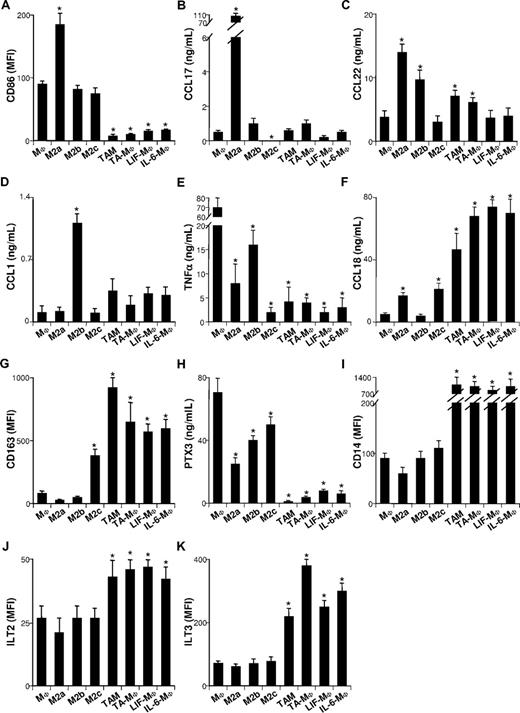
Three M2 cell subsets, M2a, M2b, and M2c, elicited by IL-4 (or IL-13), IL-1, and IL-10, respectively, have been described.5 They exhibit general M2 characteristics, some of which are more or less accentuated. It has been hypothesized that these cytokines could be involved in TAM generation.24,–26 To test this hypothesis, we quantified IL-4, IL-13, IL-1β, and IL-10 in ovarian tumor ascites (Figure 2A) and tumor-cell line SNs (Figure 2B). IL-4 and IL-13 were undetectable, while IL-1β and IL-10 were either undetectable or present at low concentrations (< 0.04 ng/mL and < 0.2 ng/mL, respectively), suggesting that they are not the main factor(s) involved in TAM polarization. To verify this assumption, we compared the phenotypes of freshly isolated TAMs to in vitro–generated TA-MΦs, M2a's, M2b's, and M2c's. As expected,5 M2a's expressed high levels of CD86 (Figure 3A) and were the main CCL17 and CCL22 producers (Figure 3B,C). M2b's were characterized by a CCL1highTNFαhigh phenotype (Figure 3D,E); they synthesized less CCL18 than M2a's and M2c's (Figure 3F).5 M2c's expressed high levels of CD163 (Figure 3G) and synthesized the antiangiogenic factor long pentraxin 3 (PTX3; Figure 3H).5 Notably, ovarian TAMs and TA-MΦs expressed less CD86 (Figure 3A) and produced less CCL17 (Figure 3B) and CCL22 (Figure 3C) than M2a's. Unlike M2b's, ovarian TAMs4,16,30 and TA-MΦs secreted high CCL18 levels (Figure 3F) but low levels of CCL1 (Figure 3D) and TNFα (Figure 3E). Finally, in contrast to M2a-c subsets, ovarian TAMs and TA-MΦs were charac-terized by low-level secretion of PTX3 (Figure 3H) and a CD14highILT2highILT3high phenotype (Figure 3I-K).
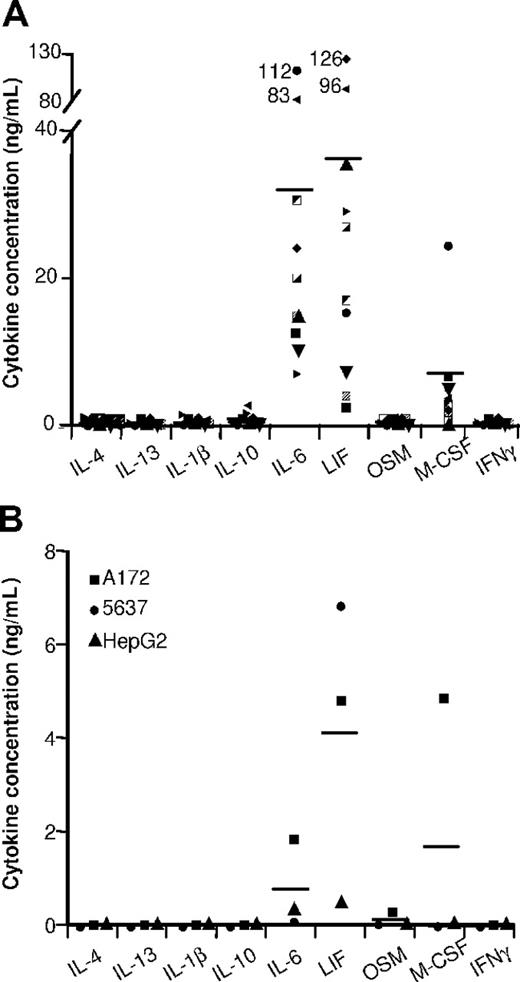
Cytokine contents in ovarian tumor ascites and in tumor-cell SNs. The indicated cytokines were quantified by ELISA in (A) 10 ascites and in (B) the SNs of the indicated tumor-cell lines. Results are expressed in ng/mL. Horizontal lines are the mean cytokine concentrations measured.
Cytokine contents in ovarian tumor ascites and in tumor-cell SNs. The indicated cytokines were quantified by ELISA in (A) 10 ascites and in (B) the SNs of the indicated tumor-cell lines. Results are expressed in ng/mL. Horizontal lines are the mean cytokine concentrations measured.
TAMs, TA-MΦs, LIF-MΦs, and IL-6-MΦs differ from M2a-c. Monocytes cultured for 5 days in CM were not (MΦs) or were exposed for 48 hours to IL-4 (M2a's), IL-1β (M2b's), or IL-10 (M2c's) or were cultured for 5 days in CM supplemented with ascites (TA-MΦs), LIF (LIF-MΦs), or IL-6 (IL-6-MΦs). CD86 (A), CD163 (G), CD14 (I), ILT2 (J), and ILT3 (K) expression was analyzed by FACS prior to (G,I) or after (A,J,K) 48 hours of LPS stimulation. CCL17 (B), CCL22 (C), CCL1 (D), TNFα (E), CCL18 (F), and PTX3 (H) were quantified by ELISA in the SNs of cells either unstimulated (B,C) or stimulated for 48 hours with LPS (D-F,H). Results are expressed in MFI (A,G,I-K) or in ng/mL (B-F,H). (A-K) Results are expressed as means plus or minus SD of data obtained with monocytes from 4 subjects (MΦs, M2a-c, LIF-MΦs, and IL-6-MΦs) or with 6 ovarian cancer patients (for TAMs and TA-MΦs). *P < .05 (considered significantly different from results obtained for MΦs).
TAMs, TA-MΦs, LIF-MΦs, and IL-6-MΦs differ from M2a-c. Monocytes cultured for 5 days in CM were not (MΦs) or were exposed for 48 hours to IL-4 (M2a's), IL-1β (M2b's), or IL-10 (M2c's) or were cultured for 5 days in CM supplemented with ascites (TA-MΦs), LIF (LIF-MΦs), or IL-6 (IL-6-MΦs). CD86 (A), CD163 (G), CD14 (I), ILT2 (J), and ILT3 (K) expression was analyzed by FACS prior to (G,I) or after (A,J,K) 48 hours of LPS stimulation. CCL17 (B), CCL22 (C), CCL1 (D), TNFα (E), CCL18 (F), and PTX3 (H) were quantified by ELISA in the SNs of cells either unstimulated (B,C) or stimulated for 48 hours with LPS (D-F,H). Results are expressed in MFI (A,G,I-K) or in ng/mL (B-F,H). (A-K) Results are expressed as means plus or minus SD of data obtained with monocytes from 4 subjects (MΦs, M2a-c, LIF-MΦs, and IL-6-MΦs) or with 6 ovarian cancer patients (for TAMs and TA-MΦs). *P < .05 (considered significantly different from results obtained for MΦs).
In summary, TAMs and TA-MΦs exhibited a CD14highCD163highCD86lowILT2highILT3high phenotype and produced high CCL18 levels but low levels of CCL1, CCL17, CCL22, TNFα, and PTX3. These data indicate that ovarian TAMs and TA-MΦs bear a unique M2-skewed myeloid suppressor phenotype and suggest that the TAM generation could be dependent on factor(s) distinct from M2a-c–polarizing cytokines.
LIF, IL-6, and oncostatin M (OSM) skew monocyte differentiation into a novel M2 subset with TAM characteristics
Ovarian tumor ascites (Figure 2A) and tumor-cell SNs (Figure 2B) contained high LIF and IL-6 concentrations. These cytokines were undetectable in primary epithelial cell SNs (which had no effect on monocyte differentiation into MΦs; data not shown). We therefore analyzed whether IL-6 and LIF might affect monocyte differentiation. LIF or IL-6 induced monocyte differentiation into MΦs expressing high CD14 (Figure 3I) and CD163 (Figure 3G) levels but not CD1a or CD83 after LPS stimulation (Table 1). These cells are called LIF-MΦs and IL-6-MΦs. LIF-MΦs and IL-6-MΦs were IL-10highIL-12low–polarized M2s (Figure 4A,B). Compared with MΦs, they presented increased IL-10 mRNA expression and decreased IL-12p35 and IL-23p19 mRNA after LPS stimulation (Figure 4C), while IL-12/IL-23p40 mRNA was undetectable (data not shown). Like TAMs, LIF-MΦs and IL-6-MΦs exhibited the CD14highCD163highCD80lowCD86lowILT2highILT3high phenotype and produced high CCL18 levels and low CCL1, CCL17, CCL22, TNFα, and PTX3 levels (Table 1; Figure 3). Lastly, LIF-MΦs and IL-6-MΦs had poor T-cell costimulatory properties (Figure 4D) and suppressed T-cell proliferation more efficiently than MΦs (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article; as positive control, DCs stimulated T-cell proliferation). Finally, LIF-MΦs, IL-6-MΦs, TA-MΦs, and TAMs induced T-cell apoptosis (Figure 4E).
LIF and IL-6 generate M2d TAM-like cells. (A,B,G,H) MΦs, ovarian TAMs, LIF-MΦs, and IL-6-MΦs were stimulated with LPS for 48 hours and IL-10, IL-12, VEGF, and TGFβ were measured by ELISA in the SNs. Results are expressed as means ± SD; n = 6. (C) MMP9, PDGFA, PDGFB, CCL2, CCL5, IL-10, IL-12p35, and IL-23p19 mRNA expression was analyzed by Q-PCR in MΦs, LIF-MΦs, and IL-6-MΦs. a indicates that cells were further stimulated for 16 hours with LPS. Results are expressed as the fold increase of mRNA expression in LIF-MΦs or IL-6-MΦs compared with MΦs (mean ± SD; n = 4). (D) MΦs, LIF-MΦs, or IL-6-MΦs were activated for 48 hours with LPS with allogenic CD4+ T cells. [3H]-thymidine incorporation was measured at day 4. Results are expressed in cpm (mean ± SD; n = 4). (E,F) LIF-MΦs, IL-6-MΦs, TA-MΦs, and TAMs were activated for 48 hours with LPS and cocultured with CFDA-SE–labeled CD4+ T cells stimulated with anti-CD3 mAb plus IL-2 in the presence or absence of 1-MT. At day 5, cells were stained with 7-AAD and annexin-V, and the percentage of living cells was determined by FACS (mean ± SD; n = 4) (E). Proliferation of living cells was evaluated by CFDA-SE dilution measured by FACS. Results are expressed as a percentage of cells in each cycle (mean ± SD; n = 3) (F). (I) PCR analysis of B7-H4 in expression of MΦs, IL-6-MΦs, LIF-MΦs, and TAMs. Result is representative of 1 of 3 experiments. *P < .05.
LIF and IL-6 generate M2d TAM-like cells. (A,B,G,H) MΦs, ovarian TAMs, LIF-MΦs, and IL-6-MΦs were stimulated with LPS for 48 hours and IL-10, IL-12, VEGF, and TGFβ were measured by ELISA in the SNs. Results are expressed as means ± SD; n = 6. (C) MMP9, PDGFA, PDGFB, CCL2, CCL5, IL-10, IL-12p35, and IL-23p19 mRNA expression was analyzed by Q-PCR in MΦs, LIF-MΦs, and IL-6-MΦs. a indicates that cells were further stimulated for 16 hours with LPS. Results are expressed as the fold increase of mRNA expression in LIF-MΦs or IL-6-MΦs compared with MΦs (mean ± SD; n = 4). (D) MΦs, LIF-MΦs, or IL-6-MΦs were activated for 48 hours with LPS with allogenic CD4+ T cells. [3H]-thymidine incorporation was measured at day 4. Results are expressed in cpm (mean ± SD; n = 4). (E,F) LIF-MΦs, IL-6-MΦs, TA-MΦs, and TAMs were activated for 48 hours with LPS and cocultured with CFDA-SE–labeled CD4+ T cells stimulated with anti-CD3 mAb plus IL-2 in the presence or absence of 1-MT. At day 5, cells were stained with 7-AAD and annexin-V, and the percentage of living cells was determined by FACS (mean ± SD; n = 4) (E). Proliferation of living cells was evaluated by CFDA-SE dilution measured by FACS. Results are expressed as a percentage of cells in each cycle (mean ± SD; n = 3) (F). (I) PCR analysis of B7-H4 in expression of MΦs, IL-6-MΦs, LIF-MΦs, and TAMs. Result is representative of 1 of 3 experiments. *P < .05.
We then evaluated whether LIF-MΦs and IL-6-MΦs expressed factors involved in immunosuppressive (eg, iNOS, Arg1, indoleamine 2,3-dioxygenase or IDO, TGFβ, and B7-H4) or trophic (eg, VEGF, PDGF, MMP9, CCL2) properties of TAMs.16,26,31,,,–35 IDO mRNA expression was detected by quantitative PCR (Q-PCR) in LIF-MΦs, IL-6-MΦs, TA-MΦs, and TAM and the expression of the protein was evidenced by Western blotting (Figure S2 and data not shown). The IDO inhibitor 1-MT enhanced the percentage of living T cells (measured by 7-AAD and annexin-V labeling; Figure 4E) and restored the proliferation of T cells cultured with LIF-MΦs, IL-6-MΦs, TA-MΦs, and TAMs (as assessed by CFSE-DA–labeling dilution in living cells) (Figures 4F and S3). In contrast, we failed in detecting Arg1 expression in the macrophage subsets tested (data not shown). iNOS was not expressed in LIF-MΦs, IL-6-MΦs, or TA-MΦs and was not expressed, or expressed at a low level, in TAMs depending on the donor (data not shown). In agreement with the absence of iNOS and Arg1 expression, L-NMMA (iNOS inhibitor) and nor-NOHA (Arg1 inhibitor), used alone or in combination, did not affect the immunosuppressive properties of the different macrophage subsets (data not shown).
Moreover, LIF-MΦs and IL-6-MΦs produced higher levels of VEGF and TGFβ (Figure 4G,H) and expressed higher levels of MMP9, PDGFA, PDGFB, CCL2 (Figure 4C), and B7-H4 mRNA (Figure 4I) than MΦs.
Similar results were obtained when IL-6 and/or LIF are used alone or in combination during the differentiation process (data not shown).
Lastly, in support of data obtained with murine TAMs,36 in the absence of detectable IFNγ in ovarian ascites (Figure 2A), LIF-MΦs and IL-6-MΦs also expressed IFNγ-inducible chemokines; they constitutively expressed CCL5 mRNA (Figure 4C) and produced CXCL10 and CXCL16 (data not shown). These data show that LIF-MΦs and IL-6-MΦs exhibit a phenotype close to those of TA-MΦs and ovarian TAMs.
LIF is a member of the IL-6 cytokine family that also includes oncostatin M (OSM), IL-11, and cardiotrophin-1. OSM induced monocyte differentiation into MΦs with a phenotype similar to those of LIF-MΦs and IL-6-MΦs (data not shown) but was either undetectable or weakly present (< 0.5 ng/mL) in ovarian cancer ascites and tumor-cell SNs (Figure 2). We detected no effect of IL-11 and cardiotrophin-1 on monocyte differentiation (data not shown). Lastly, because TGFβ was present in ovarian ascites (3 ± 1 ng/mL; mean ± SD; n = 10), we analyzed its effect on monocyte differentiation. TGFβ induced monocyte differentiation into CD14lowCD163lowILT3low unpolarized MΦs (data not shown), excluding a major role in TAM generation.
These data demonstrate that LIF, IL-6, and OSM skew monocyte differentiation into a novel M2 subset that exhibits most ovarian TAM phenotypic and functional characteristics. We propose calling this novel subset M2d.
IL-6 induces M2d generation through autocrine M-CSF consumption
In kinetic experiments, LIF and IL-6 skewed monocyte differentiation into M2d's only when added early during the differentiation process and had no effect on established MΦs (data not shown). Chomarat et al reported that IL-6 switched monocyte differentiation to MΦs rather than DCs by allowing monocytes to consume their autocrine M-CSF.18 Therefore, we evaluated the role of M-CSF in IL-6-MΦ generation. IL-6 decreased, in a dose-dependent manner, M-CSF concentrations in monocyte culture SNs (Figure 5A,B) but did not modulate M-CSF mRNA expression (Figure 5C [16 hours] and data not shown). These observations suggested that IL-6 might up-regulate autocrine M-CSF consumption by differentiating monocytes. Supporting that notion, a neutralizing anti–M-CSF mAb added at day 0, together with IL-6, prevented the generation of IL-6-MΦs as assessed by the restoration of CD86 expression (Figure 5D), IL-12 production (Figure 5E), and the costimulatory properties (Figure 5F) of differentiated cells in response to LPS. Similar data were obtained using a neutralizing anti–M-CSF-R mAb (data not shown). Moreover, the expression of M-CSF-R on monocytes was significantly reduced in response to IL-6 (during the first 2 days; Figure 5G,H), while total M-CSF-R expression (analyzed after cell permeabilization) and M-CSF mRNA expression were not modulated, regardless of the time analyzed (from 4 hours to day 3; Figure 5C and data not shown). These last observations support a role for IL-6 in M-CSF consumption through M-CSF receptor internalization. Lastly, the expression of LIF and OSM mRNA remained unchanged at all time points analyzed during monocyte differentiation with or without IL-6 (Figure 5C at 16 hours and data not shown), and LIF and OSM remained undetectable in the SNs (data not shown). Together, these data show that IL-6 triggers M2d generation by enabling autocrine M-CSF consumption by monocytes.
Involvement of autocrine M-CSF consumption in M2d generation. (A) At the indicated times, M-CSF was quantified in the SNs of monocytes cultured in CM without or with LIF, IL-6, or OSM. Results are expressed in ng/mL as means plus or minus SD; n = 4. (B) At day 2, M-CSF was quantified in the SNs of monocytes activated with 10 to 100 ng/mL LIF or IL-6. Results are expressed in ng/mL as mean plus or minus SD; n = 4. (C) PCR analysis of M-CSF, M-CSF-R, IL-6, LIF, and OSM mRNA expression in monocytes cultured for 16 hours in CM without or with IL-6, LIF, or OSM. Results are representative of 1 of 3 experiments. (D-F) Monocytes were cultured in CM without or with IL-6, LIF, or M-CSF without or with neutralizing anti–M-CSF and/or anti–IL-6 mAbs or control mAbs. On day 5, LPS was added and CD86 expression (D) and IL-12 production (E) were analyzed 48 hours later. After LPS stimulation, macrophages were cultured with allogenic CD4+ T cells, and [3H]-thymidine incorporation was measured at day 4 (F). Results are expressed as the percentages of restoration (mean ± SD; n = 4). (G) Monocytes were cultured in CM without (dotted line) or with (full line) IL-6, and M-CSF-R expression was analyzed by FACS after 24 hours. Results are representative of 1 of 4 experiments. Shaded areas correspond to the control mAb. (H) Monocytes were cultured in CM without or with LIF or IL-6, and M-CSF-R expression was analyzed by FACS after 24 and 48 hours. Results are expressed in MFI (mean ± SD; n = 4). (I) At the indicated times, IL-6 was quantified in the SNs of monocytes maintained in CM without or with LIF, OSM, or M-CSF. Results are expressed in ng/mL as means plus or minus SD; n = 4. *P < .05.
Involvement of autocrine M-CSF consumption in M2d generation. (A) At the indicated times, M-CSF was quantified in the SNs of monocytes cultured in CM without or with LIF, IL-6, or OSM. Results are expressed in ng/mL as means plus or minus SD; n = 4. (B) At day 2, M-CSF was quantified in the SNs of monocytes activated with 10 to 100 ng/mL LIF or IL-6. Results are expressed in ng/mL as mean plus or minus SD; n = 4. (C) PCR analysis of M-CSF, M-CSF-R, IL-6, LIF, and OSM mRNA expression in monocytes cultured for 16 hours in CM without or with IL-6, LIF, or OSM. Results are representative of 1 of 3 experiments. (D-F) Monocytes were cultured in CM without or with IL-6, LIF, or M-CSF without or with neutralizing anti–M-CSF and/or anti–IL-6 mAbs or control mAbs. On day 5, LPS was added and CD86 expression (D) and IL-12 production (E) were analyzed 48 hours later. After LPS stimulation, macrophages were cultured with allogenic CD4+ T cells, and [3H]-thymidine incorporation was measured at day 4 (F). Results are expressed as the percentages of restoration (mean ± SD; n = 4). (G) Monocytes were cultured in CM without (dotted line) or with (full line) IL-6, and M-CSF-R expression was analyzed by FACS after 24 hours. Results are representative of 1 of 4 experiments. Shaded areas correspond to the control mAb. (H) Monocytes were cultured in CM without or with LIF or IL-6, and M-CSF-R expression was analyzed by FACS after 24 and 48 hours. Results are expressed in MFI (mean ± SD; n = 4). (I) At the indicated times, IL-6 was quantified in the SNs of monocytes maintained in CM without or with LIF, OSM, or M-CSF. Results are expressed in ng/mL as means plus or minus SD; n = 4. *P < .05.
LIF and OSM induce M2d's through an autocrine IL-6/M-CSF loop
LIF and OSM also decreased, in a dose-dependent manner, M-CSF concentrations in monocyte culture SNs (Figure 5A,B and data not shown) but did not modulate M-CSF mRNA expression (Figure 5C). In parallel, a significant decrease of M-CSF-R expression was observed on monocytes exposed to LIF or OSM (during the first 2 days; Figure 5H and data not shown), while M-CSF-R mRNA expression was not affected (Figure 5C). Finally, the addition at day 0 of a neutralizing anti–M-CSF mAb totally restored CD86 expression, IL-12 production, and the costimulatory properties of MΦs generated in the presence of LIF or OSM (Figure 5D-F and data not shown). These data suggested that LIF and OSM may favor the differentiation of monocytes into macrophages by allowing M-CSF consumption, as reported for IL-6.18
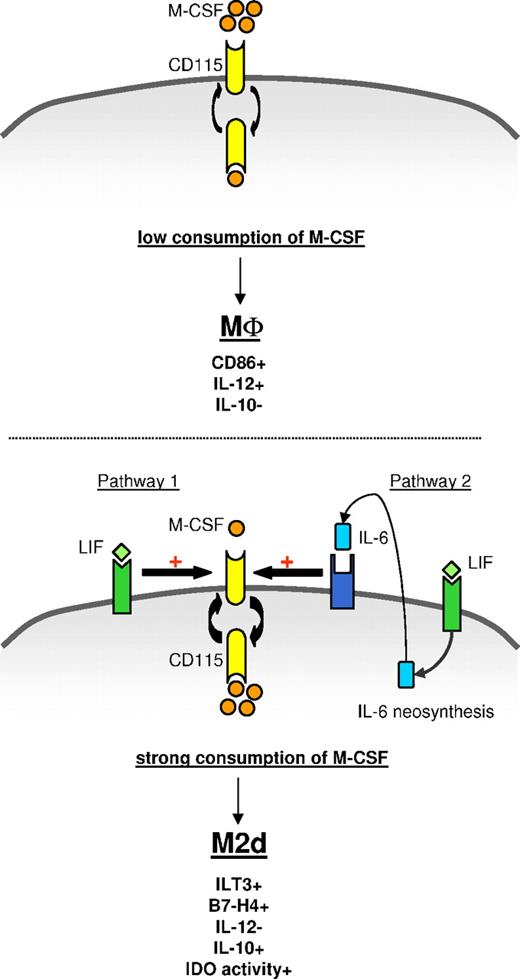
In agreement with previous studies showing that LIF or OSM induce IL-6 production by monocytes,37,,,,–42 LIF and OSM up-regulated monocyte IL-6 production with a peak at day 1 (Figure 5C,I). We then evaluated whether LIF and OSM may also act on monocyte differentiation by inducing an autocrine consumption of IL-6. A neutralizing anti–IL-6 mAb partly, but significantly, restored CD86 and IL-12 expression and the costimulatory properties of LIF-MΦs (Figure 5D-F). The concomitant addition of a neutralizing anti–M-CSF mAb to a neutralizing anti–IL-6 mAb totally restored CD86 and IL-12 expression (Figure 5D,E) and strongly restored the costimulatory properties of LIF-MΦs (Figure 5F). Similar data were obtained in the presence of OSM (data not shown). Collectively, these results show that LIF and/or OSM act on monocytes, at least in part, in an IL-6–dependent manner. Lastly, LIF did not modulate OSM expression and OSM did not affect LIF expression (Figure 5C). These observations indicate that LIF and OSM induce monocyte differentiation into M2d's through an autocrine IL-6/M-CSF loop, as represented in a schematic model (Figure 6).
Model of M2d generation by LIF through an autocrine IL-6/M-CSF loop. (Top panel) In the absence of exogenous LIF or IL-6, monocytes fail to efficiently consume autocrine M-CSF and differentiate into CD86+IL-12+IL-10− MΦs. (Bottom panel) Exogenous LIF induces secretion and autocrine consumption of IL-6. Exogenous LIF (pathway 1) and endogenous IL-6 (pathway 2) increase M-CSF-R turnover and facilitate M-CSF consumption, leading to ILT3+B7-H4+IL-12−IL-10+IDO+ M2d generation.
Model of M2d generation by LIF through an autocrine IL-6/M-CSF loop. (Top panel) In the absence of exogenous LIF or IL-6, monocytes fail to efficiently consume autocrine M-CSF and differentiate into CD86+IL-12+IL-10− MΦs. (Bottom panel) Exogenous LIF induces secretion and autocrine consumption of IL-6. Exogenous LIF (pathway 1) and endogenous IL-6 (pathway 2) increase M-CSF-R turnover and facilitate M-CSF consumption, leading to ILT3+B7-H4+IL-12−IL-10+IDO+ M2d generation.
It has been recently reported that M-CSF gives rise to M2-polarized MΦs.43 Because our findings supported a role of autocrine M-CSF in LIF, IL-6, and OSM-MΦ generation, we compared these M2d phenotypes to monocytes differentiated in the presence of rM-CSF (M-CSF-MΦs; Table 1). Regardless of rM-CSF concentration used (20-200 ng/mL), M-CSF-MΦs presented a light M2d phenotype, with a CD14highCD86lowILT3highIL-12lowIL10high profile that was less pronounced than for LIF-MΦs, IL-6-MΦs, and ovarian TAMs (Table 1 and data not shown). M-CSF-MΦs were also less potent than M2d's and TAMs at inhibiting T-cell proliferation (data not shown). M-CSF slightly up-regulated IL-6 production (Figure 5I) and IL-6 mRNA expression but did not induce LIF or OSM production or mRNA expression, at any time point analyzed (from 4 hours to day 3; data not shown). This limited autocrine production of IL-6 by M-CSF might help to explain the light M2d phenotype of M-CSF-MΦs. It is further supported by the observation that a neutralizing anti–IL-6 mAb restored CD86 and IL-12 expression and the costimulatory properties of M-CSF-MΦs (Figure 5D-F).
These data demonstrate that the presence of LIF, IL-6, and OSM during monocyte differentiation acts as a rheostat to control the degree of M-CSF consumption and thereby regulates engagement toward an M2d differentiation pathway.
LIF, IL-6, and M-CSF present in tumor microenvironment act in concert to generate TAM-like cells
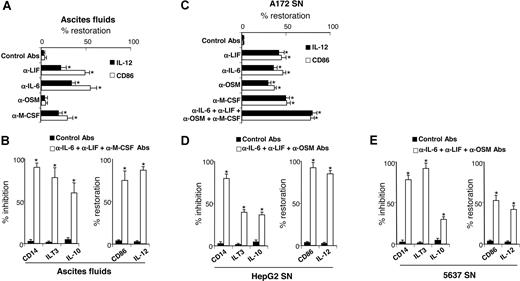
Our results showed that ovarian cancer ascites and tumor-cell line SNs skewed monocyte differentiation into TAM-like cells and contained high LIF and IL-6 levels, and that M-CSF was also present in ascites and tumor-cell SNs. Therefore, we evaluated the contribution of LIF, IL-6, and M-CSF present in ascites to TAM generation. Depletion of all 3 cytokines from ascites partly restored the capacity of differentiated MΦs to express IL-12 and CD86 (Figure 7A). OSM depletion did not prevent ascites-induced M2d generation consistent with undetectable or low OSM concentrations in ascites (< 0.5 ng/mL; Figure 2A). The concomitant depletions of IL-6, LIF, and M-CSF from ascites almost completely reversed the M2d phenotype (Figure 7B); differentiated cells retained a phenotype close to MΦs, based on CD14, ILT3, and CD86 cell-surface expression and IL-10 and IL-12 secretion (Figure 7B). Because LIF, IL-6, OSM, and M-CSF are also present in A172 SNs (Figure 2B), their individual or combined depletion from A172 SNs significantly restored the capacity of A172 SN-MΦs to express IL-12 and CD86 and to enable unpolarized MΦ differentiation, respectively (Figure 7C). Lastly, combined depletion of IL-6, LIF, and OSM in HepG2 SNs (Figure 7D) and in 5637 SNs (Figure 7E) (which did not contain detectable M-CSF levels; Figure 2B) also skewed monocyte differentiation into MΦs that are able to produce IL-12 and to express CD86 and that express less CD14, ILT3, and IL-10 than MΦs generated with undepleted SNs.
LIF, IL-6, and M-CSF depletion prevents M2d generation. (A,C) Monocytes were cultured for 5 days in CM with ascites (A) or A172 SNs (C) either concomitantly depleted or not (control mAbs) of LIF, IL-6, OSM, and/or M-CSF. After 48 hours of LPS stimulation, IL-12 production and CD86 expression were analyzed. Results are expressed in percentages of restoration as means plus or minus SD; n = 6 (in panel A, experiments were performed with 6 ascites). (B,D,E) Monocytes were cultured for 5 days in CM without or with ascites (B), HepG2 SNs (D), or 5637 SNs (E) depleted or not. CD14 expression was analyzed by FACS on day 5. IL-10 and IL-12 production and ILT3 and CD86 expression were analyzed after 48 hours of LPS stimulation. (B,D,E) Left panels represent markers whose expressions are inhibited compared with M2d's; results are expressed in percentages of inhibition of marker expression. Right panels represent markers whose expressions are increased compared with M2d's; results are expressed in percentages of restoration of marker expression. (B,D,E) Results are expressed as means plus or minus SD; n = 6 (in panel B, experiments were performed with 6 ascites). *P < .05.
LIF, IL-6, and M-CSF depletion prevents M2d generation. (A,C) Monocytes were cultured for 5 days in CM with ascites (A) or A172 SNs (C) either concomitantly depleted or not (control mAbs) of LIF, IL-6, OSM, and/or M-CSF. After 48 hours of LPS stimulation, IL-12 production and CD86 expression were analyzed. Results are expressed in percentages of restoration as means plus or minus SD; n = 6 (in panel A, experiments were performed with 6 ascites). (B,D,E) Monocytes were cultured for 5 days in CM without or with ascites (B), HepG2 SNs (D), or 5637 SNs (E) depleted or not. CD14 expression was analyzed by FACS on day 5. IL-10 and IL-12 production and ILT3 and CD86 expression were analyzed after 48 hours of LPS stimulation. (B,D,E) Left panels represent markers whose expressions are inhibited compared with M2d's; results are expressed in percentages of inhibition of marker expression. Right panels represent markers whose expressions are increased compared with M2d's; results are expressed in percentages of restoration of marker expression. (B,D,E) Results are expressed as means plus or minus SD; n = 6 (in panel B, experiments were performed with 6 ascites). *P < .05.
In summary, LIF and IL-6 present in the tumor microenvironment act in concert with M-CSF to induce the TAM-like cell generation.
Discussion
In this study, we identify one of the missing puzzle pieces in the process leading to TAM generation. We demonstrate herein that LIF and IL-6, present in the tumor microenvironment, induce monocyte differentiation into TAM-like cells by enabling monocytes to consume autocrine/paracrine M-CSF. We also show that ovarian TAMs, IL-6-MΦs, and LIF-MΦs represent a novel subset of MΦs, distinct from M2a-c, that we called M2d. These findings offer novel perspectives to increase the efficacy of T-cell–based cancer vaccines by subverting TAM-induced immunosuppression.
We observed that M2d's, like TAMs, presented immunosuppressive properties. T-cell suppression by TAMs and MDSCs, a heterogeneous myeloid cell populations (ie, immature MΦs, DCs, granulocytes, or other myeloid cells at early stages of differentiation),35,44,–46 can be mediated by different mechanisms involving soluble factors (IL-10 and TGFβ; and IDO, iNOS, and Arg1 metabolites)32,46,–48 and membrane molecules (B7-H4, CD80).26,49 In humans, MDSCs are not fully characterized, except in immature myeloid cells that express CD33 but lack expression of MHC-II and markers of mature myeloid cells.46,50 These cells inhibit IFNγ production by tumor antigen–specific T-cells.46,50
Our results showed that M2d's and TAMs expressed IDO and that the specific IDO inhibitor 1-MT restored T-cell proliferation. IDO leads to the formation of tryptophan metabolites that suppress T-cell proliferation and cause T-cell apoptosis.51,52 IDO+ DCs from tumor-draining lymph nodes suppress T cells.47 In contrast, we failed to detect Arg1 and iNOS expression in the macrophage subsets tested and, accordingly, the TAM-mediated immune suppression was not reverted by Arg1 and iNOS inhibitors.26
Immunosuppression can be also mediated by membrane molecules. B7-H4 is a potent immunosuppressive molecule described on ovarian TAMs.26 We confirmed its expression on TAMs and showed that M2d's constitutively expressed B7-H4. TAMs and M2d's express low levels of the costimulatory molecules CD86 and CD80. CD80 has been implicated in MDSC-mediated immune suppression in a mouse model of ovarian carcinoma.49 However, in our study, a neutralizing anti-CD80 antibody did not affect the immunosuppressive properties of TAMs and M2d's (data not shown). Yang et al reported that the expression of CD80 on MDSCs is dependent on a direct cell contact with epithelial tumor cells.49 In our study, we investigated the role of soluble mediators on TAM generation and we could not exclude that a contact with tumor cells may modulate the expression/function of CD80 on TAMs. Furthermore, we observed that M2d's and TAMs express low levels of the costimulatory molecule CD86 but, in contrast, express ILT3 that renders APCs tolerogenic.53 In conclusion, these data demonstrate a role for IDO in the immunosuppressive properties of M2d's and ovarian TAMs and suggest that the imbalance between costimulatory (CD86) and inhibitory (B7-H4/ILT3) molecule expression may also participate in the immunosuppressive properties of M2d's and ovarian TAMs.
We found that in vitro–generated M2d's present phenotypic and functional characteristics similar to ovarian TAMs and distinct from M2a-c. M2d's and TAMs produced low levels of TNFα, which promotes tumor growth and metastasis.16 Moreover, M2d's and TAMs, unlike M2a-c, are poor producers of PTX3, an antiangiogenic factor.54 TAMs express VEGF, MMP9, and TGFβ,16 and M2d's had also up-regulated VEGF, MMP9, and TGFβ compared with unpolarized MΦs. By expressing VEGF and MMP9, but not PTX3, M2d's might promote angiogenesis, tumor growth, and metastasis. Furthermore, M2d's, similar to TAMs and MDSCs, are M2-polarized (IL-10highIL-12low) cells44 but also exhibit M1 characteristics (expression of IFNγ-inducible chemokines such as CCL5, CXCL10, and CXCL16).36 These data define M2d's as a novel subset of MΦs that exhibit a protumorogenic phenotype and immunosuppressive properties.
LIF, IL-6, and, to a lesser extent, OSM are expressed in numerous cancers and involved in tumor progression. Malignant cells from a wide variety of tissues produce LIF,55 and serum LIF levels are elevated in cancer patients.56 As one study indicated that epithelial ovarian carcinoma cells did not express LIF,57 LIF detected in ovarian cancer ascites might be derived from stroma cells (since a variety of cell types produce LIF in response to proinflammatory stimuli28 ). LIF stimulated breast cancer cell line proliferation58 and increased tumor-cell attachment to extracellular matrix components.59 IL-6 has also been implicated in various cancer progressions. In addition to promoting the growth of numerous tumor-cell lines, IL-6 increased their resistance to apoptosis.60,61 High circulating IL-6 levels are a marker of poor prognosis in melanoma and myeloma patients. Lastly, T cells, myeloid cells, and some tumor cells secrete OSM.57 Despite its cytostatic effect on some tumor cells, OSM also up-regulated angiogenesis and the metastatic potential of different cell lines.62 Our findings highlight that, in addition to a trophic role on tumor cells, LIF, IL-6, and OSM may favor tumor acquisition of immune tolerance through TAM generation. Lastly, in agreement with our data, it was observed that LIF transfection into tumor cells prevented the in vivo development of an antitumor immune response.63
M-CSF plays an important role in the physiology of several cancers, and its expression has been correlated with tumor-cell invasiveness and poor prognosis.64 Our findings indicate that LIF and IL-6 induced TAM generation by enabling autocrine/paracrine M-CSF consumption. Previously reported results demonstrated that M-CSF favored monocyte recruitment at the tumor site and murine TAM survival.12,16 Data obtained with M-CSF–deficient mice also supported a role for M-CSF in the malignant transformation of tumors and their ability to metastasize through the intermediary of TAM. These observations suggest that M-CSF plays a pivotal role in TAM generation and accumulation. Our data suggest that M-CSF alone is not sufficient to induce TAM generation. Some authors previously reported that M-CSF induced monocyte differentiation into M2 cells.43 Our extensive analysis of M-CSF-MΦ phenotype and function revealed that they exhibited an M2d light phenotype (intermediate differentiation state between MΦs and M2d's). Our results also indicated that LIF and IL-6 enabled M-CSF consumption and were required during differentiation to induce the generation of fully differentiated M2d's. Based on these findings, we concluded that LIF and IL-6 act as rheostats that allow M-CSF consumption and thus TAM generation.
Pertinently, constitutive LIF expression is rarely observed except in the uterus and airway epithelia.27,28 In both of these tissues, the immune system is tightly regulated (to avoid excessive responses to repeated contact with exogenous agents and to maintain maternal tolerance of embryos, respectively). Moreover, decidual and alveolar MΦs are immunosuppressive (ie, produce high IL-10 levels, express low CD86 levels, and have poor APC capacities).65,66 Lastly, because LIF and M-CSF are concomitantly present in the endometrium,67 it is tempting to hypothesize that LIF might contribute to maintaining immunologic tolerance by favoring the generation of immunosuppressive MΦs. In line with an immunoregulatory role of LIF, some authors previously reported that Tregs produce more LIF than Th1 cells, thereby implicating LIF in the regulation of posttransplantation tolerance.68 These observations suggest that LIF might contribute locally to control immunologic tolerance by acting on monocytes.
In summary, herein we identified LIF and IL-6 as tumor microenvironmental factors involved in TAM-like cell generation. These observations highlighted a novel tumor escape mechanism mediated through LIF and IL-6 production and open new insights into how to subvert TAM-induced immune tolerance and thereby boost the efficacy of cancer vaccines based on cytotoxic T lymphocyte (CTL) induction.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by INSERM (Avenir program) and the Cancéropôle Grand Ouest. D.D. and F.T. received financial support from the Comité Départemental du Maine-et-Loire of La Ligue contre le Cancer and from INSERM/région Pays de la Loire, respectively.
We thank members of the EFS for help with blood collection; Michelle Boisdron-Celle, who supervised collection of some samples from patients; Simon Blanchard and Marie-Hélène Guilleux, for technical assistance; Franck Morel and Jean-Claude Lecron (UPRES-EA 3806, Poitiers, France), for the supernatants of primary epithelial cells; and Janet Jacobson, for comments on the paper.
Authorship
Contribution: D.D. designed and performed research, analyzed the data, and wrote the paper; Y.D. made a major intellectual and critical contribution to the study design; F.T., M.-P.M., and J.L. performed research; L.G. and L.P. provided technical assistance and were supervised by H.G.; I.A. analyzed IDO expression and made an intellectual contribution; L.C., N.I., P.D., E.G., and M.H. provided biologic samples and made intellectual comments on the study; and P.J. devised the study, critically evaluated the data, drafted the paper, and takes responsibility for integrity of the work as a whole.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Pascale Jeannin, Equipe Avenir, Inserm Unit 564, Bâtiment Montéclair, Centre Hospitalier Universitaire, 4 rue Larrey, 49933 Angers, France; e-mail:pascale.jeannin@univ-angers.fr.

![Figure 1. TA-MΦs exhibit ovarian TAM phenotypic characteristics. (A) Analysis of CD14 and CD163 expression on freshly isolated ovarian tumor CD14+ cells (TAMs) and on healthy donor monocytes cultured for 5 days in CM without (MΦs) or with ovarian tumor ascites (TA-MΦs), or with the SNs of the 5637, A172, or HepG2 tumor-cell lines (SN-MΦs). (B) MΦs, TAMs, TA-MΦs, and SN-MΦs were stimulated for 48 hours with LPS before FACS analysis of CD86 and ILT3 expressions. (C) TAMs, MΦs, TA-MΦs, and SN-MΦs were stimulated for 48 hours with LPS before IL-10 and IL-12 quantification. Results are expressed in MFI (A,B) or in ng/mL or pg/mL (C), as means plus or minus SD of experiments performed with TAMs from 10 patients, or experiments performed with monocytes from 4 healthy donors treated either with 4 different ascites fluids or with tumor-cell supernatants. (D) MΦs, TAMs, and TA-MΦs were activated for 48 hours with LPS and incubated, in graded doses, with allogenic CD4+ T cells. (E) MΦs, TAMs, TA-MΦs, and DCs were stimulated for 48 hours with LPS and incubated with allogenic CD4+ T cells plus anti-CD3 mAb and IL-2. (D,E) [3H]-thymidine incorporation was measured on day 4. Results are expressed in cpm (D) or in variation of T-cell proliferation (E) as means plus or minus SD of experiments realized with TAMs and ascites of 5 ovarian cancer patients. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-02-072587/3/m_zh80010811000001.jpeg?Expires=1769085917&Signature=gHoCMy3179QlV9lEGjjhf7oqC6d5y7F54qHqMc6TQLcaUj7~LH1~AE6GBLOegeuFgzWDvSg6YgFezTTunNsnaRZjUvIGlyM8TUgO0oz0F3yE2fpZAcWf7IbAeeZWwofiyqV8iZAmCj9xpklJOXPNmtm9Jzj~K55U2mC2vI6~WAPJ8piB-PJG2kqixrTgb4yl48WBxsNRIT2tiorYsGRElIjVH877qf0EqJyI~Lq09x5vEGglthVnMH3hP3Qectvg2KflSMtmdXDAqOeV~H4d-h69Nk9cW2Pvt6vjY2Sw03xbmG4DC1pP8q0e8Jzpe~nCizg9NhCZzFqq6sY7IJFP2g__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 4. LIF and IL-6 generate M2d TAM-like cells. (A,B,G,H) MΦs, ovarian TAMs, LIF-MΦs, and IL-6-MΦs were stimulated with LPS for 48 hours and IL-10, IL-12, VEGF, and TGFβ were measured by ELISA in the SNs. Results are expressed as means ± SD; n = 6. (C) MMP9, PDGFA, PDGFB, CCL2, CCL5, IL-10, IL-12p35, and IL-23p19 mRNA expression was analyzed by Q-PCR in MΦs, LIF-MΦs, and IL-6-MΦs. a indicates that cells were further stimulated for 16 hours with LPS. Results are expressed as the fold increase of mRNA expression in LIF-MΦs or IL-6-MΦs compared with MΦs (mean ± SD; n = 4). (D) MΦs, LIF-MΦs, or IL-6-MΦs were activated for 48 hours with LPS with allogenic CD4+ T cells. [3H]-thymidine incorporation was measured at day 4. Results are expressed in cpm (mean ± SD; n = 4). (E,F) LIF-MΦs, IL-6-MΦs, TA-MΦs, and TAMs were activated for 48 hours with LPS and cocultured with CFDA-SE–labeled CD4+ T cells stimulated with anti-CD3 mAb plus IL-2 in the presence or absence of 1-MT. At day 5, cells were stained with 7-AAD and annexin-V, and the percentage of living cells was determined by FACS (mean ± SD; n = 4) (E). Proliferation of living cells was evaluated by CFDA-SE dilution measured by FACS. Results are expressed as a percentage of cells in each cycle (mean ± SD; n = 3) (F). (I) PCR analysis of B7-H4 in expression of MΦs, IL-6-MΦs, LIF-MΦs, and TAMs. Result is representative of 1 of 3 experiments. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-02-072587/3/m_zh80010811000004.jpeg?Expires=1769085917&Signature=rREROQSOBx~gydUImcHSi8IOihgq86y-1F-Llzm6yCPUkAvB2oZQ7ayNkRzzm1WtnHjPlkNXKaATOFKiafXjsT4t6zcr0R3C2-tVTRzX6Y5Nsy~XjmAMTqyC-SA8jhVmIRJ0D0qTLVjgYe3xcuYeMBJIPg7N0j7k8qySAhMknj~3EBHWU8bgfdV7MlBTKZkzSbTPRTUrQ~Eo4wv2edSV8koQW5NdCdY-tNr4DHxBHiCEUQNlk6wqGdlYwmVuegYo-CxiJQ4~2291oa9grlo3u2b5Mnx7HIENAIh6SS0GeMGJvfsqXkX~qRQjVFY4XH4l4DHm3x8FRvyAjuhH1GoXyA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 5. Involvement of autocrine M-CSF consumption in M2d generation. (A) At the indicated times, M-CSF was quantified in the SNs of monocytes cultured in CM without or with LIF, IL-6, or OSM. Results are expressed in ng/mL as means plus or minus SD; n = 4. (B) At day 2, M-CSF was quantified in the SNs of monocytes activated with 10 to 100 ng/mL LIF or IL-6. Results are expressed in ng/mL as mean plus or minus SD; n = 4. (C) PCR analysis of M-CSF, M-CSF-R, IL-6, LIF, and OSM mRNA expression in monocytes cultured for 16 hours in CM without or with IL-6, LIF, or OSM. Results are representative of 1 of 3 experiments. (D-F) Monocytes were cultured in CM without or with IL-6, LIF, or M-CSF without or with neutralizing anti–M-CSF and/or anti–IL-6 mAbs or control mAbs. On day 5, LPS was added and CD86 expression (D) and IL-12 production (E) were analyzed 48 hours later. After LPS stimulation, macrophages were cultured with allogenic CD4+ T cells, and [3H]-thymidine incorporation was measured at day 4 (F). Results are expressed as the percentages of restoration (mean ± SD; n = 4). (G) Monocytes were cultured in CM without (dotted line) or with (full line) IL-6, and M-CSF-R expression was analyzed by FACS after 24 hours. Results are representative of 1 of 4 experiments. Shaded areas correspond to the control mAb. (H) Monocytes were cultured in CM without or with LIF or IL-6, and M-CSF-R expression was analyzed by FACS after 24 and 48 hours. Results are expressed in MFI (mean ± SD; n = 4). (I) At the indicated times, IL-6 was quantified in the SNs of monocytes maintained in CM without or with LIF, OSM, or M-CSF. Results are expressed in ng/mL as means plus or minus SD; n = 4. *P < .05.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/13/10.1182_blood-2007-02-072587/3/m_zh80010811000005.jpeg?Expires=1769085917&Signature=omU5M2Qdu4-tc7Sr2b7SgivXtFcj8bKiqIlMjJKT7km~UAiXPpwdkrPC88kQ-ZdtO5zrl8gdZXOhhcdOvYDl1cklIg4LNQ9ybg1CeAdkdGV6qt-weg29LUAsZf6KQ--EAUEziB9MDXkJGOag6qwEZaiPOvAXEBDXuR9FxlezirNgimsvbtB3iUhPWN68-aYMdO7H3oZpEpQs4m0rtYepeD49Uc-KNDuh5y4HPdLudGPIZQwy5wTm9ovrPcZA8eXmUiUF0ESjg0ekqLu~hxO1iDqyIHDo0-ZakNuOJhyPOlDq6ujseYMV1jnCL707EXOq3qGw4TZiHVidgILGb4cHgg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal