Abstract
Systemic mastocytosis (SM) is a myeloid neoplasm characterized by increased survival and accumulation of neoplastic mast cells (MCs). In most patients, the D816V-mutated variant of KIT is detectable. We report here that heat shock protein 32 (Hsp32), also known as heme oxygenase-1 (HO-1), is a novel KIT-inducible survival factor in neoplastic MCs. As assessed by reverse transcription-polymerase chain reaction (RT-PCR), immunocytochemistry, and Western blotting, the KIT D816V+ MC line HMC-1.2 as well as highly enriched primary neoplastic MCs were found to express Hsp32 mRNA and the Hsp32 protein. Moreover, KIT D816V and stem cell factor (SCF)–activated wild-type KIT were found to induce Hsp32 promoter activity, expression of Hsp32 mRNA, and expression of the Hsp32 protein in Ba/F3 cells. Correspondingly, the KIT D816V-targeting drug PKC412 decreased the expression of Hsp32 as well as proliferation/survival in neoplastic MCs. The inhibitory effects of PKC412 on the survival of HMC-1.2 cells were counteracted by the HO-1 inductor hemin or lentiviral-transduced HO-1. Moreover, 2 Hsp32-targeting drugs, pegylated zinc protoporphyrin (PEG-ZnPP) and styrene maleic acid copolymer micelle-encapsulated ZnPP (SMA-ZnPP), were found to inhibit proliferation and to induce apoptosis in neoplastic MCs. Furthermore, both drugs were found to cooperate with PKC412 in producing growth inhibition. Together, these data show that Hsp32 is an important survival factor and interesting new therapeutic target in neoplastic MCs.
Introduction
Systemic mastocytosis (SM) is a myeloid neoplasm characterized by increased survival and accumulation of neoplastic mast cells (MCs) in internal organs.1-5 Indolent and aggressive variants of SM have been described.1-7 In patients with aggressive SM (ASM) or MC leukemia (MCL), the response to conventional drugs is poor, and the prognosis is grave.4-7 These patients are candidates for cytoreductive or experimental therapy.4-10 Indeed, several attempts have been made to identify novel therapeutic targets in neoplastic MCs.4,5,8-10
In most patients with SM, including those with ASM or MCL, the KIT mutation D816V is detectable.5-7,11-15 This mutation is associated with ligand-independent activation of KIT.16 Therefore, a number of attempts have been made to identify drugs interfering with the tyrosine kinase (TK) activity of KIT D816V.9,10,17-21 One of these drugs is PKC412, which counteracts in vitro growth of neoplastic MCs harboring KIT D816V.17,18 In addition, PKC412 was found to inhibit growth of neoplastic MCs in a patient with MCL.19 However, despite its impressive effects, PKC412 alone may not be sufficient to induce long-lasting complete responses in ASM/MCL.19 Therefore, current research focuses on additional drug targets in neoplastic MCs and respective targeted drugs.9 One attractive type of target are the survival factors expressed in neoplastic MCs.
Heat shock protein 32 (Hsp32), also known as heme oxygenase-1 (HO-1), is a stress-related survival factor22-24 that is expressed constitutively in various neoplastic cells.25-31 Thus, whereas previous studies have pointed to the protective role of Hsp32 in mesenchymal cells in inflammatory reactions and the related stress response,22-24 more recent data suggest that neoplastic cells also use Hsp32 as a survival-related molecule.25-31 Moreover, Hsp32-targeting drugs have been described as counteracting in vivo growth of experimental tumors in mice.25-27 We have recently shown that Hsp32/HO-1 is constitutively expressed in neoplastic cells in chronic myeloid leukemia (CML), and that the CML-specific oncoprotein BCR-ABL promotes expression of Hsp32.29
With regard to MCs, little is known about the expression of Hsp32. In rats, normal tissue MCs express low baseline levels of Hsp32, and its expression may increase after cell activation.32 It has also been shown that activated rat MCs can use Hsp32 as a cytoprotective survival factor.32 So far, however, expression of Hsp32 has not been examined in the context of mastocytosis. In the current study, we show that Hsp32 is an important survival factor and interesting new target in neoplastic MCs.
Materials and methods
Reagents, antibodies, and plasmids
Imatinib (STI571)33 and PKC412 (midostaurin)34 were kindly provided by Dr Paul W. Manley, Dr Elisabeth Buchdunger, and Dr Doriano Fabbro (Novartis Pharma AG, Basel, Switzerland). Stock solutions of PKC412 were prepared by dissolving the reagent in DMSO. Water-soluble pegylated zinc protoporphyrin (PEG-ZnPP)27 and styrene-maleic acid copolymer micelle-encapsulated zinc protoporphyrin (SMA-ZnPP)28 were produced at the Biodynamics Research Laboratory (Kumamoto, Japan). Encapsulation of ZnPP into SMA micelles was performed as described.28,35,36
The PI3-kinase inhibitor LY294002, MEK inhibitor PD98059, and rapamycin were purchased from Calbiochem (San Diego, CA); recombinant stem cell factor (SCF) was purchased from Strathmann Biotech (Hannover, Germany); RPMI 1640 medium and fetal calf serum (FCS) were purchased from PAA Laboratories (Pasching, Austria); L-glutamine and Iscove modified Dulbecco medium (IMDM) were purchased from Gibco Life Technologies (Gaithersburg, MD); 3H-thymidine was purchased from Amersham (Buckinghamshire, United Kingdom); and hemin was purchased from Sigma (St Louis, MO). The phycoerythrin (PE)-labeled monoclonal antibody (mAb) YB5.B8 (CD117) and FITC-labeled CD34 mAb 581 were purchased from Becton Dickinson (San Jose, CA); a polyclonal rabbit anti-Hsp32 antibody and Hsp32-specific blocking peptide were purchased from Stressgen Biotechnology (Victoria, BC, Canada); and an anti–β-actin antibody was purchased from Sigma.
pHHO-137 was kindly provided by Dr Shigeki Shibahara (Tohoku University School of Medicine, Sendai, Japan). The Hsp32 reporter construct (Hsp32-luc)38 was a kind gift of Dr Shigeru Takahashi (Scripps Research Institute, La Jolla, CA). The plasmids pLV-tTR-KRAB-dsRed,39 pWPT-GFP,39 psPAX2,39 pMD2G,39 pRSVrev,39 and pLVTHM39 were kindly provided by Dr Didier Trono (University of Geneva, Switzerland). To construct a lentiviral vector enabling doxycycline-dependent gene expression, the tetO cassette from pLVTHM was polymerase chain reaction (PCR)-amplified and cloned into pWPT-GFP upstream of the CMV promoter. Then, green fluorescent protein (GFP) cDNA was replaced by Hsp32 cDNA.37 The resulting vector was called pWPtet-HO-1 and was used to express Hsp32 in HMC-1.2 cells.
Cell lines and culture of primary cells
The MCL cell line HMC-140 was kindly provided by Dr Joseph H. Butterfield (Mayo Clinic, Rochester, MN). Two subclones were used, namely HMC-1.1 harboring KIT V560G but not KIT D816V,41 and HMC-1.2 harboring KIT V560G as well as KIT D816V.40,41 HMC-1 were grown in IMDM with 10% FCS, L-glutamine, and antibiotics at 37°C and 5% CO2. In addition, the following cell lines were used: KU812 (KIT+), MO7e (KIT+), U937, and K562. These cells were maintained in RPMI 1640 supplemented with 10% FCS. In control experiments, peripheral blood mononuclear cells (MNCs; 3 healthy donors), cultured lung fibroblasts, and cultured human umbilical vein endothelial cells (HUVECs) were used. Human MCs were cultured from their peripheral blood progenitors as described.42
The generation of Ba/F3 cells with doxycycline-inducible expression of wild-type (wt) KIT (Ton.Kit.wt) or KIT D816V (Ton.Kit.D816V) has been described elsewhere.18,43 In brief, Ba/F3 cells expressing the reverse tet-transactivator44,45 were cotransfected with a pTRE2 vector (Clontech, Palo Alto, CA) containing KIT D816V cDNA or wt KIT cDNA and pTK-Hyg (Clontech).18,43 Stably transfected cells were selected by growing in hygromycin and cloned by limiting dilution. In this study, clone Ton.Kit.D816V.27 was used. Cells were maintained in RPMI 1640 medium supplemented with 10% FCS and IL-3. Expression of KIT D816V is induced in Ton.Kit.D816V.27 cells within 12 hours on exposure to doxycycline (1 μg/mL).18,43
Hsp32 reporter gene assay
The Hsp32 promoter activity assay was performed as described.29 In brief, the HO-1-luc construct was transfected together with pCMV-βGAL into Ton.Kit.D816V.27 cells by electroporation. Then, cells were maintained in the presence or absence of doxycycline (1 μg/mL) for 18 hours. Plasmid pCMV-βGAL (Invitrogen, Carlsbad, CA) was used as a reporter for transfection efficiency. Hsp32/HO-1 reporter gene activity was expressed as a ratio of luciferase activity:βGAL activity.
Treatment with signal transduction inhibitors
HMC-1.2 cells and doxycycline-exposed Ton.Kit.D816V.27 cells were incubated with LY294002 (20 μM), PD98059 (50 μM), or rapamycin (20 nM) in IMDM (HMC-1) or RPMI 1640 medium (Ton.Kit.D816V.27) plus 10% FCS for 24 hours. After incubation, cells were subjected to Western blot analysis.
Generation of HMC-1 cells with doxycycline-inducible expression of Hsp32
Recombinant lentiviruses were produced by transient transfection of HEK-293FT cells as described.39,46 Briefly, subconfluent cells were cotransfected with a lentiviral vector carrying the gene of interest (tTR-KRAB, pWPTet-HO-1, or pWPT-GFP) and with psPAX2, pMD2G, and pRSVrev by Lipofectamine 2000 (Invitrogen) as reported.46,47 After 16 hours, the medium was changed, and recombinant lentivirus vectors were harvested 24 and 48 hours later. HMC-1.2 cells were transduced by spin infection in the presence of polybrene (8 μg/mL). Transduction efficacy of HMC-1 cells was less than 90% by fluorescence-activated cell-sorting (FACS) analysis GFP expression). To generate HMC-1.2 cells with conditional expression of HO-1/Hsp32, we took advantage of a tetracycline-controlled hybrid protein, tTR-KRAB, in which the tetracycline repressor (tTR) from Escherichia coli Tn10 is fused to the KRAB domain of human Kox1.39,48 In a first step, HMC-1.2 cells were transduced with pLV-tTR-KRAB-dsRed. Then, HMC-1.2 cells stably expressing tTR-KRAB were transduced with pWPTet-HO-1. Expression of Hsp32/HO-1 in these cells (called HMC-1.2.K-HO-1) was induced by doxycycline (1 μg/mL) and was confirmed by Western blotting.
Design and application of siRNA
A small interfering RNA (siRNA) against Hsp32 described by Zhang et al (sequence: 5′-GGAGAU UGAGCGCAACAAGdTdT-3′)49 was used. This siRNA, as well as a control siRNA against luciferase (5′-CUUACGCUGAGUACUUCGAdTdT), were synthesized in a 2′-deprotected duplexed, desalted, and purified form by Dharmacon (Lafayette, CO), and were transfected into HMC-1.2 cells using lipofectin (Invitrogen) according to the manufacturer's instructions. Expression of Hsp32 was confirmed by Western blotting.
Isolation of primary neoplastic MCs
In 5 patients with mastocytosis, neoplastic MCs were examined. One patient had indolent SM, 1 had smoldering SM (SSM), 1 had ASM, 1 had MCL, and 1 had MC sarcoma (MCS). The study was approved by the institutional review board of the Medical University of Vienna, Austria, and conducted in accordance with the declaration of Helsinki. Informed consent was obtained for each patient. Cell isolation was performed as described.18,50 In 2 patients (1 with MCL, 1 with MCS), neoplastic MCs were purified to homogeneity as reported.50 In brief, MCs (defined as CD117++/CD34− cells) were sorted by flow cytometry on a FACS-Vantage (Becton Dickinson) using the PE-labeled CD117 mAb YB5.B8 and a FITC-conjugated CD34 mAb. The purity of sorted MC amounted to more than 98%.
Northern blot analysis and RT-PCR
Total RNA was isolated from HMC-1 cells using Trizol (Invitrogen) according to the manufacturer's instructions. Northern blotting was performed as described29,42,47 using 32P-labeled cDNA probes specific for Hsp32 or β-actin. PCR primers used to generate Northern blot probes (VBC Genomics, Vienna, Austria) were as follows: human Hsp32, 5′-ATGGAGCGTCCGCAACCCGA-3′ (forward) and 5′-GCATAAAGCCCTACAGCAAC-3′ (reverse); and β-actin, 5′-ATGGATGATGATATCGCCGCG-3′ (forward) and 5′-CTAGAAGCATTTGCGGTGGACGATGGAGGGGCC-3′ (reverse). Labeling was performed using the Megaprime kit (Amersham). Bound radioactivity was visualized by exposure to Biomax MS film (Kodak, Rochester, NY) at −80°C using intensifying screens. mRNA expression levels were quantified by densitometry using E.A.S.Y. Win32 software (Herolab, Wiesloch, Germany).
For reverse transcription (RT)-PCR analysis, total RNA was extracted from MCs using the RNeasy Mini Kit (Qiagen, Hilden, Germany). One-step RT-PCR reactions were performed on total RNA using the Titan 1 tube RT-PCR system (Roche, Mannheim, Germany) and primers specific for human Hsp32 (same as used for Northern blotting). PCR conditions were 31 cycles, annealing temperature 60°C. Equal loading was confirmed by determining β-actin mRNA levels using published primers29 (PCR conditions: 23 cycles, annealing temperature 60°C). Hsp32 mRNA expression levels were quantified by densitometry and were expressed after correcting for β-actin mRNA expression levels.
Western blot experiments and immunocytochemistry
HMC-1 cells and Ba/F3 cells inducibly expressing either wt KIT (Ton. Kit.wt) or KIT D816V (Ton.Kit.D816V.27) were incubated with PKC412 (1 μM), imatinib (1 μM), or control medium at 37°C for 16 to 24 hours. Prior to drug exposure, Ton.Kit.wt cells and Ton.Kit.D816V.27 cells were kept in IL-3 and were incubated with doxycycline (1 μg/mL) at 37°C for 24 hours to induce expression of KIT. Then, Ton.Kit cells were incubated with inhibitors or control medium for another 16 hours without IL-3. In the case of Ton.Kit.wt cells, KIT phosphorylation was induced by adding SCF (100 ng/mL). HMC-1 cells were incubated with control medium or hemin (10 μM) at 37°C for 4 hours. In a separate set of experiments, various cell lines (KU812, MO7e, U937, and K562) and cultured human MCs were exposed to control medium, SCF (100 ng/mL), or hemin (10 μM) for 24 hours. Western blotting was performed as described18,29 using a polyclonal rabbit anti-Hsp32 antibody and anti-β-actin antibody. To confirm that incubation of KU812 with SCF is followed by KIT activation, immunoprecipitation experiments were performed as described.18 In brief, KU812 cells were incubated in control medium or SCF (100 ng/mL) for 24 hours. Then, lysates were prepared as reported18 and subjected to immunoprecipitation with the anti-KIT antibody 1C1 (kindly provided by Dr Hans-Jörg Bühring, University of Tübingen, Germany). Western blotting was performed using the antiphosphoprotein antibody 4G10 (Upstate Biotechnology, Lake Placid, NY) or 1C1 antibody.
Immunocytochemistry was performed on cytospin preparations of primary neoplastic MCs and HMC-1 cells as described29 using a polyclonal rabbit anti-Hsp32 antibody (dilution, 1:1000; Stressgen) and biotinylated goat-antirabbit IgG antibody (Biocarta, San Diego, CA). As chromogen, streptavidin-alkaline-phosphatase complex (Biocarta) was used. Antibody reactivity was made visible using Neofuchsin (Nichirei, Tokyo, Japan). In control experiments, a Hsp32-blocking peptide was applied.
Measurement of 3H-thymidine uptake
HMC-1 cells were incubated with various concentrations of SMA-ZnPP (1-30 μM), PEG-ZnPP (1-30 μM), or PKC412 (0.02-0.2 μM) in 96-well culture plates (TPP, Trasadingen, Switzerland) at 37°C for 48 hours. Primary neoplastic MCs were exposed to SMA-ZnPP (1-50 μM) or PEG-ZnPP (1-50 μM) at 37°C for 48 hours. After incubation, 1 μCi (0.037 MBq) 3H-thymidine was added (37°C for 12 hours). Cells were then harvested on filter membranes (Packard Bioscience, Meriden, CT) in a Filtermate 196-harvester (Packard Bioscience). Filters were air-dried, and the bound radioactivity was counted in a β-counter (Top-Count NXT; Packard Bioscience). To determine additive or synergistic drug effects, HMC-1 cells were exposed to various combinations of PKC412 and PEG-ZnPP or PKC412 and SMA-ZnPP (at fixed ratios of drug concentrations). Cooperative drug effects (additive, synergistic) were determined by calculating combination index values using the Calcusyn software (Biosoft, Ferguson, MO).51 All experiments were performed in triplicates.
Evaluation of apoptosis by microscopy and TUNEL assay
In typical experiments, cells were incubated with various concentrations of PEG-ZnPP (1-10 μM), SMA-ZnPP (1-10 μM), PKC412 (1 μM or 5 μM), or control medium at 37°C for 48 hours. In select experiments, HMC-1 cells were exposed to PKC412 (1 or 5 μM) in the presence or absence of hemin (10 μM). In other experiments, HMC-1 cells transfected with Hsp32-specific siRNA were analyzed. Apoptotic cells52 were quantified on Wright-Giemsa-stained cytospin preparations. To confirm apoptosis, electron microscopy was performed as described53-55 using HMC-1 cells exposed to SMA-ZnPP (10 μM) for 72 hours. After fixation, cells were washed, suspended in agar, “postfixed” in 1.3% OsO4, and stained en bloc in 2% uranyl acetate and sodium maleate buffer. After embedding in EPON-812, ultrathin sections were cut and placed on gold grids. Sections were contrasted in uranyl acetate and lead citrate, and viewed in a JEOL 1200-EX-II transmission electron microscope (JEOL, Tokyo, Japan).53-55 To further confirm apoptosis in HMC-1 cells exposed to PEG-ZnPP or SMA-ZnPP (10 μM; 72 hours), a TUNEL assay was performed using the in situ cell death detection kit-fluorescein (Roche) as reported.55,56 Cells were analyzed with a Nikon Eclipse-E-800 fluorescence microscope (Tokyo, Japan).
Statistical analysis
To determine the significance in differences in proliferation and apoptosis in cells exposed to control-medium or inhibitors, the Student t test for dependent samples was applied. Results were considered statistically significant when P was less than .05.
Results
Neoplastic MCs express Hsp32 mRNA and the Hsp32 protein
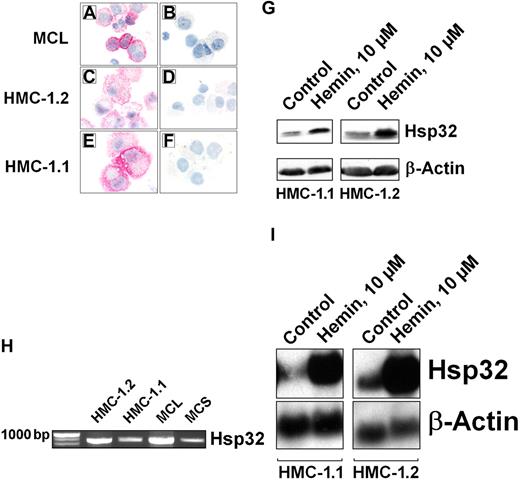
As assessed by immunocytochemistry, primary neoplastic MCs as well as HMC-1 cells (HMC-1.1 and HMC-1.2) were found to express the Hsp32 protein (Figure 1A-F). Preincubation of anti-Hsp32 antibody with a specific blocking peptide resulted in a negative stain (Figure 1B, D, F). Expression of the Hsp32 protein in HMC-1 cells was also demonstrable by Western blotting (Figure 1G). In addition, we were able to detect Hsp32 mRNA in primary neoplastic MC (Figure 1H) as well as in HMC-1 cells (Figure 1I). Hemin, a specific Hsp32 inductor, was found to up-regulate expression of Hsp32 in these cells (Figure 1G,I).
Expression of Hsp32 in neoplastic mast cells. Immunocytochemical detection of Hsp32 in primary neoplastic MCs in a patient with mast cell leukemia (A-B), in HMC-1.2 cells expressing KIT D816V (C-D), and in HMC-1.1 cells lacking KIT D816V (E-F), using an antibody against Hsp32. Before being applied, the antibody was preincubated with control-buffer (A,C,E) or a specific blocking-peptide (B,D,F). Acquisition of figures was performed by an Olympus DP11 camera connected to an Olympus BX50F4 microscope equipped with 100 ×/1.35 UPlan-Apo objective lenses (Olympus, Hamburg, Germany). Images were acquired with Adobe Photoshop CS2 software version 9.0 (Adobe Systems, San Jose, CA) and processed with PowerPoint software (Microsoft, Redmond, WA). (G) Western blot analysis of Hsp32 expression in HMC-1.1 cells and HMC-1.2 cells after exposure to control medium or hemin (10 μM; 37°C, 4 hours). Western blotting was performed using antibodies specific for Hsp32 or β-actin (loading control). (H) Detection of Hsp32 mRNA by RT-PCR in HMC-1 cells and highly enriched KIT-sorted neoplastic MCs obtained from 1 patient with mast cell leukemia (MCL) and 1 patient with mast cell sarcoma (MCS). RT-PCR was performed as described in “Materials and methods, Northern blot analysis and RT-PCR.” (I) Northern blot analysis of expression of Hsp32 mRNA in HMC-1.1 and HMC-1.2 cells after incubation in control medium or hemin (10 μM, 37°C) for 4 hours. Northern blotting was performed using DNA probes specific for Hsp32 or β-actin. As assessed by densitometry, hemin induced a 10-fold up-regulation of Hsp32 mRNA in HMC-1.1 cells, and a 20-fold up-regulation in HMC-1.2 cells.
Expression of Hsp32 in neoplastic mast cells. Immunocytochemical detection of Hsp32 in primary neoplastic MCs in a patient with mast cell leukemia (A-B), in HMC-1.2 cells expressing KIT D816V (C-D), and in HMC-1.1 cells lacking KIT D816V (E-F), using an antibody against Hsp32. Before being applied, the antibody was preincubated with control-buffer (A,C,E) or a specific blocking-peptide (B,D,F). Acquisition of figures was performed by an Olympus DP11 camera connected to an Olympus BX50F4 microscope equipped with 100 ×/1.35 UPlan-Apo objective lenses (Olympus, Hamburg, Germany). Images were acquired with Adobe Photoshop CS2 software version 9.0 (Adobe Systems, San Jose, CA) and processed with PowerPoint software (Microsoft, Redmond, WA). (G) Western blot analysis of Hsp32 expression in HMC-1.1 cells and HMC-1.2 cells after exposure to control medium or hemin (10 μM; 37°C, 4 hours). Western blotting was performed using antibodies specific for Hsp32 or β-actin (loading control). (H) Detection of Hsp32 mRNA by RT-PCR in HMC-1 cells and highly enriched KIT-sorted neoplastic MCs obtained from 1 patient with mast cell leukemia (MCL) and 1 patient with mast cell sarcoma (MCS). RT-PCR was performed as described in “Materials and methods, Northern blot analysis and RT-PCR.” (I) Northern blot analysis of expression of Hsp32 mRNA in HMC-1.1 and HMC-1.2 cells after incubation in control medium or hemin (10 μM, 37°C) for 4 hours. Northern blotting was performed using DNA probes specific for Hsp32 or β-actin. As assessed by densitometry, hemin induced a 10-fold up-regulation of Hsp32 mRNA in HMC-1.1 cells, and a 20-fold up-regulation in HMC-1.2 cells.
Effect of KIT D816V on expression of Hsp32 in Ba/F3 cells
To analyze the effects of KIT D816V and wt KIT on expression of Hsp32, Ba/F3 cells with doxycycline-inducible expression of KIT were used. As shown in Figure 2, KIT D816V was found to up-regulate Hsp32 promoter activity (Figure 2A) as well as expression of the Hsp32 protein (Figure 2B) in these cells. Interestingly, SCF-activated wt KIT was also found to augment Hsp32 promoter activity (Figure 2C) and expression of the Hsp32 protein (Figure 2D). Moreover, SCF was found to promote expression of Hsp32 in cultured human MCs. By contrast, SCF failed to promote the expression of Hsp32 in KIT+ leukemic cell lines (KU812, MO7e) or KIT− control cells (Figure 2E-F).
Effect of KIT D816V on expression of HO-1 in Ba/F3 cells. (A) Ton.Kit.D816V-27 cells were transfected with a Hsp32 promoter construct (HO-1-luc) and pCMV-βGal as described in “Materials and methods, Hsp32 reporter gene assay.” Cells were starved from IL-3 and kept in control medium or induced to express KIT D816V by exposure to doxycycline (1 μg/mL) for 16 hours. Then, cells were analyzed for luciferase and βGal activities. Luciferase activity was reported as the ratio HO-1-luc/pCMV/βGal and was expressed as a percentage of control. Results represent the mean (± standard deviation [SD]) of 3 independent experiments. (B) Western blot analysis of Ton.Kit.D816V-27 cells after starvation from IL-3 and incubation in control medium or doxycycline (to induce expression of KIT D816V). Western blotting was performed with antibodies against Hsp32 or β-actin (loading control). (C) wt KIT-transfected Ba/F3 cells (Ton.Kit.wt) were starved from IL-3 and kept in control medium or were induced to express KIT D816V by exposure to doxycycline. To activate wt KIT, cells were kept in SCF (100 ng/mL). After incubation, cells were analyzed for Hsp32 reporter gene activity. Results represent the mean (± SD) of 3 independent experiments. (D) Western blot analysis of Ton.Kit.wt cells after starvation from IL-3 and incubation in control medium, doxycycline (to induce expression of KIT), or doxycycline and SCF (100 ng/mL). (E) Western blot analysis of expression of Hsp32 in cultured (21 days) human blood cell-derived mast cells (d21 MC), KU812 cells (KIT+), MO7e cells (KIT+), U937 cells (KIT−), and K562 cells (KIT−), after exposure to control medium, SCF (100 ng/mL), or hemin (10 μM) at 37°C for 24 hours. Western blotting was performed with antibodies against Hsp32 or β-actin (loading control). (F) KU812 cells were exposed to control medium (control) or SCF (100 ng/mL) for 24 hours. Then, lysates were prepared and subjected to immunoprecipitation with anti-KIT antibody 1C1 followed by Western blot analysis using 4G10 antibody (p-KIT) or 1C1 antibody (controlling total KIT expression). In the same experiment, cell lysates were also subjected to Western blot analysis using antibodies against Hsp32 and β-actin.
Effect of KIT D816V on expression of HO-1 in Ba/F3 cells. (A) Ton.Kit.D816V-27 cells were transfected with a Hsp32 promoter construct (HO-1-luc) and pCMV-βGal as described in “Materials and methods, Hsp32 reporter gene assay.” Cells were starved from IL-3 and kept in control medium or induced to express KIT D816V by exposure to doxycycline (1 μg/mL) for 16 hours. Then, cells were analyzed for luciferase and βGal activities. Luciferase activity was reported as the ratio HO-1-luc/pCMV/βGal and was expressed as a percentage of control. Results represent the mean (± standard deviation [SD]) of 3 independent experiments. (B) Western blot analysis of Ton.Kit.D816V-27 cells after starvation from IL-3 and incubation in control medium or doxycycline (to induce expression of KIT D816V). Western blotting was performed with antibodies against Hsp32 or β-actin (loading control). (C) wt KIT-transfected Ba/F3 cells (Ton.Kit.wt) were starved from IL-3 and kept in control medium or were induced to express KIT D816V by exposure to doxycycline. To activate wt KIT, cells were kept in SCF (100 ng/mL). After incubation, cells were analyzed for Hsp32 reporter gene activity. Results represent the mean (± SD) of 3 independent experiments. (D) Western blot analysis of Ton.Kit.wt cells after starvation from IL-3 and incubation in control medium, doxycycline (to induce expression of KIT), or doxycycline and SCF (100 ng/mL). (E) Western blot analysis of expression of Hsp32 in cultured (21 days) human blood cell-derived mast cells (d21 MC), KU812 cells (KIT+), MO7e cells (KIT+), U937 cells (KIT−), and K562 cells (KIT−), after exposure to control medium, SCF (100 ng/mL), or hemin (10 μM) at 37°C for 24 hours. Western blotting was performed with antibodies against Hsp32 or β-actin (loading control). (F) KU812 cells were exposed to control medium (control) or SCF (100 ng/mL) for 24 hours. Then, lysates were prepared and subjected to immunoprecipitation with anti-KIT antibody 1C1 followed by Western blot analysis using 4G10 antibody (p-KIT) or 1C1 antibody (controlling total KIT expression). In the same experiment, cell lysates were also subjected to Western blot analysis using antibodies against Hsp32 and β-actin.
Role of the TK activity of KIT in expression of Hsp32 in neoplastic cells
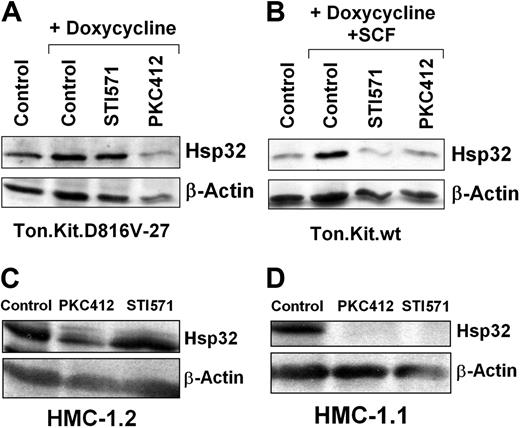
To define the role of KIT D816V in expression of Hsp32, we examined the effects of PKC412, an inhibitor of KIT D816V. For control purpose, we also applied imatinib, known to suppress the TK activity of wt KIT, but not KIT D816V.41,57,58 In these experiments, PKC412 (1 μM) was found to inhibit KIT D816V-induced expression of Hsp32 in Ton.Kit.D816V cells (Figure 3A) as well as the SCF-induced expression of Hsp32 in Ton.Kit.wt cells (Figure 3B). In contrast, imatinib (1 μM) was found to counteract only the wt KIT-induced expression of Hsp32, but not KIT D816V-induced expression of Hsp32 (Figure 3A-B). Corresponding results were obtained with HMC-1 cells. In particular, exposure of HMC-1.2 or HMC-1.1 cells to PKC412 resulted in a decreased expression of Hsp32, whereas imatinib decreased the levels of Hsp32 only in HMC-1.1 cells (Figure 3C-D). These data suggest that KIT TK activation contributes to expression of Hsp32 in neoplastic MCs.
Effects of PKC412 and imatinib on expression of Hsp32 in neoplastic cells. (A) Ton.Kit.D816V-27 cells were starved from IL-3 and kept in control medium or were induced to express KIT D816V by exposure to doxycycline (1 μg/mL) in the presence or absence of PKC412 (1 μM) or STI571 (1 μM) at 37°C for 16 hours. Then, cells were harvested and subjected to Western blotting using antibodies against Hsp32 or β-actin (loading control). (B) Ton.Kit.wt cells were starved (control) or were induced to express wt KIT by exposure to doxycycline. To activate wt KIT, cells were exposed to SCF (100 ng/mL). Then, cells were kept in control medium, PKC412 (1 μM), or STI571 (1 μM) at 37°C for 18 hours, and then subjected to Western blotting using antibodies against murine Hsp32 or β-actin. (C-D) HMC-1.2 cells (C) and HMC-1.1 cells (D) were incubated in control medium, PKC412 (1 μM), or STI571 (1 μM) at 37°C for 24 hours. After exposure to drugs, cells were harvested, and Western blots were performed using antibodies against Hsp32 or β-actin.
Effects of PKC412 and imatinib on expression of Hsp32 in neoplastic cells. (A) Ton.Kit.D816V-27 cells were starved from IL-3 and kept in control medium or were induced to express KIT D816V by exposure to doxycycline (1 μg/mL) in the presence or absence of PKC412 (1 μM) or STI571 (1 μM) at 37°C for 16 hours. Then, cells were harvested and subjected to Western blotting using antibodies against Hsp32 or β-actin (loading control). (B) Ton.Kit.wt cells were starved (control) or were induced to express wt KIT by exposure to doxycycline. To activate wt KIT, cells were exposed to SCF (100 ng/mL). Then, cells were kept in control medium, PKC412 (1 μM), or STI571 (1 μM) at 37°C for 18 hours, and then subjected to Western blotting using antibodies against murine Hsp32 or β-actin. (C-D) HMC-1.2 cells (C) and HMC-1.1 cells (D) were incubated in control medium, PKC412 (1 μM), or STI571 (1 μM) at 37°C for 24 hours. After exposure to drugs, cells were harvested, and Western blots were performed using antibodies against Hsp32 or β-actin.
KIT D816V promotes expression of Hsp32 through multiple signaling pathways
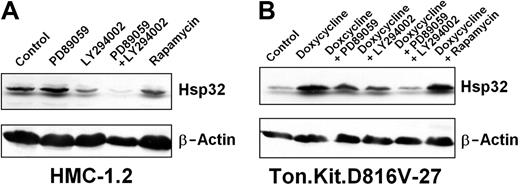
The PI3-kinase inhibitor LY294002 was found to down-regulate expression of Hsp32 in HMC-1.2 cells (Figure 4A) and Ton.Kit.D816V-27 cells (Figure 4B). The MEK-inhibitor PD98059 slightly decreased Hsp32 expression in Ton.Kit.D816V-27 cells, but did not inhibit expression of Hsp32 in HMC-1.2 cells (Figure 4). Interestingly, a combination of LY294002 and PD98059 resulted in a more pronounced inhibition of Hsp32 expression compared with single agents (Figure 4A-B). These data suggest that KIT D816V-dependent expression of Hsp32 involves multiple signaling molecules. Interestingly, rapamycin showed no effects on Hsp32 expression (Figure 4).
Effects of signal transduction inhibitors on expression of Hsp32 in neoplastic cells. (A) HMC-1.2 cells were cultured in control-medium, the MEK inhibitor PD89059 (50 μM), the PI3-kinase inhibitor LY294002 (20 μM), a combination of PD89059 (50 μM) and LY294002 (20 μM), or the mTOR inhibitor rapamycin (20 nM) at 37°C for 24 hours. Then, cells were subjected to Western blot analysis using antibodies against Hsp32 or β-actin. (A) Ton.Kit.D816V-27 cells were starved (control) or were induced to express KIT D816V by exposure to doxycycline. KIT D816V-expressing cells were kept in control medium, PD89059 (50 μM), LY294002 (20 μM), a combination of PD89059 (50 μM) and LY294002 (20 μM), or rapamycin (20 nM) at 37°C for 18 hours. Then, cells were harvested and subjected to Western blotting using antibodies against Hsp32 or β-actin (loading control).
Effects of signal transduction inhibitors on expression of Hsp32 in neoplastic cells. (A) HMC-1.2 cells were cultured in control-medium, the MEK inhibitor PD89059 (50 μM), the PI3-kinase inhibitor LY294002 (20 μM), a combination of PD89059 (50 μM) and LY294002 (20 μM), or the mTOR inhibitor rapamycin (20 nM) at 37°C for 24 hours. Then, cells were subjected to Western blot analysis using antibodies against Hsp32 or β-actin. (A) Ton.Kit.D816V-27 cells were starved (control) or were induced to express KIT D816V by exposure to doxycycline. KIT D816V-expressing cells were kept in control medium, PD89059 (50 μM), LY294002 (20 μM), a combination of PD89059 (50 μM) and LY294002 (20 μM), or rapamycin (20 nM) at 37°C for 18 hours. Then, cells were harvested and subjected to Western blotting using antibodies against Hsp32 or β-actin (loading control).
Functional consequences of overexpression of Hsp32 in neoplastic MCs
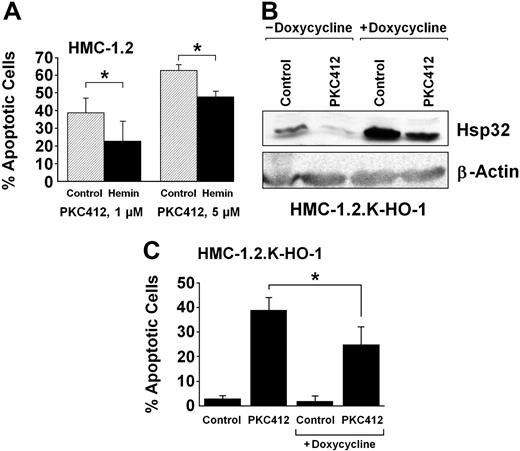
To define the role of Hsp32 as a potential survival factor in neoplastic MCs, drug-rescue experiments were performed. In a first step, HMC-1.2 cells were exposed to either control medium or hemin (10 μM), and to PKC412 to induce apoptosis. In line with previous data,18 PKC412 was found to induce a marked increase in apoptotic cells. As visible in Figure 5A, hemin was found to partially rescue HMC-1.2 cells from this apoptosis-inducing effect of PKC412 (Figure 5A; P < .05). To further document the role of Hsp32 as a survival and rescue factor, HMC-1.2 cells with doxycycline-inducible expression of Hsp32 (HMC-1.2.K-HO-1) were examined. As assessed by Western blotting, exposure to doxycycline resulted in a significant increase in expression of Hsp32 in these cells (Figure 5B). When HMC-1.2.K-HO-1 cells (kept in doxycycline) were then exposed to PKC412 (500 nM), the levels of Hsp32 decreased, but expression of the survival factor was not completely blocked. Furthermore, the enforced (doxycycline-induced) expression of Hsp32 in HMC-1.2.K-HO-1 cells was found to be associated with a significantly reduced rate of PKC412-induced apoptosis compared with cells kept in control medium (Figure 5C). Doxycycline per se did not induce expression of Hsp32 in HMC-1 cells or untransfected Ba/F3 cells, and did not rescue HMC-1.2 cells from PKC412-induced apoptosis (not shown).
Hsp32-mediated rescue of HMC-1 cells from the effects of PKC412. (A) HMC-1.2 cells were cultured in control medium or hemin (10 μM) at 37°C and then were exposed to PKC412 at 1 μM (left panel) or 5 μM (right panel) for 48 hours. Thereafter, the number of apoptotic cells were determined by light microscopy. Results show the percentage of apoptotic cells and represent the mean (± SD) of 3 independent experiments. *P <.05. In the absence of PKC412, the percentage of apoptotic cells ranged between 1% and 3% (not shown). (B) Doxycycline-induced expression of Hsp32/HO-1 in HMC-1.2.K-HO-1 cells. HMC-1.2.K-HO-1 cells were incubated in the absence or presence of doxycycline (1 μg/mL) at 37°C for 24 hours. When added, PKC412 (1 μM, 24 hours) was found to down-regulate the expression of Hsp32 to trace amounts in the absence of doxycycline, whereas the doxycycline-induced expression of Hsp32 in these cells could not be completely abrogated by PKC412. (C) HMC-1.2.K-HO-1 cells were incubated in the absence or presence of doxycycline (1 μg/mL) at 37°C for 24 hours. Then, cells were exposed to control medium or PKC412 (0.5 μM) at 37°C for 24 hours. Thereafter, the numbers of apoptotic cells were assessed by light microscopy. Results represent the mean (± SD) of 3 independent experiments. *P < .05.
Hsp32-mediated rescue of HMC-1 cells from the effects of PKC412. (A) HMC-1.2 cells were cultured in control medium or hemin (10 μM) at 37°C and then were exposed to PKC412 at 1 μM (left panel) or 5 μM (right panel) for 48 hours. Thereafter, the number of apoptotic cells were determined by light microscopy. Results show the percentage of apoptotic cells and represent the mean (± SD) of 3 independent experiments. *P <.05. In the absence of PKC412, the percentage of apoptotic cells ranged between 1% and 3% (not shown). (B) Doxycycline-induced expression of Hsp32/HO-1 in HMC-1.2.K-HO-1 cells. HMC-1.2.K-HO-1 cells were incubated in the absence or presence of doxycycline (1 μg/mL) at 37°C for 24 hours. When added, PKC412 (1 μM, 24 hours) was found to down-regulate the expression of Hsp32 to trace amounts in the absence of doxycycline, whereas the doxycycline-induced expression of Hsp32 in these cells could not be completely abrogated by PKC412. (C) HMC-1.2.K-HO-1 cells were incubated in the absence or presence of doxycycline (1 μg/mL) at 37°C for 24 hours. Then, cells were exposed to control medium or PKC412 (0.5 μM) at 37°C for 24 hours. Thereafter, the numbers of apoptotic cells were assessed by light microscopy. Results represent the mean (± SD) of 3 independent experiments. *P < .05.
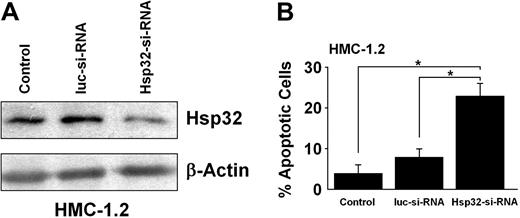
Effect of disruption of Hsp32 expression in neoplastic MCs by specific siRNA
As assessed by Western blotting, transfection of HMC-1.2 cells with specific siRNA resulted in a marked decrease in expression of Hsp32 compared with cells transfected with siRNA against luciferase (Figure 6A). As shown in Figure 6B, the siRNA-induced knockdown of Hsp32 in HMC-1 cells was associated with a significant decrease in cell viability and increase in apoptotic cells compared with luc siRNA (P < .05). These data suggest that Hsp32 is a critical survival factor in neoplastic MCs.
Effect of siRNA induced down-regulation of Hsp32 in HMC-1.2 cells. (A) HMC-1.2 cells were transfected with a control siRNA (luc-siRNA) or a Hsp32-specific siRNA (Hsp32-siRNA) using lipofectin as described. Then, cells were examined for expression of the Hsp32 protein by Western blotting using antibodies against Hsp32 and β-actin (loading control). Results from nontransfected control HMC-1.2 cells (control) are also shown. As assessed by densitometry, transfection with Hsp32-siRNA resulted in a decrease in expression of the Hsp32 protein to 35% compared with untransfected cells, whereas no decrease was found in case of the Luc siRNA (165%). (B) Nontransfected HMC-1.2 cells and HMC-1.2 cells transfected with control siRNA (luc-siRNA) or an Hsp32-specific siRNA (Hsp32-siRNA) were examined for the percentage of apoptotic cells by light microscopy. Results represent the mean (± SD) of 3 independent experiments. *P < .05.
Effect of siRNA induced down-regulation of Hsp32 in HMC-1.2 cells. (A) HMC-1.2 cells were transfected with a control siRNA (luc-siRNA) or a Hsp32-specific siRNA (Hsp32-siRNA) using lipofectin as described. Then, cells were examined for expression of the Hsp32 protein by Western blotting using antibodies against Hsp32 and β-actin (loading control). Results from nontransfected control HMC-1.2 cells (control) are also shown. As assessed by densitometry, transfection with Hsp32-siRNA resulted in a decrease in expression of the Hsp32 protein to 35% compared with untransfected cells, whereas no decrease was found in case of the Luc siRNA (165%). (B) Nontransfected HMC-1.2 cells and HMC-1.2 cells transfected with control siRNA (luc-siRNA) or an Hsp32-specific siRNA (Hsp32-siRNA) were examined for the percentage of apoptotic cells by light microscopy. Results represent the mean (± SD) of 3 independent experiments. *P < .05.
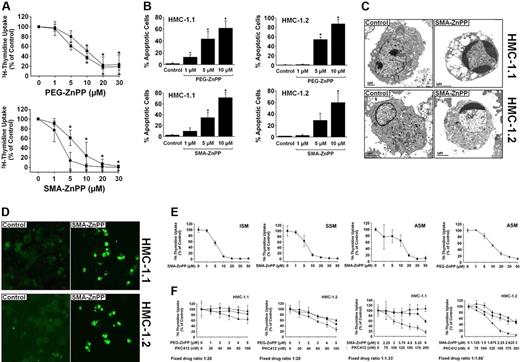
Targeting of Hsp32 by specific pharmacologic inhibitors (SMA-ZnPP, PEG-ZnPP) is associated with growth inhibition and apoptosis in HMC-1 cells
We next examined the effects of 2 pharmacologic inhibitors of Hsp32, SMA-ZnPP and PEG-ZnPP, on the growth and viability of HMC-1.1 cells and HMC-1.2 cells. As visible in Figure 7A, SMA-ZnPP and PEG-ZnPP were found to suppress the proliferation of HMC-1.1 cells and HMC-1.2 cells in a dose-dependent manner with similar IC50 values. In each case, growth inhibition was found to be associated with induction of apoptosis (Figure 7B). Apoptosis-inducing effects of the Hsp32-targeting drugs were demonstrable by light microscopy (Figure 7B), electron microscopy (Figure 7C), and in a TUNEL assay (Figure 7D). PEG-ZnPP and SMA-ZnPP were also found to inhibit SCF-induced development of MCs from their circulating progenitors and induced apoptosis in immature cultured MCs, whereas no effects were seen in mature blood MNCs, lung fibroblasts, or HUVECs (supplemental data are available online at the Blood website; see the Supplemental Figures link at the top of the online article).
Effects of PEG-ZnPP and SMA-ZnPP on proliferation and viability of neoplastic mast cells. (A) HMC-1.1 cells (●) and HMC-1.2 cells (■) were incubated in control-medium (0) or in various concentrations of PEG-ZnPP (top panel) or SMA-ZnPP (bottom panel) as indicated at 37°C for 48 hours. Uptake of 3H-thymidine was then measured. Results show the percentage of 3H-thymidine uptake compared with medium control (0 on x-axis = 100%) and represent the mean (± SD) of 3 independent experiments. (B) HMC-1.1 cells (left panels) and HMC-1.2 cells (right panels) were incubated in control medium (control) or various concentrations of PEG-ZnPP (upper panels) or SMA-ZnPP (lower panels) as indicated at 37°C for 48 hours. Then, the numbers (percentages) of apoptotic cells were determined by light microscopy. Results represent the mean (± SD) of 3 independent experiments. *P < .05 compared with control. (C) HMC-1.1 cells and HMC-1.2 cells were cultured in control medium (left panels) or medium containing SMA-ZnPP (10 μM) (right panels) at 37°C for 72 hours. Then, cells were harvested and subjected to electron microscopic examination. Typical cells are depicted. Those from cultures kept in SMA-ZnPP showed characteristic signs of apoptosis. Original magnification, 5000×. Images were captured using a Gatan Bioscan Camera model 792 and Digital Micrograph acquisition software (Gatan, Pleasanton, CA). (D) HMC-1.1 cells and HMC-1.2 cells were cultured in control medium (left panels) or medium containing SMA-ZnPP (10 μM) (right panels) at 37°C for 72 hours. Then, apoptosis was examined by a TUNEL assay. Images were obtained using a Nikon Plan Apo 40×/1.0 numeric aperture oil objective. Images were acquired from FITC-labeled cells using a Hamamatsu high-resolution digital camera (model C4242-95; Hamamatsu, Japan) and HPD-CPX32 Microsoft Windows 95 software (Microsoft, Redmond, WA). Citifluor (Agar Science, Stansted, United Kingdom) was used as imaging solution. (E) Primary neoplastic cells obtained from the bone marrow of patients with ISM, SSM, and ASM were cultured in control medium or medium containing various concentrations of SMA-ZnPP at 37°C for 48 hours. In one patient (ASM), PEG-ZnPP was also applied. After exposure to drugs, uptake of 3H-thymidine was measured. Results show the percentage of 3H-thymidine uptake compared with medium control (0 on x-axis = 100%) and represent the mean (± SD) of triplicates. (F) HMC-1.1 cells (left panels) and HMC-1.2 cells (right panels) were cultured in control medium (0 on x-axis) or medium containing either PEG-ZnPP alone or SMA-ZnPP alone (■), PKC412 alone (●), or a combination (at fixed ratio) between PKC412 and one of the 2 Hsp32/HO-1-targeting compounds (PEG-ZnPP, top panels; SMA-ZnPP, bottom panels) (▴) at the concentrations indicated. After incubation (37°C for 48 (hours), 3H-thymidine was measured. Results show the percentage of 3H-thymidine uptake compared with medium control (0 on x-axis = 100%) and represent the mean (± SD) of triplicates from 1 typical experiment.
Effects of PEG-ZnPP and SMA-ZnPP on proliferation and viability of neoplastic mast cells. (A) HMC-1.1 cells (●) and HMC-1.2 cells (■) were incubated in control-medium (0) or in various concentrations of PEG-ZnPP (top panel) or SMA-ZnPP (bottom panel) as indicated at 37°C for 48 hours. Uptake of 3H-thymidine was then measured. Results show the percentage of 3H-thymidine uptake compared with medium control (0 on x-axis = 100%) and represent the mean (± SD) of 3 independent experiments. (B) HMC-1.1 cells (left panels) and HMC-1.2 cells (right panels) were incubated in control medium (control) or various concentrations of PEG-ZnPP (upper panels) or SMA-ZnPP (lower panels) as indicated at 37°C for 48 hours. Then, the numbers (percentages) of apoptotic cells were determined by light microscopy. Results represent the mean (± SD) of 3 independent experiments. *P < .05 compared with control. (C) HMC-1.1 cells and HMC-1.2 cells were cultured in control medium (left panels) or medium containing SMA-ZnPP (10 μM) (right panels) at 37°C for 72 hours. Then, cells were harvested and subjected to electron microscopic examination. Typical cells are depicted. Those from cultures kept in SMA-ZnPP showed characteristic signs of apoptosis. Original magnification, 5000×. Images were captured using a Gatan Bioscan Camera model 792 and Digital Micrograph acquisition software (Gatan, Pleasanton, CA). (D) HMC-1.1 cells and HMC-1.2 cells were cultured in control medium (left panels) or medium containing SMA-ZnPP (10 μM) (right panels) at 37°C for 72 hours. Then, apoptosis was examined by a TUNEL assay. Images were obtained using a Nikon Plan Apo 40×/1.0 numeric aperture oil objective. Images were acquired from FITC-labeled cells using a Hamamatsu high-resolution digital camera (model C4242-95; Hamamatsu, Japan) and HPD-CPX32 Microsoft Windows 95 software (Microsoft, Redmond, WA). Citifluor (Agar Science, Stansted, United Kingdom) was used as imaging solution. (E) Primary neoplastic cells obtained from the bone marrow of patients with ISM, SSM, and ASM were cultured in control medium or medium containing various concentrations of SMA-ZnPP at 37°C for 48 hours. In one patient (ASM), PEG-ZnPP was also applied. After exposure to drugs, uptake of 3H-thymidine was measured. Results show the percentage of 3H-thymidine uptake compared with medium control (0 on x-axis = 100%) and represent the mean (± SD) of triplicates. (F) HMC-1.1 cells (left panels) and HMC-1.2 cells (right panels) were cultured in control medium (0 on x-axis) or medium containing either PEG-ZnPP alone or SMA-ZnPP alone (■), PKC412 alone (●), or a combination (at fixed ratio) between PKC412 and one of the 2 Hsp32/HO-1-targeting compounds (PEG-ZnPP, top panels; SMA-ZnPP, bottom panels) (▴) at the concentrations indicated. After incubation (37°C for 48 (hours), 3H-thymidine was measured. Results show the percentage of 3H-thymidine uptake compared with medium control (0 on x-axis = 100%) and represent the mean (± SD) of triplicates from 1 typical experiment.
Targeting of Hsp32 in primary neoplastic MCs results in growth inhibition
We next confirmed the Hsp32-targeting effects of SMA-ZnPP and PEG-ZnPP for primary neoplastic MCs in patients with SM. For this purpose, primary MCs obtained from patients with ISM, SSM, and ASM were exposed to various concentrations of SMA-ZnPP or PEG-ZnPP. As visible in Figure 7E, SMA-ZnPP was found to inhibit proliferation of primary neoplastic MCs in a dose-dependent manner in all patients, with slightly higher IC50 values in ASM compared with that in ISM or SSM. PEG-ZnPP was also found to inhibit the growth of neoplastic MCs (Figure 7E).
SMA-ZnPP and PEG-ZnPP cooperate with PKC412 in producing growth inhibition in HMC-1 cells
Recent data suggest that optimal antineoplastic effects can be achieved by combining targeted drugs with each other. We therefore applied combinations of SMA-ZnPP (or PEG-ZnPP) and PKC412. As shown in Figure 7F, both SMA-ZnPP and PEG-ZnPP were found to cooperate with PKC412 in producing growth inhibition in HMC-1.1 cells and HCM-1.2 cells. These cooperative drug effects were found to be mostly additive, whereas no clear synergistic drug interactions were observed.
Discussion
Hsp32 is a stress-related survival factor that has recently been implicated in growth of neoplastic cells.25-31 We report that neoplastic MCs express Hsp32 in a constitutive manner and use this molecule as a survival factor. Our data also show that SCF-activated wt KIT as well as KIT D816V promote the expression of Hsp32 in MCs. Moreover, pharmacologic inhibitors of Hsp32 produce growth inhibition in neoplastic MCs. These data suggest that Hsp32 is a novel interesting target in neoplastic MCs. In this regard, it is noteworthy that neoplastic MCs have recently been described as also expressing another functionally relevant Hsp, namely Hsp90, that may also serve as a molecular target.59
So far, little is known about the regulation of expression of Hsp32 in neoplastic cells. In CML, the disease-specific oncoprotein BCR-ABL was found to contribute to expression of Hsp32 in leukemic cells.29 In the present study, we were able to show that the SM-related oncoprotein KIT D816V promotes expression of Hsp32 in Ba/F3 cells, and that the KIT D816V-targeting drug PKC412 decreases the expression of Hsp32 in HMC-1.2 cells. By contrast, exposure of HMC-1.2 cells to imatinib, a drug without a major effect on KIT D816V,41,57,58 did not result in a decreased expression of Hsp32. These data suggest that the D816V-mutated variant of KIT is involved in the regulation of expression of Hsp32 in neoplastic MCs.
However, the Hsp32-inducing effect was not found to be specific for KIT D816V, but was also seen with (SCF-activated) wt KIT. In addition, the HMC-1.1 subclone, which exhibits KIT V560G but not KIT D816V, was also found to express the Hsp32 protein in a constitutive manner. Interestingly, both TK inhibitors, namely PKC412 and imatinib, were found to down-regulate expression of Hsp32 in HMC-1.1 cells. These data suggest that KIT V560G may also contribute to expression of Hsp32 in neoplastic MCs. The differential effect of imatinib on Hsp32 expression in the 2 HMC-1 subclones is best explained by the fact that KIT D816V is associated with imatinib resistance.41,57,58
In contrast to neoplastic MCs, normal MCs may express Hsp32 only upon cell activation or as immature proliferating progenitors, and then can use this protein as a survival-enhancing (cytoprotective) molecule.32 Correspondingly, we were able to show that SCF induces the expression of Hsp32 in Ton.Kit.wt cells as well as in cultured MC progenitors. All in all, these data suggest that KIT activation is associated with expression of Hsp32 in normal and neoplastic MCs. By contrast, in non-MC lineage cells, ligation of KIT by SCF was not followed by enhanced expression of Hsp32.
A number of downstream-signaling pathways are considered to play an important role in KIT D816V-dependent growth and function of neoplastic MCs.60 In the present study, the PI3-kinase inhibitor LY294002 was found to suppress KIT D816V-induced expression of Hsp32. The MEK inhibitor PD98059 was also found to inhibit KIT D816V-dependent expression of Hsp32, but the effect of this compound was less pronounced. The important role of the PI3-kinase pathway in Hsp32 expression in neoplastic MCs is consistent with data obtained for stress-induced expression of Hsp32 in other cell types.61 A remarkable observation was that the combination of LY294002 and PD98059 resulted in a more pronounced inhibition of Hsp32 expression compared with LY294002 as a single agent. These observations suggest that multiple signaling molecules may contribute to KIT D816V-dependent expression of Hsp32 in neoplastic MCs. An unexpected observation was that the mTOR-targeting drug rapamycin did not inhibit expression of Hsp32 in neoplastic MCs. Thus, mTOR acts downstream of PI3-kinase, and the mTOR-inhibitor rapamycin has been described to counteract growth of HMC-1 cells.62
A number of observations suggest that Hsp32 is an important survival factor in various physiologic and neoplastic cells.22-31 To define the functional role of Hsp32 in neoplastic MCs, we examined the consequences of overexpression and of a specific knockdown of Hsp32 in HMC-1 cells. Overexpression of Hsp32 induced by hemin or by doxycycline-exposure in HMC-1.2.K-HO-1 cells was found to be associated with a decrease in PKC412-induced apoptosis. These data suggest that Hsp32 expression is associated with cytoprotection and rescue against KIT-targeting drugs (drug resistance). We then applied a specific siRNA and found that the Hsp32 knockdown blocks the survival in HMC-1 cells. Thus, targeting of Hsp32 may be a promising antineoplastic treatment-strategy in SM.
We next examined the effects of 2 specific water-soluble pharmacologic inhibitors of Hsp32, namely SMA-ZnPP and PEG-ZnPP. Both drugs were found to down-regulate growth of HMC-1 cells (both subclones) in a dose-dependent manner, with IC50 values achievable in vivo.27,28 As expected, the growth-inhibitory effects of SMA-ZnPP were found to be associated with apoptosis of HMC-1 cells.
KIT-targeting drugs such as PKC412 may be useful for the treatment of ASM/MCL.5,8-10,17-21 However, recent data suggest that drug resistance may develop during therapy.19 To overcome resistance, a number of pharmacologic strategies may be envisaged. One possibility is to apply drug combinations.18 We were therefore interested to learn whether PKC412 and SMA-ZnPP or PKC412 and PEG-ZnPP would exhibit cooperative antiproliferative effects. Indeed, our data show that PKC412 cooperates with Hsp32-targeting drugs in producing growth inhibition in HMC-1 cells.
Based on our data, targeting of Hsp32 by may be a novel interesting approach in patients with advanced MC neoplasms. However, although in vivo data in mice suggest that the respective drugs can be administered safely without significant side effects and without cytopenia,27,28 no data on antineoplastic effects or side effects occurring in patients are available. In this regard, the effects of these drugs on normal (resting) cells are of interest. Notably, whereas mature resting cells may not be affected by Hsp32-targeting drugs, activated cells (eg, in inflammatory reactions) or proliferating (MC) progenitors may express significant amounts of Hsp32, and therefore may by affected. Thus, the pharmacologic profile of Hsp32-targeting drugs may be less favorable during an ongoing inflammation (infection).
In summary, we show that Hsp32 is a novel important survival factor and promising therapeutic target expressed in neoplastic MCs. because targeted drugs are available, it may be a straightforward approach to test this new concept in clinical trials in patients with ASM/MCL.
The online version of this article contains a data supplement.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Fonds zur Förderung der Wissenschaftlichen Forschung in Österreich (FWF) grant no. P-17205-B14; the Austrian Federal Ministry for Science and Research (grants no. GZ 200.136/1-VI/1/2005 and no. GZ 200.142/1-IV/1/2006); grant no. 20030 from the Center of Molecular Medicine (CeMM), Austrian Academy of Sciences; and a Grant for Cancer Research from the Ministry of Education, Science, Culture, and Sports of Japan (no. 17016076).
Authorship
Contribution: R.K. performed key research experiments, established the research plan, analyzed data, and contributed to paper drafting. K.V.G. performed key laboratory experiments, including Western blots and proliferation experiments, and analyzed data. M.M., H.E., and C.S. established vital new analytical tools (cell lines with inducible expression of KIT). K.J.A., A.G., and W.F.P. performed key laboratory experiments on cell growth and proliferation. A.V. contributed PCR and Northern blot experiments. M.-T.K. performed immunocytochemistry experiments. P.S. performed electron microscopy experiments and the Tunel assay. K.G. and H.M. designed and provided essential new reagents (Hsp32-targeting drugs). P.V. designed the study, established the research plan, provided logistic and budget support, drafted the paper, and approved the data and the final version of the manuscript.
R.K. and K.V.G. contributed equally to the study.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Valent, Department of Internal Medicine I, Division of Hematology & Hemostaseology, Medical University of Vienna, AKH-Wien, Waehringer Guertel 18-20, A-1090 Vienna, Austria; e-mail: peter.valent@meduniwien.ac.at.

![Figure 2. Effect of KIT D816V on expression of HO-1 in Ba/F3 cells. (A) Ton.Kit.D816V-27 cells were transfected with a Hsp32 promoter construct (HO-1-luc) and pCMV-βGal as described in “Materials and methods, Hsp32 reporter gene assay.” Cells were starved from IL-3 and kept in control medium or induced to express KIT D816V by exposure to doxycycline (1 μg/mL) for 16 hours. Then, cells were analyzed for luciferase and βGal activities. Luciferase activity was reported as the ratio HO-1-luc/pCMV/βGal and was expressed as a percentage of control. Results represent the mean (± standard deviation [SD]) of 3 independent experiments. (B) Western blot analysis of Ton.Kit.D816V-27 cells after starvation from IL-3 and incubation in control medium or doxycycline (to induce expression of KIT D816V). Western blotting was performed with antibodies against Hsp32 or β-actin (loading control). (C) wt KIT-transfected Ba/F3 cells (Ton.Kit.wt) were starved from IL-3 and kept in control medium or were induced to express KIT D816V by exposure to doxycycline. To activate wt KIT, cells were kept in SCF (100 ng/mL). After incubation, cells were analyzed for Hsp32 reporter gene activity. Results represent the mean (± SD) of 3 independent experiments. (D) Western blot analysis of Ton.Kit.wt cells after starvation from IL-3 and incubation in control medium, doxycycline (to induce expression of KIT), or doxycycline and SCF (100 ng/mL). (E) Western blot analysis of expression of Hsp32 in cultured (21 days) human blood cell-derived mast cells (d21 MC), KU812 cells (KIT+), MO7e cells (KIT+), U937 cells (KIT−), and K562 cells (KIT−), after exposure to control medium, SCF (100 ng/mL), or hemin (10 μM) at 37°C for 24 hours. Western blotting was performed with antibodies against Hsp32 or β-actin (loading control). (F) KU812 cells were exposed to control medium (control) or SCF (100 ng/mL) for 24 hours. Then, lysates were prepared and subjected to immunoprecipitation with anti-KIT antibody 1C1 followed by Western blot analysis using 4G10 antibody (p-KIT) or 1C1 antibody (controlling total KIT expression). In the same experiment, cell lysates were also subjected to Western blot analysis using antibodies against Hsp32 and β-actin.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/2/10.1182_blood-2006-10-054411/4/m_zh80140704870002.jpeg?Expires=1769085906&Signature=JzX4BY9GXqBnHQGHLWA1MM-c7vBS7fYWBbmD9aSeRZUIOq4lVBQZOyQERIf4FPu6TGiGtfqpMYQNhOzsVS5xLvzNUKjZ4XOLzzL58Uva7y4K4yCiIj6U24~vHakywlPmgj4cL66qi3T4ivuwObWnmrVgy9EpTTRBkjSxHmejRvcyIj6lRoSWgZgIKprU53mJat83xV71qppIEzUrKve8l94Lf-bdLfkgMaBH3mWO1OXJHJosp6rsZLqr02XmAZ~-cjmi5cA73WTDJhxJ2FUr4aul5Ksv8jHQbpsgkrf3058F6vXDqnWLMskU0prF3nMss0~pCE8aYqTmt6hz3huQ2Q__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal