Abstract
Iron (Fe) plays a critical role in proliferation, and Fe deficiency results in G1/S arrest and apoptosis. However, the precise role of Fe in cell-cycle control remains unclear. We observed that Fe depletion increased the mRNA of the universal cyclin-dependent kinase inhibitor, p21CIP1/WAF1, while its protein level was not elevated. This observation is unique to the G1/S arrest seen after Fe deprivation, as increased p21CIP1/WAF1 mRNA and protein are usually found when arrest is induced by other stimuli. In this study, we examined the posttranscriptional regulation of p21CIP1/WAF1 after Fe depletion and demonstrated that its down-regulation was due to 2 mechanisms: (1) inhibited translocation of p21CIP1/WAF1 mRNA from the nucleus to cytosolic translational machinery; and (2) induction of ubiquitin-independent proteasomal degradation. Iron chelation significantly (P < .01) decreased p21CIP1/WAF1 protein half-life from 61 (± 4 minutes; n = 3) to 28 (± 9 minutes, n = 3). Proteasomal inhibitors rescued the chelator-mediated decrease in p21CIP1/WAF1 protein, while lysosomotropic agents were not effective. In Fe-replete cells, p21CIP1/WAF1 was degraded in an ubiquitin-dependent manner, while after Fe depletion, ubiquitin-independent proteasomal degradation occurred. These results are important for considering the mechanism of Fe depletion–mediated cell-cycle arrest and apoptosis and the efficacy of chelators as antitumor agents.
Introduction
Iron (Fe) is critical for many processes, such as DNA synthesis and energy production.1,2 Tumor cells require more Fe for DNA synthesis than normal cells, which is probably related to their rapid proliferation.3,4 This is supported by the fact that tumor cells, compared with their normal counterparts, have significantly higher expression of transferrin receptor 1 (TfR1), a molecule involved in Fe uptake from the Fe-transport protein, transferrin.3
Many studies have demonstrated that Fe chelators have inhibitory effects on growth of tumor cells.2,5 Chelators, such as desferrioxamine (DFO), show effective anticancer activity.6-8 However, use of DFO is limited by its poor membrane permeability and short half-life.9 Currently, Fe chelators that show much greater antiproliferative activity than DFO are in development and include thiosemicarbazones5,10-13 and tachpyridine.14,15
There are multiple mechanisms involved in the antitumor activity of Fe chelators.9,15 Fe depletion results in inhibition of the Fe-containing enzyme, ribonucleotide reductase, which is critical for DNA synthesis.9 Treatment of cells with Fe chelators down-regulates Bcl-2 levels, up-regulates the proapoptotic protein Bax, and significantly increases caspase-3, caspase-8, and caspase-9.11,16 Consequently, these concerted effects induce apoptosis.11 Previous studies demonstrated that Fe depletion also alters expression of many molecules that cause cell-cycle arrest.9,17-26
Cyclins and cyclin-dependent kinases (cdks) form active cyclin-cdk complexes to phosphorylate the retinoblastoma protein (pRb),27 which regulates G1/S progression. Iron chelators down-regulate both cyclin D1 and cdk2 expression.20,21,23 Consequently, phosphorylated pRb decreases after Fe chelation, which may contribute to G1/S arrest.21,28
Cell-cycle regulation is achieved by sequential activation and inactivation of cdks.27,29,30 Cdk inhibitors are involved in inactivating cdks and include the WAF1/CIP/KIP family. A key member of this group of molecules is the cyclin-dependent kinase inhibitor p21CIP1/WAF1, which acts to prevent G1/S transition.31-33 Cell-cycle progression through the G1 phase into S is a major checkpoint for proliferating cells and is under multiple levels of control by p21CIP1/WAF1 and other regulators.27,29
Interestingly, p21CIP1/WAF1 has both positive and negative effects on G1 progression.29 In fact, assembly and activation of cyclin-cdk complexes requires basal levels of p21CIP1/WAF1.34 However, overexpression and induction of p21CIP1/WAF1 dominantly inhibits the activity of cdks, especially the cyclin E/cdk2 complex, resulting in cell-cycle arrest.33 Paradoxically, p21CIP1/WAF1 can function as an assembly factor for cyclin D/cdk complexes.34 Thus, p21CIP1/WAF1 also has positive effects on G1 phase progression.34 In fact, p21CIP1/WAF1 can stabilize and mediate nuclear accumulation of cyclin D–cdk4 or cyclin D–cdk6 complexes, which are important for G1/S progression.35 Finally, p21CIP1/WAF1 also has an antiapoptotic function, and its down-regulation leads to apoptosis in tumor cells.36,37
Previous studies have shown that Fe chelators induce a G1/S arrest and up-regulate p21CIP1/WAF1 mRNA in a p53-independent manner.21,22,24 Furthermore, transcriptional and posttranscriptional mechanisms were shown to be involved in the up-regulation of p21CIP1/WAF1 mRNA after Fe chelation.17 In addition, p21CIP1/WAF1 mRNA expression was markedly increased after Fe depletion with chelators, while paradoxically, its protein level remained similar to the control or decreased.18 This has not been observed with other Fe-regulated molecules, and the mechanism(s) involved remain unclear.
The current investigation focuses on the posttranscriptional mechanisms involved in the decrease in p21CIP1/WAF1 protein after Fe chelation, as it could play roles in the G1/S arrest and apoptosis observed. Our studies demonstrate that the decrease in p21CIP1/WAF1 protein upon Fe depletion was due to a decrease in nuclear translocation of p21CIP1/WAF1 mRNA to the cytosol and the induction of an ubiquitin-independent pathway of proteasomal degradation. The results are important for understanding the G1/S arrest and apoptosis observed after Fe depletion and the mechanism of Fe chelator–mediated antitumor activity.
Materials and methods
Reagents
Cell culture
A31N-ts20 cells40 were from Dr H. Ozer (University of Medicine and Dentistry, New Jersey (UMDNJ)-New Jersey Medical School, Newark, NJ). All other cells were from the American Type Culture Collection (Manassas, VA) and were cultured as described.19 Cells were incubated with chelators in the presence of medium containing 10% fetal calf serum (FCS).
RNA isolation and semiquantitative RT-PCR
RNA was isolated using TRIzol (Invitrogen, Melbourne, Australia). Reverse transcription–polymerase chain reaction (RT-PCR) was performed via standard methods10 using the primers in Table 1. RT-PCR was shown to be semiquantitative by a protocol that demonstrated it was in the log-phase of amplification. Expression of β-actin was used as an RNA-loading control.
Primers for amplification of human p21CIPI/WAF1 and β-actin
| Pair no. . | Primer name . | Genotype/accession no. . | Oligonucleotides (5′ to 3′) . | Product size, bp . | |
|---|---|---|---|---|---|
| Forward . | Reverse . | ||||
| 1 | p21CIP1/WAF1 | U03106 | CTCAGAGGAGGCGCCATGTC | GAGTGGTAGAAATCTGTCATGCTGG | 473 |
| 2 | β-actin | NM_001101 | CCCGCCGCCAGCTCACCATGG | AAGGTCTCAAACATGATCTGGGTC | 397 |
In situ RT-PCR
Western analysis and immunoprecipitation
Western analysis was performed as described.19,21 Lysate protein concentrations were assayed using the Pierce BCA Protein Assay (Rockford, IL). Optimization of antibody conditions were performed using a range of antibody concentrations. Quantitation of results was performed using Biorad Quantity One software (Hercules, CA). To ensure equal loading of proteins, membranes were probed with anti-β-actin antibody. Immunoprecipitation was achieved using standard methods.20,43 The antibodies were from Santa Cruz Biotechnology (Santa Cruz, CA) or Sigma-Aldrich.
Transient transfections and treatments
The pCS2+p21WT and pCS2+p21-K6R plasmids with human wild-type and mutant p21CIP1/WAF1 inserts were transiently transfected into mouse NIH3T3 cells using Lipofectin (Invitrogen). At 24 hours after transfection, cells were treated with 311, MG132, or 311 and MG132 for 24 hours. Total protein was extracted, and human p21CIP1/WAF1 protein was analyzed by Western blot using antihuman p21CIP1/WAF1 (Zymed, Melbourne, Australia), which does not detect endogenous mouse p21CIP1/WAF1.
Statistics
Experimental data were compared using the Student t test. Results were considered statistically significant when P was less than .05.
Results
p21CIP1/WAF1 mRNA and protein levels after Fe chelation
Many previous investigations have clearly shown that Fe chelators such as DFO and 31138 induce marked Fe depletion by causing increased Fe efflux from cells and inhibition of Fe uptake from transferrin.17,24,44,45 In addition, 311 is a far more effective Fe chelator than DFO due to its greater lipophilicity, enabling ready access to intracellular Fe pools.24,44-46 This results in a marked increase in expression of genes such as TfR1 and Ndrg-1 that are regulated by Fe.19,24,46
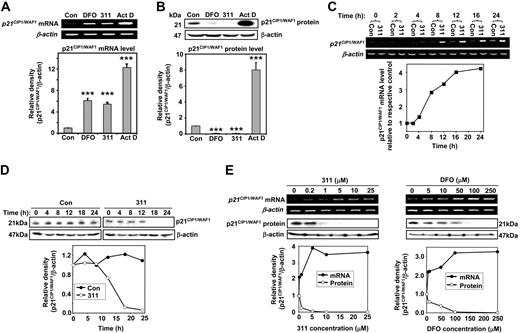
In initial studies, MCF-7 cells were incubated for 24 hours with concentrations of DFO (250 μM) and 311 (25 μM) that have been shown to induce cellular Fe depletion.18-20 These studies demonstrated that p21CIP1/WAF1 mRNA levels were significantly elevated in DFO- or 311-treated cells compared with cells grown in control medium (6 and 5.6 times higher, respectively; Figure 1A). However, Western blot results indicated that p21CIP1/WAF1 protein was significantly decreased after Fe chelation (9 and 8 times lower than control, respectively; Figure 1B). As a positive control, a low Act D concentration (5 nM) was implemented as a DNA-damaging agent to induce p21CIP1/WAF1 expression,47 and this increased both p21CIP1/WAF1 mRNA and protein levels (Figure 1A-B). The paradoxical increase in p21CIP1/WAF1 mRNA and decrease in protein expression after Fe depletion was found in various cell types (data not shown), indicating a general effect rather than being cell-type specific. Collectively, the effect of Fe chelation on p21CIP1/WAF1 expression appeared different to the DNA-damaging agent, Act D, and was in good agreement with our previous studies.18,21,24
Iron chelation paradoxically up-regulates p21CIP1/WAF1 mRNA levels and down-regulates protein expression. MCF-7 cells were incubated with 311 (25 μM), DFO (250 μM), or Act D (5 nM) for 24 hours at 37°C. Total mRNA and protein were then extracted, and RT-PCR and Western blotting were conducted to detect p21CIP1/WAF1 mRNA and protein levels, respectively. β-actin was used as a loading control. (A) Effect of DFO, 311, and Act D on p21CIP1/WAF1 mRNA levels (***P < .001 relative to the control; n = 3). (B) Effect of 311 and DFO on p21CIP1/WAF1 protein levels (***P < .001 relative to the control; n = 3). Error bars are mean ± SD (standard deviation). (C) p21CIP1/WAF1 mRNA and (D) p21CIP1/WAF1 protein expression as a function of incubation time with 311 (25 μM) at 37°C. (E) Effect of 311 and DFO concentration on p21CIP1/WAF1 mRNA and protein expression after an incubation of 24 hours at 37°C. Results are typical of 3 independent experiments.
Iron chelation paradoxically up-regulates p21CIP1/WAF1 mRNA levels and down-regulates protein expression. MCF-7 cells were incubated with 311 (25 μM), DFO (250 μM), or Act D (5 nM) for 24 hours at 37°C. Total mRNA and protein were then extracted, and RT-PCR and Western blotting were conducted to detect p21CIP1/WAF1 mRNA and protein levels, respectively. β-actin was used as a loading control. (A) Effect of DFO, 311, and Act D on p21CIP1/WAF1 mRNA levels (***P < .001 relative to the control; n = 3). (B) Effect of 311 and DFO on p21CIP1/WAF1 protein levels (***P < .001 relative to the control; n = 3). Error bars are mean ± SD (standard deviation). (C) p21CIP1/WAF1 mRNA and (D) p21CIP1/WAF1 protein expression as a function of incubation time with 311 (25 μM) at 37°C. (E) Effect of 311 and DFO concentration on p21CIP1/WAF1 mRNA and protein expression after an incubation of 24 hours at 37°C. Results are typical of 3 independent experiments.
Changes to p21CIP1/WAF1 mRNA and protein expression after incubation with chelators was time and dose dependent
Our previous studies examining the effect of Fe chelation on p21CIP1/WAF1expression were performed after 24 or 30 hours.18,21 To examine the kinetics involved in the expression of p21CIP1/WAF1, MCF-7 cells were incubated with 311 (25 μM) for 2 to 24 hours, and then p21CIP1/WAF1 mRNA (Figure 1C) and protein (Figure 1D) were assessed. In these experiments, 311 was used in preference to DFO due to its much more rapid permeation of cells to chelate and deplete Fe pools.24,44 Relative to the control, p21CIP1/WAF1 mRNA started to increase after an 8-hour incubation with 311 and was more than 4 times greater in the presence of the chelator after 24 hours (Figure 1C). In contrast, p21CIP1/WAF1 protein levels started to decrease after a 12-hour incubation with 311 relative to the control (Figure 1D). The p21CIP1/WAF1 protein levels decreased to nearly undetectable levels after an 18- or 24-hour incubation with 311 (Figure 1D). These results suggested that the effects of 311 on p21CIP1/WAF1 mRNA and protein levels in MCF-7 cells were time dependent and paradoxic. Moreover, because the increase in mRNA expression occurred well before the decrease in protein levels, it appeared these 2 events were not closely coupled.
Further studies examined the effect on p21CIP1/WAF1 expression of incubating MCF-7 cells with increasing concentrations of 311 (0.2-25 μM) or DFO (5-250 μM) for 24 hours at 37°C. The effect of chelators on p21CIP1/WAF1 mRNA and protein levels were dose dependent (Figure 1E). Notably, 311 was more potent than DFO, with low concentrations of 311 (1 μM) causing significant changes in p21CIP1/WAF1 mRNA and protein, while DFO required much higher concentrations (50 μM) to induce similar effects.
Reconstitution of p21CIP1/WAF1 protein levels after Fe chelation by Fe supplementation
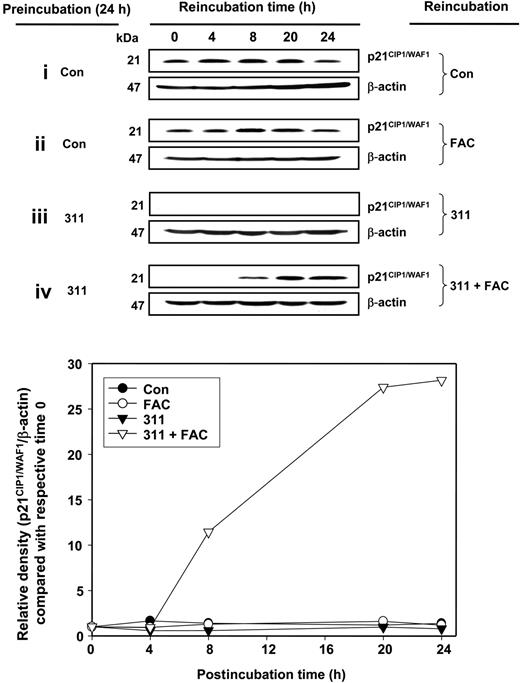
To assess if Fe chelator–mediated down-regulation of p21CIP1/WAF1 protein was definitely due to cellular Fe depletion, we examined the effect of Fe reconstitution by incubating cells with ferric ammonium citrate (FAC).19,20 In these studies, after preincubation for 24 hours with or without 311 (25 μM), MCF-7 cells were then reincubated for up to a further 24 hours with or without FAC (100 μg/mL; [Fe] = 280 μM; Figure 2). The results showed that p21CIP1/WAF1 protein levels remained relatively constant when cells were incubated in control medium for 24 hours and then reincubated with the same medium for another 24 hours (Figure 2; row i). When cells were incubated in control medium for 24 hours and then reincubated with FAC for up to 24 hours (Figure 2; row ii), there was little change in p21CIP1/WAF1 protein expression. This suggested that FAC alone did not affect basal p21CIP1/WAF1 protein expression in Fe-replete cells. In contrast, when cells were incubated with 311 for 24 hours to induce Fe depletion and then reincubated with this chelator for up to 24 hours (Figure 2; row iii), p21CIP1/WAF1 was not detectable. However, when cells were treated with 311 for 24 hours and then reincubated for up to 24 hours with FAC (100 μg/mL), there was a gradual increase in p21CIP1/WAF1 protein from 8 hours to 24 hours (Figure 2; row iv). This indicated that addition of Fe after incubation with 311 resulted in restoration of p21CIP1/WAF1. These results suggested that p21CIP1/WAF1 protein expression was regulated by Fe levels.
Reconstitution of p21CIP1/WAF1 protein expression after Fe chelation with the Fe supplement FAC. MCF-7 cells were preincubated with control medium (Con) or 311 (25 μM) for 24 hours at 37°C. Medium was then removed, and the cells were reincubated with either control medium (Con), FAC (100 μg/mL), 311 (25 μM), or 311 (25 μM) and FAC (100 μg/mL) for 0, 4, 8, 20, and 24 hours at 37°C. Cells were then harvested, total protein was extracted, and Western blot was performed to measure p21CIP1/WAF1 protein expression. Results are typical of 3 independent experiments.
Reconstitution of p21CIP1/WAF1 protein expression after Fe chelation with the Fe supplement FAC. MCF-7 cells were preincubated with control medium (Con) or 311 (25 μM) for 24 hours at 37°C. Medium was then removed, and the cells were reincubated with either control medium (Con), FAC (100 μg/mL), 311 (25 μM), or 311 (25 μM) and FAC (100 μg/mL) for 0, 4, 8, 20, and 24 hours at 37°C. Cells were then harvested, total protein was extracted, and Western blot was performed to measure p21CIP1/WAF1 protein expression. Results are typical of 3 independent experiments.
Fe depletion decreases nuclear export of p21CIP1/WAF1 mRNA to the cytosol
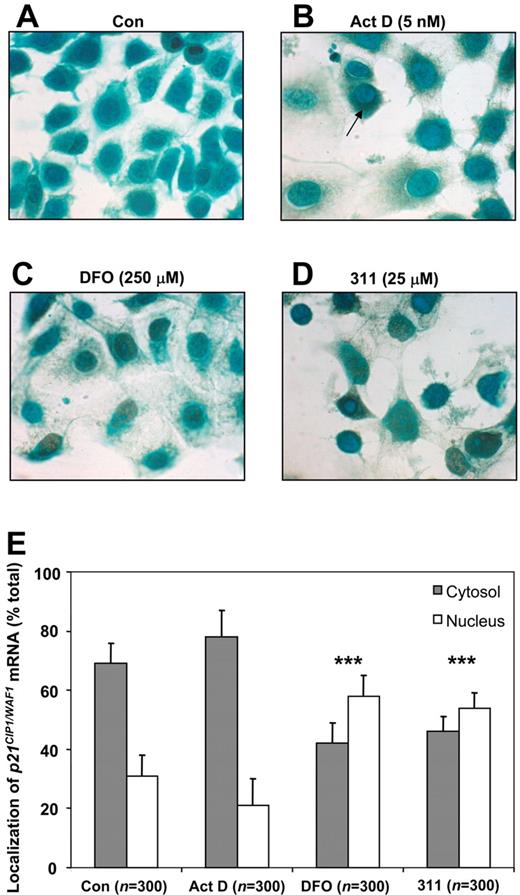
Considering p21CIP1/WAF1 mRNA expression was increased after Fe chelation while its protein level was decreased, this suggested that nuclear p21CIP1/WAF1 mRNA was not being efficiently transported to the cytosol for translation. This was examined by in situ RT-PCR (Figure 3). MCF-7 cells were treated with control medium (Con), Act D (5 nM), DFO (250 μM), or 311 (25 μM) for 24 hours, and the cellular localization of p21CIP1/WAF1 mRNA was then analyzed (observed as a dark green/brown stain within cells; see arrow in Figure 3). These results demonstrated that compared with Fe-replete control cells, where little dark green/brown staining occurred (Figure 3A), there was a marked increase in staining of p21CIP1/WAF1 mRNA in cells treated with Act D, DFO, or 311 (Figure 3B-D). These data confirm the RT-PCR results in Figure 1A, C, and E. Examining p21CIP1/WAF1 mRNA distribution between the nucleus and cytosol, in Fe-replete control cells, 69% (± 7%, cell number: n = 300) of p21CIP1/WAF1 mRNA was located in the cytoplasm, while 31%(± 7%, n = 300) was in the nucleus (Figure 3A). As a positive control, a low Act D concentration (5 nM) was used to induce DNA damage, which transcriptionally up-regulates p21CIP1/WAF1 mRNA.19,47 In this case, increased p21CIP1/WAF1 mRNA was mainly located within the cytosol, with only 21% (± 9%, n = 300) in the nucleus (Figure 3B). In comparison to Fe-replete cells, after Fe depletion, the proportion of p21CIP1/WAF1 mRNA localized in the nucleus was significantly (P < .001) increased to 58% (± 7%, n = 300) and 54% (± 5%, n = 300) for DFO- and 311-treated cells, respectively (Figure 3C-D). Hence, export of p21CIP1/WAF1 mRNA from the nucleus for translation was inhibited after Fe chelation, which may, in part, account for decreased p21CIP1/WAF1 protein levels. However, because p21CIP1/WAF1 mRNA was still identified within the cytosol after Fe depletion, this mechanism alone could not account for the decreased p21CIP1/WAF1 protein expression. Therefore, the possible role of Fe depletion in the degradation of p21CIP1/WAF1 was then studied.
In situ RT-PCR for localization of p21CIP1/WAF1 mRNA demonstrates that its nuclear translocation to the cytosol is partially inhibited after Fe chelation with DFO or 311. MCF-7 cells were grown on slides and incubated with control medium (Con), 311 (25 μM), DFO (250 μM), or Act D (5 nM) for 24 hours at 37°C. Cells were then fixed, and in situ RT-PCR was performed. Visual assessment of 300 cells was performed to examine cytosolic or nuclear localization of p21CIP1/WAF1 mRNA (dark green/brown stain), and the percentages were calculated (***P < .001 relative to the control). (A) Con; (B) Act D–treated cells; (C) 311-treated cells; and (D) DFO-treated cells. (E) Estimation of the nuclear and cytosolic localization of p21CIP1/WAF1 mRNA by cell counts (n = 300). Results are presented as means ± SD. Images in panels A-D were taken by Olympus microscope BX51 with UPLFLN 40× objective (0.75 numeric aperture) and attached DP30BW digital camera (Olympus, Tokyo, Japan). Images were analyzed using NIH Image J program (Bethesda, MD).
In situ RT-PCR for localization of p21CIP1/WAF1 mRNA demonstrates that its nuclear translocation to the cytosol is partially inhibited after Fe chelation with DFO or 311. MCF-7 cells were grown on slides and incubated with control medium (Con), 311 (25 μM), DFO (250 μM), or Act D (5 nM) for 24 hours at 37°C. Cells were then fixed, and in situ RT-PCR was performed. Visual assessment of 300 cells was performed to examine cytosolic or nuclear localization of p21CIP1/WAF1 mRNA (dark green/brown stain), and the percentages were calculated (***P < .001 relative to the control). (A) Con; (B) Act D–treated cells; (C) 311-treated cells; and (D) DFO-treated cells. (E) Estimation of the nuclear and cytosolic localization of p21CIP1/WAF1 mRNA by cell counts (n = 300). Results are presented as means ± SD. Images in panels A-D were taken by Olympus microscope BX51 with UPLFLN 40× objective (0.75 numeric aperture) and attached DP30BW digital camera (Olympus, Tokyo, Japan). Images were analyzed using NIH Image J program (Bethesda, MD).
Turnover studies using cycloheximide: iron depletion induces the degradation of p21CIP1/WAF1
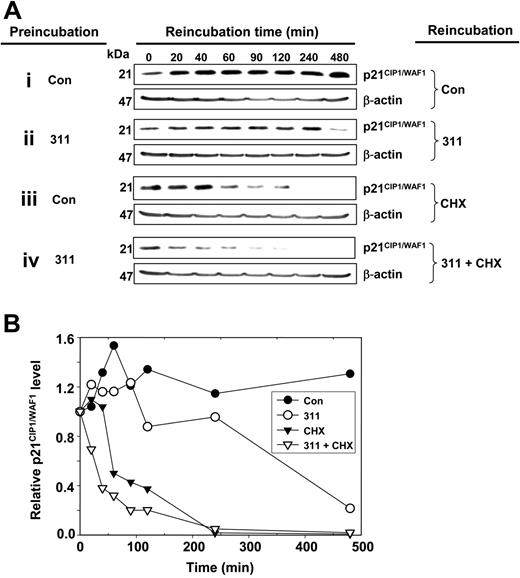
The effect of Fe chelation on p21CIP1/WAF1 protein degradation was examined using the protein synthesis inhibitor cycloheximide (CHX; 10 μg/mL) via standard methodology20,48 (Figure 4). MCF-7 cells were preincubated with or without 311 (25 μM) for 12 hours and then reincubated with or without CHX in the presence or absence of 311 (25 μM) for up to 480 minutes (Figure 4). Compared with Fe-replete control cells (Figure 4A, row i and 4B), the degradation of p21CIP1/WAF1 protein was accelerated after incubation with 311, there being a marked decrease after 480 minutes of reincubation (Figure 4A, row ii, and B). When Fe-replete control cells were reincubated with CHX, there was a decrease in p21CIP1/WAF1 protein expression that was totally ablated after 240 minutes (Figure 4A, row iii, and B), with a protein half-life of 61 minutes (± 4 minutes, n = 3). This estimation of p21CIP1/WAF1 half-life is similar to previous reports in Fe-replete cells.49,50 However, after preincubation of cells with 311 and then reincubation with 311 and CHX, there was a more rapid decrease in p21CIP1/WAF1 protein levels (Figure 4A, row iv, and B) than in the presence of CHX alone (Figure 4A, row iii, and B). In fact, in the presence of 311 and CHX, there was a significant (P < .01) decrease in the half-life of p21CIP1/WAF1 protein to 28 minutes (± 9 minutes, n = 3), indicating Fe depletion induced p21CIP1/WAF1 protein degradation.
Iron chelation decreases p21CIP1/WAF1 protein half-life. MCF-7 cells were preincubated with either control medium or medium containing 311 (25 μM) for 12 hours at 37°C and then reincubated for 20 to 480 minutes at 37°C with either control media alone (Con), 311 (25 μM), the protein synthesis inhibitor, cycloheximide (CHX; 10 μg/mL), or CHX (10 μg/mL) and 311 (25 μM). Cells were harvested and Western blot analysis was performed. (A) Western blot analysis. (B) Densitometric analysis of the results in panel A. Results are representative of 3 experiments.
Iron chelation decreases p21CIP1/WAF1 protein half-life. MCF-7 cells were preincubated with either control medium or medium containing 311 (25 μM) for 12 hours at 37°C and then reincubated for 20 to 480 minutes at 37°C with either control media alone (Con), 311 (25 μM), the protein synthesis inhibitor, cycloheximide (CHX; 10 μg/mL), or CHX (10 μg/mL) and 311 (25 μM). Cells were harvested and Western blot analysis was performed. (A) Western blot analysis. (B) Densitometric analysis of the results in panel A. Results are representative of 3 experiments.
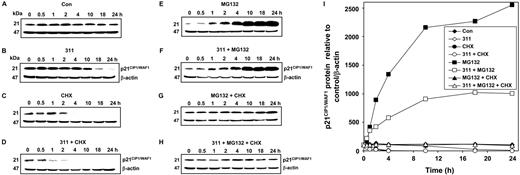
The degradation of p21CIP1/WAF1 protein after Fe depletion is mainly proteasome dependent
Considering that previous studies have suggested that p21CIP1/WAF1 is degraded by the proteasome by a variety of stimuli,39,51-53 and to assess the process of degradation after Fe chelation, experiments were performed using the proteasomal inhibitor, MG-13254-56 (Figure 5). Incubation of MCF-7 cells with control medium for up to 24 hours had no appreciable effect on p21CIP1/WAF1 protein (Figure 5A), while 311 (25 μM) markedly decreased its level only after an 18-hour incubation (Figure 5B). In contrast, CHX alone (Figure 5C) or CHX and 311 (Figure 5D) decreased p21CIP1/WAF1 protein expression after 4 hours and 1 hour, respectively. Incubation of cells with medium containing MG132 (20 μM)54-56 alone or MG132 and 311 increased p21CIP1/WAF1 protein after 1 hour (Figure 5E-F). Furthermore, addition of MG132 to 311 totally prevented the decrease in p21CIP1/WAF1 protein observed with 311 alone (Figure 5B), and in fact led to accumulation of the protein. This indicated that p21CIP1/WAF1 degradation was via a proteasome-dependent mechanism after Fe depletion. Comparing p21CIP1/WAF1 levels in Figure 5E and 5F, it is clear that in the presence of 311 and MG132 (Figure 5F), the accumulation of p21CIP1/WAF1 was less than that found with MG132 alone (Figure 5E). This suggested that the chelator also decreased p21CIP1/WAF1 protein expression via a proteasome-independent pathway. Potentially, this could be explained by the effect of the chelator at decreasing export of p21CIP1/WAF1 mRNA from the nucleus to the cytosol (Figure 3).
p21CIP1/WAF1 degradation is via the proteasomal pathway. (A-H) The effect of 311 on p21CIP1/WAF1 expression in MCF-7 cells in the presence or absence of the proteasomal inhibitor MG132 and the protein synthesis inhibitor cycloheximide (CHX). MCF-7 cells were incubated for 0.5 to 24 hours at 37°C with: (A) control medium (Con); (B) 311 (25 μM); (C) CHX (10 μg/mL); (D) 311 (25 μM) and CHX (10 μg/mL); (E) MG132 (20 μM); (F) 311 (25 μM) and MG132 (20 μM); (G) MG132 (20 μM) and CHX (10 μg/mL); or (H) 311 (25 μM), MG132 (20 μM), and CHX (10 μg/mL). (I) Densitometric analysis of the results in panels A-H. Cells were harvested and Western blot analysis was performed. Results shown are from a representative experiment of 3 performed.
p21CIP1/WAF1 degradation is via the proteasomal pathway. (A-H) The effect of 311 on p21CIP1/WAF1 expression in MCF-7 cells in the presence or absence of the proteasomal inhibitor MG132 and the protein synthesis inhibitor cycloheximide (CHX). MCF-7 cells were incubated for 0.5 to 24 hours at 37°C with: (A) control medium (Con); (B) 311 (25 μM); (C) CHX (10 μg/mL); (D) 311 (25 μM) and CHX (10 μg/mL); (E) MG132 (20 μM); (F) 311 (25 μM) and MG132 (20 μM); (G) MG132 (20 μM) and CHX (10 μg/mL); or (H) 311 (25 μM), MG132 (20 μM), and CHX (10 μg/mL). (I) Densitometric analysis of the results in panels A-H. Cells were harvested and Western blot analysis was performed. Results shown are from a representative experiment of 3 performed.
When control cells were incubated with MG132 and CHX to inhibit proteasomal degradation and protein synthesis, respectively, there was no appreciable change in protein expression with time (Figure 5G). This demonstrated these CHX and MG132 concentrations appropriately inhibited protein synthesis and proteasomal degradation, as the protein level was relatively constant up to 24 hours (Figure 5G). Similar observations were found when cells were incubated with MG132 and CHX in the presence of 311 (Figure 5H). Overall, the results in Figure 5 with MG132 suggested the proteasome played a major role in p21CIP1/WAF1 degradation under Fe-replete and -depleted conditions. Furthermore, experiments with another proteasomal inhibitor, namely lactacystin (10 and 20 μM),54-56 led to similar results found with MG132 (data not shown), confirming the role of the proteasome in p21CIP1/WAF1 protein degradation.
It could be suggested that the decreased p21CIP1/WAF1 protein expression after incubation with the chelator could be mediated by a direct increase in proteasomal activity. However, our previous studies20 performed under similar experimental conditions demonstrated no alteration in 20S or 26S proteasomal activity after Fe depletion. Hence, while the proteasome was involved in degradation of p21CIP1/WAF1 after Fe chelation, this was not mediated by an alteration in the rate of its proteolytic activity.
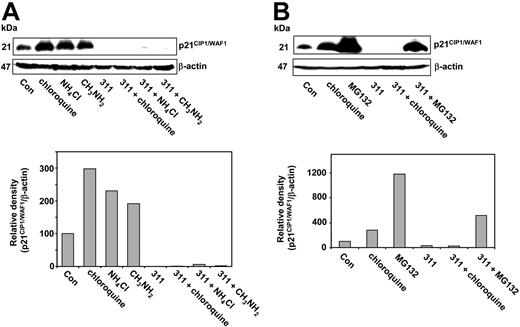
Levels of p21CIP1/WAF1 in Fe-replete cells are increased by lysosomotropic agents, but these compounds cannot prevent the chelator-induced decrease in p21CIP1/WAF1
The lysosome can degrade soluble proteins via chaperone-mediated autophagy.57,58 To investigate the possible role of a lysosomal mechanism in p21CIP1/WAF1 degradation, the well-characterized lysosomotropic agents chloroquine (250 μM), NH4Cl (15 mM), and CH3NH2 (15 mM)59-61 were incubated with Fe-replete MCF-7 cells for 24 hours. The results showed that p21CIP1/WAF1 protein levels increased after incubation with all these agents, with chloroquine being most effective (Figure 6A). However, in contrast to the ability of MG132 to prevent the chelator-mediated decrease in p21CIP1/WAF1 (Figures 5F, 6B), when the lysosomotropic agents were added with the chelator they could not rescue the decrease in p21CIP1/WAF1 (Figure 6A-B). It is notable that when chloroquine (250 μM) and MG132 (20 μM) were compared in the same experiment (Figure 6B), the increase in p21CIP1/WAF1 protein expression using chloroquine was considerably less than with MG132. Collectively, these results suggested p21CIP1/WAF1 can be degraded by both pathways in Fe-replete cells, but that the proteasome is dominant. In addition, chelator-induced p21CIP1/WAF1 degradation was lysosome independent.
Expression of p21CIP1/WAF1 protein is increased by lysosomotropic agents in Fe-replete cells, while chelator-induced down-regulation of p21CIP1/WAF1 can only be effectively rescued by proteasomal inhibitors. (A) MCF-7 cells were incubated for 24 hours at 37°C with either control medium (Con), chloroquine (250 μM), NH4Cl (15 mM), CH3NH2 (15 mM), 311 (25 μM), or the combination of 311 (25 μM) with chloroquine (250 μM), NH4Cl (15 mM), or CH3NH2 (15 mM). (B) MCF-7 cells were incubated for 24 hours at 37°C with either control medium (Con), chloroquine (250 μM), MG132 (20 μM), 311 (25 μM), or 311 (25 μM) combined with either chloroquine (250 μM) or MG132 (20 μM). Cells were harvested and Western blot was performed. Results are representative from 3 experiments formed.
Expression of p21CIP1/WAF1 protein is increased by lysosomotropic agents in Fe-replete cells, while chelator-induced down-regulation of p21CIP1/WAF1 can only be effectively rescued by proteasomal inhibitors. (A) MCF-7 cells were incubated for 24 hours at 37°C with either control medium (Con), chloroquine (250 μM), NH4Cl (15 mM), CH3NH2 (15 mM), 311 (25 μM), or the combination of 311 (25 μM) with chloroquine (250 μM), NH4Cl (15 mM), or CH3NH2 (15 mM). (B) MCF-7 cells were incubated for 24 hours at 37°C with either control medium (Con), chloroquine (250 μM), MG132 (20 μM), 311 (25 μM), or 311 (25 μM) combined with either chloroquine (250 μM) or MG132 (20 μM). Cells were harvested and Western blot was performed. Results are representative from 3 experiments formed.
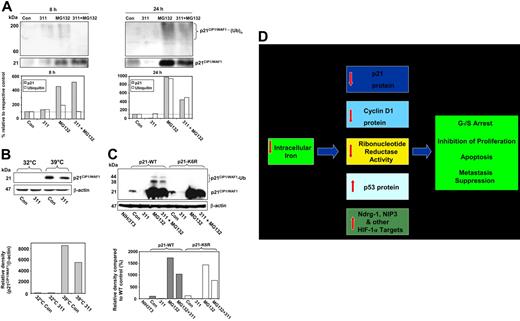
Polyubiquitination of p21CIP1/WAF1 is decreased in 311-treated cells during inhibition of proteasomal activity
Proteasomal degradation of p21CIP1/WAF1 under other conditions has been reported to occur by both ubiquitin-dependent and -independent mechanisms.39,51-53 To investigate how Fe depletion induces proteasome-mediated degradation, we examined the effect of chelation on p21CIP1/WAF1 polyubiquitination. In these studies, p21CIP1/WAF1 protein was immunoprecipitated with anti-p21CIP1/WAF1, and the complex was collected, washed, and separated using SDS-PAGE.20,43 Western analysis was then performed using anti-p21CIP1/WAF1 or antiubiquitin (Figure 7A). Cells were incubated for 8 or 24 hours with medium alone or medium containing 311 (25 μM) in the presence or absence of MG132 (20 μM). These time points were chosen because there was little effect of 311 on p21CIP1/WAF1 protein expression at 8 hours, while a pronounced decrease occurred after 24 hours (Figure 1D).
Role of ubiquitination in degradation of p21CIP1/WAF1 after Fe chelation. (A-C) Iron chelators decrease p21CIP1/WAF1 polyubiquitination when proteasomal activity is inhibited. (D) A simplified schematic illustration showing the multiple molecular effectors involved in G1/S arrest of the cell cycle, inhibition of proliferation, metastasis suppression, and induction of apoptosis. MCF-7 cells were incubated with control media (Con) or media containing 311 (25 μM) in the presence or absence of MG132 (20 μM) for 8 or 24 hours. Immunoprecipitation was performed using anti-p21CIP1/WAF1 antibody. Complexes were then separated by SDS-PAGE and probed with anti-p21CIP1/WAF1 or antiubiquitin antibody using Western analysis. (B) Iron chelators induce ubiquitin-independent degradation of p21CIP1/WAF1 in A31N-ts20 cells. A31N-ts20 cells were used, as they have a temperature-sensitive E1 ubiquitin–activating enzyme that is active at 32°C, but which inactivates at 39°C, preventing ubiquitin-dependent proteasomal degradation.40,62 Cells were grown at 32°C or 39°C for 24 hours before the experiment. The media was then removed, and the cells were incubated for 24 hours at either temperature with control media, or media containing 311 (25 μM), and Western analysis was performed. (C) Iron chelators induce ubiquitin-independent degradation of p21CIP1/WAF1 in p21-K6R–transfected cells. Mouse fibroblast NIH3T3 cells were transiently transfected with pCS2+p21WT or pCS2+p21-K6R plasmids, which contain wild-type (p21-WT) or mutant forms for human p21CIP1/WAF1, respectively. At 24 hours after transfection, cells were incubated for 24 hours with either control medium, 311 (25 μM), MG132 (20 μM), or the combination of 311 (25 μM) and MG132 (20 μM). Western blotting was performed using antihuman p21CIP1/WAF1 antibody, which only detects the transfected wild-type and mutant human p21CIP1/WAF1. Results are a representative experiment from 3 performed. (D) Multiple molecular effectors involved in G1/S arrest, inhibition of proliferation, apoptosis, and metastasis suppression are modulated in response to Fe deprivation. These responses include down-regulation of p21CIP1/WAF1 protein,18,21 down-regulation of cyclin D1 protein,20,21,23 decreased activity of ribonucleotide reductase,63,64 increased p53 protein expression,17,25 increased expression of the metastasis suppressor protein Ndrg-1,19 and the up-regulation of the apoptosis-inducing protein NIP3.65 Both NIP3 and Ndrg-1 are targets of the hypoxia inducible factor-1α (HIF-1α) transcription factor.19,65 Other HIF-1α targets are probably also involved in the Fe depletion–induced inhibition of proliferation and induction of apoptosis.30
Role of ubiquitination in degradation of p21CIP1/WAF1 after Fe chelation. (A-C) Iron chelators decrease p21CIP1/WAF1 polyubiquitination when proteasomal activity is inhibited. (D) A simplified schematic illustration showing the multiple molecular effectors involved in G1/S arrest of the cell cycle, inhibition of proliferation, metastasis suppression, and induction of apoptosis. MCF-7 cells were incubated with control media (Con) or media containing 311 (25 μM) in the presence or absence of MG132 (20 μM) for 8 or 24 hours. Immunoprecipitation was performed using anti-p21CIP1/WAF1 antibody. Complexes were then separated by SDS-PAGE and probed with anti-p21CIP1/WAF1 or antiubiquitin antibody using Western analysis. (B) Iron chelators induce ubiquitin-independent degradation of p21CIP1/WAF1 in A31N-ts20 cells. A31N-ts20 cells were used, as they have a temperature-sensitive E1 ubiquitin–activating enzyme that is active at 32°C, but which inactivates at 39°C, preventing ubiquitin-dependent proteasomal degradation.40,62 Cells were grown at 32°C or 39°C for 24 hours before the experiment. The media was then removed, and the cells were incubated for 24 hours at either temperature with control media, or media containing 311 (25 μM), and Western analysis was performed. (C) Iron chelators induce ubiquitin-independent degradation of p21CIP1/WAF1 in p21-K6R–transfected cells. Mouse fibroblast NIH3T3 cells were transiently transfected with pCS2+p21WT or pCS2+p21-K6R plasmids, which contain wild-type (p21-WT) or mutant forms for human p21CIP1/WAF1, respectively. At 24 hours after transfection, cells were incubated for 24 hours with either control medium, 311 (25 μM), MG132 (20 μM), or the combination of 311 (25 μM) and MG132 (20 μM). Western blotting was performed using antihuman p21CIP1/WAF1 antibody, which only detects the transfected wild-type and mutant human p21CIP1/WAF1. Results are a representative experiment from 3 performed. (D) Multiple molecular effectors involved in G1/S arrest, inhibition of proliferation, apoptosis, and metastasis suppression are modulated in response to Fe deprivation. These responses include down-regulation of p21CIP1/WAF1 protein,18,21 down-regulation of cyclin D1 protein,20,21,23 decreased activity of ribonucleotide reductase,63,64 increased p53 protein expression,17,25 increased expression of the metastasis suppressor protein Ndrg-1,19 and the up-regulation of the apoptosis-inducing protein NIP3.65 Both NIP3 and Ndrg-1 are targets of the hypoxia inducible factor-1α (HIF-1α) transcription factor.19,65 Other HIF-1α targets are probably also involved in the Fe depletion–induced inhibition of proliferation and induction of apoptosis.30
Immunoprecipitation and Western blotting with anti-p21CIP1/WAF1 demonstrated that compared with the control after 8 hours, there was little effect on p21CIP1/WAF1 expression of incubating cells with 311 (Figure 7A). However, Fe chelation caused near ablation of p21CIP1/WAF1 compared with the control after 24 hours (Figure 7A). Incubation with MG132 alone or MG132 and 311 increased p21CIP1/WAF1 relative to the control at 8 and 24 hours. However, after 24 hours, there was a decrease in p21CIP1/WAF1 expression in the presence of 311 and MG132 relative to MG132 alone (Figure 7A), in agreement with Figure 5E and 5F.
Western blotting with antiubiquitin antibody demonstrated that p21CIP1/WAF1 polyubiquitination resulted in high Mr [molecular weight] species between 60 and 200 kDa (Figure 7A), in agreement with previous work.39 These latter bands were above the immunoglobulin G heavy chain at 50 kDa (data not shown). Incubation with 311 alone caused no significant change in p21CIP1/WAF1 ubiquitination compared with the control at 8 hours over 3 experiments. After 24 hours of incubation with 311, there was no increase in p21CIP1/WAF1 ubiquitination relative to the control, despite complete ablation of p21CIP1/WAF1 (Figure 7A). However, compared with the controls, there was an increase in p21CIP1/WAF1 polyubiquitination in the presence of MG132 after an 8-hour and 24-hour incubation, due to the ability of this agent to inhibit p21CIP1/WAF1 degradation by the proteasome. Hence, under Fe-replete control conditions, p21CIP1/WAF1 was ubiquitinated before proteasomal degradation.
In the presence of 311 and MG132, there was a decrease in p21CIP1/WAF1 ubiquitination in comparison to MG132 alone after an 8- and 24-hour incubation (Figure 7A). Decreased p21CIP1/WAF1 ubiquitination mediated by Fe depletion would not favor proteasomal degradation via the ubiquitin pathway and could not explain the decreased p21CIP1/WAF1 after chelation. Considering this, further studies examined the role of ubiquitin-independent proteasomal degradation of p21CIP1/WAF1 after Fe depletion.
Iron depletion–induced degradation of p21CIP1/WAF1 via the proteasome is independent of ubiquitination
To determine the role of ubiquitin-independent p21CIP1/WAF1 protein degradation after Fe chelation, we used the A31N-ts20 cell line that has a temperature-sensitive E1-ubiquitin–activating enzyme.40,62 The E1 enzyme is inactivated at 39°C, preventing ubiquitin-dependent proteasomal degradation, but is active at 32°C.40 Cells were grown for 24 hours at 32°C or 39°C before the experiment to ensure maximum activation or inactivation of E1, respectively. The media was then removed and the cells incubated for 24 hours at either temperature with control media or 311 (25 μM).
The results showed that in Fe-replete control cells, p21CIP1/WAF1 protein was markedly increased when grown at 39°C compared with 32°C, again demonstrating p21CIP1/WAF1 protein degradation was via a ubiquitin-dependent proteasomal pathway when cells were Fe replete (Figure 7B). At 32°C, p21CIP1/WAF1 was not detected in cells incubated with control medium or 311, demonstrating the efficacy of the ubiquitin-dependent mechanism at degrading the protein at this temperature.
Incubation with 311 down-regulated p21CIP1/WAF1 protein levels when A31N-ts20 cells were grown at 39°C, where ubiquitin-dependent proteasomal degradation was prevented (Figure 7B). This observation indicated that the effect of Fe depletion on p21CIP1/WAF1 protein expression was at least partially ubiquitin-independent (Figure 7B).
To further assess the role of a ubiquitin-independent process in the proteasomal degradation of p21CIP1/WAF1, we examined a widely implemented model39,51,66 consisting of murine NIH3T3 cells transiently transfected with either: (1) a mutant form of human p21CIP1/WAF1 (p21-K6R) where the potential ubiquitin-conjugation sites consisting of 6 lysine residues were mutated to arginine; or (2) human wild-type p21CIP1/WAF1 (p21-WT), which can be ubiquitinated39 (Figure 7C).
Western analysis results using antihuman p21CIP1/WAF1 antibody and murine NIH3T3 cells transfected with p21-WT demonstrated that endogenous mouse p21CIP1/WAF1 could not be detected (Figure 7C). Indeed, only transfected human p21CIP1/WAF1 could be observed under these conditions. It was clear in cells transfected with p21-WT that after a 24-hour incubation with 311 (25 μM), there was a marked decrease in p21CIP1/WAF1 expression compared with cells incubated with control medium (Figure 7C). Incubation of these cells with MG132 alone led to a pronounced increase in p21CIP1/WAF1 protein at 21 kDa and also the appearance of 2 higher Mr bands at 38 and 44 kDa, consistent with the ubiquitinated protein.39 Incubation of p21-WT transfected cells with 311 and MG132 also led to a pronounced increase in p21CIP1/WAF1 and its ubiquitinated products, although the intensity of these bands were less than in the presence of MG132 alone. Hence, as shown in Figure 7A, incubation with 311 and MG132 compared with 311 alone decreased the expression of p21CIP1/WAF1 and its ubiquitinated forms. These data may suggest decreased p21CIP1/WAF1 ubiquitination during Fe depletion. However, this would not explain the increased degradation of p21CIP1/WAF1 if the ubiquitin-mediated pathway were involved. Alternatively, these observations are consistent with a ubiquitin-independent process of p21CIP1/WAF1 degradation by the proteasome.
Considering these 2 possibilities, when 311 was incubated with cells transfected with p21-K6R that cannot be ubiquitinated,39 there was a decrease in p21CIP1/WAF1 relative to the untreated control, indicating chelator treatment led to ubiquitin-independent degradation of p21CIP1/WAF1 (Figure 7C). Using these cells, incubation with MG-132 led to a marked increase in p21CIP1/WAF1, but unlike cells transfected with p21-WT, there was no evidence of high-Mr ubiquitinated products. These results confirm that p21-K6R could not be ubiquitinated, in agreement with previous studies.39 Incubation of p21-K6R–transfected cells with 311 and MG132 reduced p21CIP1/WAF1 expression relative to MG132 alone, demonstrating induction of ubiquitin-independent proteasomal degradation after Fe depletion (Figure 7C).
Discussion
Despite the fact that Fe depletion induces a G1/S arrest20 and apoptosis,67 it is surprising that little is known concerning the role of Fe in cell-cycle regulation. Furthermore, it has become clear that some Fe chelators show promising anticancer activity, inducing cell-cycle arrest and apoptosis.2,5 However, the mechanisms involved in these effects remain uncertain and important to investigate. The cdk inhibitor p21CIP1/WAF1 plays a key role in regulating the cell cycle and inducing apoptosis36,68 and the alteration of its expression after chelation could play important roles in these processes.
For the first time, this study examines in detail the posttranscriptional regulation of p21CIP1/WAF1 after Fe depletion and has demonstrated that its down-regulation is due to 2 mechanisms, namely (1) inhibited translocation of p21CIP1/WAF1 mRNA from the nucleus to the translational machinery in the cytosol, and (2) the induction of a ubiquitin-independent mechanism of proteasomal degradation. Considering these processes, the translocation of p21CIP1/WAF1 mRNA from the nucleus involves many factors and proteins, such as the small GTPase Ran, which is involved in regulating RNA nuclear export.69 At present, the exact pathway Fe depletion affects to modulate p21CIP1/WAF1 mRNA export remains unknown. However, it is relevant to note that the effect of Fe chelation on reducing p21CIP1/WAF1 mRNA export does not appear to be a general response, as the mRNA expression of other genes (eg, TfR1, Ndrg-1, and VEGF-1) are increased under these conditions, leading to increased translation.9,19,20
It was clear from in situ RT-PCR studies that while there was decreased nuclear translocation of p21CIP1/WAF1 mRNA to the cytosol comparing control and 311-treated cells (from 69% to 46%), this could not totally account for the decrease in p21CIP1/WAF1 expression. Indeed, a proteasome-mediated process was the major mechanism involved in the reduction of p21CIP1/WAF1 protein after Fe chelation. We suggest this because the Fe depletion–mediated decrease in p21CIP1/WAF1 expression could be effectively rescued by proteasomal inhibitors (Figures 5, 6B). Proteasomal-mediated degradation of p21CIP1/WAF1 has been reported to occur via ubiquitin-dependent or -independent pathways, depending on the conditions.39,51-53 In this investigation, we showed that p21CIP1/WAF1 protein degradation after Fe depletion was via a ubiquitin-independent process, while in Fe-replete cells, a ubiquitin-dependent mechanism was responsible.
The induction of a ubiquitin-independent mechanism of proteasomal degradation was suggested by immunoprecipitation, where p21CIP1/WAF1 ubiquitination was reduced in cells incubated with 311 and MG132 relative to cells incubated with MG132 alone (Figure 7A). The implementation of 2 other models confirmed this observation. First, using A31N-ts20 cells, 311 decreased p21CIP1/WAF1 protein even when the ubiquitin-dependent pathway was inhibited at 39°C (Figure 7B). Second, Fe depletion via 311 was able to down-regulate mutant p21CIP1/WAF1 (p21-K6R) protein, which cannot not bind ubiquitin for degradation.39 Taken together, these results indicate chelator-mediated down-regulation of p21CIP1/WAF1 was by the proteasome via a ubiquitin-independent process. This ubiquitin-independent pathway of p21CIP1/WAF1 degradation may be mediated by NAD(P)H:quinone-oxidoreductase-170,71 or antizyme,72 which are responsible for degradation of other proteins by this process.
Examination of Fe chelator–mediated down-regulation of p21CIP1/WAF1 is important, as apart from being a cdk inhibitor31-33 and positive regulator of G1 phase progression,34,35 this molecule also has antiapoptotic activity.36,68 Considering the role of p21CIP1/WAF1 in promoting G1 progression, this is due to its ability to stabilize cyclin D1–cdk complexes.34 Since Fe chelation decreases expression of both cyclin D120 and p21CIP1/WAF1, this will prevent the formation of such complexes and promote G1/S arrest. Regarding the additional role of p21CIP1/WAF1 in apoptosis, it is known that high p21CIP1/WAF1 expression in some cancers may provide a growth advantage capable of subverting apoptosis induced by DNA-damaging chemotherapeutics.36 In fact, by decreasing p21CIP1/WAF1 expression using antisense oligonucleotides, cancer cell apoptosis can be induced.37,73 Hence, p21CIP1/WAF1 has been proposed as a target for developing novel anticancer agents.36 Since Fe chelators effectively inhibit p21CIP1/WAF1 expression, this is important for understanding their marked antitumor activity10 and the ability to induce apoptosis.44,67 However, the effect of chelators at inhibiting cancer proliferation and inducing apoptosis is probably due to effects on multiple molecular targets (Figure 7D).9 These include, but are not limited to, inhibition of the rate-limiting step of DNA synthesis, ribonucleotide reductase,63,64 the capacity of Fe depletion to decrease cyclin D1 expression,20 and the up-regulation of the growth and metastasis suppressor Ndrg-1.19 In fact, the existence of multiple targets of chelators that play key roles in proliferation and inducing apoptosis may explain their pronounced activity and ability to overcome resistance to antitumor agents.10
It is of interest that up-regulation of p21CIP1/WAF1 mRNA but down-regulation of its protein after Fe depletion are different to the effects on its expression during cell-cycle arrest in response to other conditions. For instance, previous studies showed that p21CIP1/WAF1 mRNA and protein were up-regulated during cell-cycle arrest,74-77 suggesting increased expression at the transcriptional and posttranscriptional levels. Moreover, in the current study, G1/S arrest induced by FCS starvation caused down-regulation of both p21CIP1/WAF1 mRNA and protein levels in MCF-7 cells (data not shown). Collectively, these observations indicate that the paradoxic chelator-mediated up-regulation of p21CIP1/WAF1 mRNA and down-regulation of p21CIP1/WAF1 protein is distinctive to Fe depletion. The mechanism responsible for the transcriptional up-regulation of p21CIP1/WAF1 remains unclear and beyond the scope of this study, although the existence of redox-sensitive regulatory regions (eg, hypoxia-response element and AP2 and Sp1 DNA-binding sites) within its promoter provides clues for further investigation.17
It is well known that cyclin D1 plays an important role in G1/S progression78 and, like p21CIP1/WAF1, we have shown that it was down-regulated after Fe chelation by the proteasome via a ubiquitin-independent process.20 Thus, one could speculate that Fe depletion may induce a ubiquitin-independent mechanism of proteasomal degradation, leading to breakdown of select proteins signaling arrest and apoptosis.21 Why a ubiquitin-independent mechanism is used remains unclear at present, but the existence of this pathway in addition to the well-known ubiquitin-dependent mechanism could be important in terms of controlling protein degradation. The ubiquitin-independent process does not appear to be a general response to Fe deprivation, leading to the degradation of all proteins, as under these same conditions, the expression of other Fe-regulated proteins is increased.9,19,20 It is also relevant to discuss that a previous study suggested cyclin D1 inhibited p21CIP1/WAF1 degradation by competing for the binding site of proteasomes.49 Therefore, cyclin D1 down-regulation after Fe depletion20 may contribute to decreased p21CIP1/WAF1.
In summary, Fe depletion significantly inhibited p21CIP1/WAF1 mRNA export from the nucleus and induced proteasomal degradation via a ubiquitin-independent mechanism. Together, these processes were responsible for decreasing p21CIP1/WAF1 protein expression in spite of increased p21CIP1/WAF1 mRNA. These results are relevant to understanding the molecular alterations in G1/S arrest during Fe deficiency and the mechanism of chelators at inducing antiproliferative activity and apoptosis.
An Inside Blood analysis of this article appears at the front of this article.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Dr Erika Becker, Dr David Lovejoy, Dr Robert Sutak, Dr Daniel Vyoral, Ms Sarah Champion, Ms Danuta Kalinowski, Ms Zaklina Kovacevic, Mr Yohan Suryo Rahmanto, and Ms Yu Yu (Iron Metabolism and Chelation Program, Department of Pathology) are thanked for their kind help in reviewing the paper prior to submission.
This work was supported by a fellowship and grants from the National Health and Medical Research Council (NHMRC) and Australian Rotary Health Research Fund to D.R.R.
Authorship
Contribution: D.R.R. designed the study, obtained grant funding, and wrote the manuscript. D.F. designed studies, wrote the paper, and performed experiments.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: D. R. Richardson, Department of Pathology, University of Sydney, Sydney, New South Wales, 2006, Australia; e-mail: d.richardson@med.usyd.edu.au.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal