Abstract
Although proinflammatory cytokines are key mediators of tissue damage during graft-versus-host disease (GVHD), IFNγ has previously been attributed with both protective and pathogenic effects. We have resolved this paradox by using wild-type (wt), IFNγ−/−, and IFNγR−/− mice as donors or recipients in well-described models of allogeneic stem cell transplantation (SCT). We show that donor-derived IFNγ augments acute GVHD via direct effects on (1) the donor T cell to promote T helper 1 (Th1) differentiation and (2) the gastrointestinal (GI) tract to augment inflammatory cytokine generation. However, these detrimental effects are overwhelmed by a protective role of IFNγ in preventing the development of idiopathic pneumonia syndrome (IPS). This is the result of direct effects on pulmonary parenchyma to prevent donor cell migration and expansion within the lung. Thus, IFNγ is the key cytokine differentially controlling the development of IPS and gastrointestinal GVHD after allogeneic SCT.
Introduction
Allogeneic bone marrow transplantation (BMT) is a definitive curative therapy for most hematologic malignancies and severe immunodeficiencies. The major complication of allogeneic BMT remains graft-versus-host disease (GVHD) in which the skin, gastrointestinal (GI) tract, liver, and lung are preferentially damaged by the transplanted donor immune system.1 GVHD occurs in most (50%-70%) recipients and is largely responsible for the high mortality associated with allogeneic BMT. Idiopathic pneumonia syndrome (IPS) is an acute noninfectious lung injury that typically occurs 3 to 4 weeks after BMT, responds poorly to therapy, and is associated with a high mortality.2 There is thus a pressing need for new treatment approaches to both prevent and treat the full spectrum of GVHD, based on a logical understanding of the underlying disease pathophysiology.
Current paradigms suggest that GVHD occurs via a complex cellular network initiated by the interaction of antigen-presenting cells (APCs) and naive donor T cells.3-5 Subsequent T helper 1 (Th1) differentiation leads to the generation of donor cytotoxic T lymphocytes (CTLs) and large amounts of inflammatory cytokines that damage host tissue by both major histocompatibility complex (MHC)-dependent and -independent pathways.6 Of the Th1 cytokines, IFNγ is perhaps the most immunologically dominant, influencing a plethora of cell subsets during allograft rejection.7 However the effects of this cytokine on GVHD are unclear, with a number of contradictory studies8-11 suggesting that a clearer understanding of the mechanisms involved are needed. We have re-examined this issue using both IFNγ−/− and IFNγR−/− stem cell transplantation (SCT) donors or recipients following myeloablative conditioning. We demonstrate that donor-derived IFNγ indeed has both positive and negative effects on GVHD due to differential effects on donor and host tissue, and individual target organs. First, IFNγ augments acute GVHD via direct affects on the donor T cell to promote Th1 differentiation and the GI tract to augment inflammatory cytokine generation. However, these detrimental effects are overwhelmed by a protective role of IFNγ in preventing the development of IPS and cutaneous GVHD. These studies provide additional mechanistic data that clarify the conflicting studies to date and provide a logical system to identify a novel and previously unrecognized inhibitory pathway that prevents the development of IPS after SCT.
Materials and methods
Mice
Female B6 (H-2b, CD45.2+), Balb/c (H-2d), and B6D2F1 (H-2b/d, CD45.2+) mice were purchased from the Animal Resources Center (Perth, Western Australia, Australia). IFNγ−/− (B6, H-2b) and IFNγR−/− (B6, H-2b) were supplied from The Jackson Laboratories (Bar Harbor, ME). Inducible nitric oxide synthase (iNOS−/−;129, H-2b), endothelial nitric oxide synthase (eNOS−/−; B6, H-2b) mice and relevant control wild-type (wt) mice were supplied by The Australian National University. The age of mice used as donors and recipients were between 7 and 10 weeks. Mice were housed in sterilized microisolator cages and received acidified autoclaved water (pH 2.5) after transplantation.
Cytokine mobilization
Recombinant human granulocyte colony-stimulating factor (G-CSF; Amgen, Thousand Oaks, CA) was given subcutaneously at 10 μg/animal on days −6 to −1. Donor spleens were harvested on day 0.
SCT
Mice received transplantations as described previously.12-14 Briefly, on day −1, B6D2F1 mice received 1100 rad total body irradiation or C57BL/6 and IFNγR−/− received 1000 rad total body irradiation (137Cs source at 108 rad/min), split into 2 doses separated by 3 hours to minimize gastrointestinal toxicity. B6 (107) or Balb/c (2.5 × 107) donor splenocytes, corrected to administer equivalent numbers of CD3+ T cells (typically 40% of grafts) between groups, were injected intravenously on day 0. Animal procedures were undertaken using protocols approved by the institutional (Queensland Institute of Medical Research) animal ethics committee. Mice that received transplants were monitored daily; those with GVHD clinical scores of 6 or higher were euthanized, and the date of death was registered as the next day in accordance with institutional animal ethics committee guidelines. We generated mixed chimeric mice by transplanting 5 × 106 wt or IFNγR−/− bone marrow cells into irradiated (1000 rad) wt or IFNγR−/− recipients, which were then allowed to reconstitute over 3 to 4 months before use as allograft recipients. In some experiments, TNFR:Fc (Enbrel; Amgen) at 100 μg/dose intraperitoneally on −2 to 21 alternate days or equivalent doses of human IgG were administered as previously described.15,16
Assessment of GVHD
The degree of systemic GVHD was assessed by scoring as previously described (maximum index = 10).17
Cell preparation
T cells were purified using magnetic bead depletion of non–T-cell splenocytes. Briefly, following red cell lysis, splenocytes were incubated with purified mAb (CD19, B220, Gr-1, CD11b, and Ter119). After incubation with antibodies, cells were incubated with goat anti-rat IgG BioMag beads (Qiagen, Melbourne, Australia) for 20 minutes on ice, and then placed on a magnet. Subsequent CD3+ T-cell purities were more than 90%, and 2 to 3 × 106 T cells were added to T-cell–depleted (TCD) grafts per animal. For total T-cell depletion, splenocytes were incubated with hybridoma supernatants containing anti-CD4 (RL172), anti-CD8 (TIB211), and Thy1.2 (HO-13-4) mAbs followed by incubation with rabbit complement (Cedarlane Laboratories, Burlington, ON, Canada) as previously described.12 Note that this also eliminates professional APCs, which results in reduced GVHD severity. Resulting cell suspensions contained less than 1% contamination of viable CD3+ T cells. Splenic dendritic cells (DCs) were purified using CD11c+ magnetic-activated cell-sorting (MACS) beads and MiniMACS positive selection columns (Miltenyi Biotech Pty Ltd, Sydney, NSW, Australia) according to the manufacturer's protocol. DCs were obtained at more than 70% purity. Donor T cells (H-2Dd+) were sorted by fluorescence-activated cell sorting (FACS) based on CD4 or CD8 staining by Moflo (DakoCytomation, Fort Collins, CO) to more than 95% purity. To study cell infiltration in the lung, the right upper lobe was removed en bloc and digested in collagenase and DNAse. Cells were counted then phenotyped, and results were expressed as total cells per right upper lobe of lung.
Chromium 51 release assay
Targets were labeled with chromium 51 (51Cr) as previously described. Target cells (host-type EL4, H-2b; donor-type A20, H-2d) were cultured with donor CD8+ effectors and sort-purified from the spleen of recipients 9 days after SCT for 5 hours at 37°C and 5% CO2. 51Cr release into supernatants was determined via gamma counter (TopCount microplate scintillation counter; Packard Instrument Co, Shelton, CT). Spontaneous release was defined from wells receiving targets only, and total release from wells receiving targets plus 1% Triton X-100. Percentage cytotoxicity was calculated as % cytotoxicity = (experimental release − spontaneous release) / (total release − spontaneous release) × 100.
Mixed lymphocyte culture
Mixed lymphocyte cultures (MLCs) were set up in triplicate in round-bottom 96-well plates (BD Falcon, Bedford, MA). Purified T cells from Balb/c mice were stimulated with C57BL/6 or IFNγR−/− CD11c+ DCs or peritoneal F4/80+ macrophages. Plates were incubated at 37°C for 72 hours before pulsing with 3[H]-thymidine (0.037 MBq/well [1 μCi/well]). At 12 to 16 hours later, cultures were harvested onto glass fiber filter mats (Wallac, Turku, Finland) and 3[H]-thymidine incorporation determined using a 1205 Betaplate reader (Wallac).
FACS analysis
The following mAbs were purchased from BioLegend (San Diego, CA): Fluorescein isothiocyanate (FITC)-conjugated CD45.2(104) and IgG2a isotype control; phycoerythrin (PE)-conjugated CD3 (2C11), CD4 (GK1.5), CD8a (53-6.7), CD11b (M1/70), CD11c (HL3), CD19 (1D3), CD45.1 (A20), CD45R/B220 (RA3-6B2), I-A/I-E (2G9), and IgG2b isotype control; and biotinylated CD45.1 and IgG2a isotype control. FITC-conjugated F4/80 was purchased from Serotec (Oxford, United Kingdom). Streptavidin-PE-Cy5 was from DAKO (Carpinteria, CA). Purified mAb against CD3 (KT3), CD19 (HB305), Gr1 (RB6-8C5), Thy1.2 (HO-13-4), Ter119, and FcγR II/III (2.4G2) were produced “in house.”
Cytokine and chemokine analysis
IFNγ, IL-5, IL-4, IL-2, and TNFα were determined using the BD Cytometric Bead Array system (BD Biosciences Pharmingen, San Diego, CA). All assays were performed according to the manufacturer's protocol. Chemokine enzyme-linked immunosorbent assays (ELISAs) were conducted using antibodies and standards from R&D systems (Minneapolis, MN) as described previously.18-20
Histology
Formalin-preserved skin, liver, small bowel, and lung were embedded in paraffin, and 5-μm-thick sections were stained with hematoxylin and eosin for histologic examination. Slides were coded and examined in a blinded fashion by A.D.C. using the semiquantitative scoring system as previously described.12,16,17,21 Scores were added to provide a total score of 24 for skin, 28 for small bowel, 40 for liver, and 18 for lung. To undertake semiquantitative analysis of leukocyte adhesion and migration across endothelium in lung, the number of adherent or migrating cells were counted in 10 venules per lung section (by A.D.C. in a blinded fashion) and the mean for each was determined. Images of GVHD target tissue were acquired at various times after transplantation using an Olympus BX51 microscope (Olympus Japan, Tokyo) UPlanFLN × 10/0.3 and × 40/0.75 objective lens (Olympus), an Evolution MP 5.0 camera (Media Cybernetic, Bethesda, MD), and Qcapture software (Qimaging, Surrey, BC, Canada). For immunohistochemistry, anti–ICAM-1 and anti–VCAM-1 mAbs were purchased from BD Biosciences (Franklin Lakes, NJ). Staining was conducted on 6-μm acetone-fixed lung sections, and primary antibodies were detected with appropriate secondary detection reagents and horseradish peroxidase according to the manufacturer's instructions (Vector Laboratories, Peterborough, United Kingdom). Sections were stained with hematoxylin and eosin, dehydrated, and mounted prior to microscopic examination. Images were then acquired on a Scanscope T2 (Aperio Technologies, Vista, CA) with 20 × magnification at room temperature, then magnified 3.1 × and cropped to size with Imagescope (Aperio Technologies) software.
IDO inhibitor treatment
Slow-release polymer pellets containing the indoleamine-2,3-dioxygenase (IDO) inhibitor 1-MT (420 mg) or placebo pellets that result in systemic IDO blockade for 21 days were inserted into recipient mice at day −4 as per the manufacturer's instructions (Innovative Research, Sarasota, FL).
Real-time PCR
Total RNA was extracted from lung tissue using TRIzol reagent (Invitrogen, Mount Waverley, Australia), according to the manufacturer's instructions, and contaminants were removed by passing the RNA over RNeasy mini columns with on-column DNase treatment (Qiagen). RNA 6000 Nano Assay Kits (Agilent Technologies, Forest Hill, Australia) were used to assess RNA quality and integrity on a Bioanalyzer 2100 (Agilent Technologies). Samples were included in the study based on 260:280 nm absorbance readings. Individual RNA samples were reverse-transcribed into cDNA using the cDNA Archive Kit (Applied Biosystems, Scoresby, Australia). Real-time polymerase chain reaction (PCR) analyses were performed on a Corbett Rotorgene 3000 (Corbett Life Sciences, Sydney, Australia). IDO, eNOS, iNOS, and HOX-1 mRNA levels were measured using Taqman Gene Expression Assays (Applied Biosystems). For each assay, standard curves were generated to allow a calculation of mRNA transcript number. All measurements were normalized against the expression of the housekeeping gene, B2m.
Statistical analysis
Survival curves were plotted using Kaplan-Meier estimates and compared by log-rank analysis. The Mann-Whitney U test was used for the statistical analysis of cytokine data and clinical scores. A P value less than .05 was considered statistically significant. Data are presented as means plus or minus SEM.
Results
Donor-derived IFNγ protects against GVHD independent of effects on the donor graft
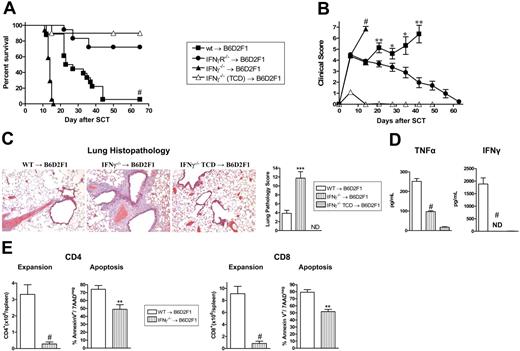
To investigate the role of IFNγ in GVHD, G-CSF–mobilized IFNγ−/−, IFNγR−/−, and wt grafts were transplanted into lethally irradiated allogeneic B6D2F1 recipients. Recipients of wt grafts died at a median of 27 days after transplantation from GVHD. In contrast, recipients of IFNγ−/− grafts died significantly faster at a median of 14 days after transplantation (Figure 1A) with clinical features of severe GVHD (Figure 1B). Recipients of TCD IFNγ−/− grafts survived the period of observation, demonstrating that the amplification of mortality in recipients of IFNγ−/− grafts was due to GVHD. Surprisingly, GVHD was significantly attenuated in recipients of allogeneic grafts that could not respond to IFNγ due to the deletion of the receptor (Figure 1A,B). Importantly, this was not associated with any reduction in engraftment in these animals (99.9% ± 0.1% vs 99.8% ± 0.1% in recipients of wt vs IFNγR−/− grafts, peripheral blood at day 30). Thus, IFNγ produced by the donor graft has 2 independent and paradoxic effects. First, in the complete absence of IFNγ generation, GVHD mortality is enhanced. Second, since the opposite occurs when there is no IFNγ signaling through the donor graft, we speculate that the protective effects of IFNγ are mediated through host tissue in a dominant fashion.
Donor-derived IFNγ prevents GVHD independently of effects on donor cells. Splenocytes from G-CSF–mobilized wt, IFNγ−/−, and IFNγR−/− B6 donors were transplanted into irradiated (1100 rad) B6D2F1 recipients (n = 18 per group). TCD splenocytes from IFNγ−/− donors were transplanted into B6D2F1 recipients (n = 10) as non-GVHD controls. Data pooled from 2 identical experiments. (A) Survival determined by Kaplan-Meier analysis. #P < .001, recipients of IFNγ−/− versus wt donor grafts and IFNγR−/− versus wt. (B) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals. #P < .001, recipients of IFNγ−/− versus wt donor grafts and IFNγR−/− versus wt. **P < .01 and *P < .05, recipients of wt grafts versus IFNγR−/− donor grafts. Data expressed as means ± standard error (SE). (C) Semiquantitative histopathology and representative images (100 ×) of lung 14 days after BMT in recipients of wt (n = 7), IFNγ−/− (n = 8), and IFNγ−/− TCD grafts (n = 4). Data expressed as means ± SE of individual animals. ***P < .001, wt versus IFNγ−/− grafts. (D) TNFα and IFNγ levels in sera of recipients of wt (n = 10), IFNγ−/− (n = 10), and IFNγ−/− TCD (n = 4) grafts 7 days after SCT. Data expressed as means ± SE of individual animals. ND indicates not detected. #P < .001, wt versus IFNγ−/− grafts. (E) Donor CD4 and CD8 T-cell expansion and proportions of apoptosis within spleen of recipients of wt (n = 8) and IFNγ−/− (n = 9) grafts 14 days after SCT. Data expressed as means ± SE of individual animals. #P < .001, **P < .01.
Donor-derived IFNγ prevents GVHD independently of effects on donor cells. Splenocytes from G-CSF–mobilized wt, IFNγ−/−, and IFNγR−/− B6 donors were transplanted into irradiated (1100 rad) B6D2F1 recipients (n = 18 per group). TCD splenocytes from IFNγ−/− donors were transplanted into B6D2F1 recipients (n = 10) as non-GVHD controls. Data pooled from 2 identical experiments. (A) Survival determined by Kaplan-Meier analysis. #P < .001, recipients of IFNγ−/− versus wt donor grafts and IFNγR−/− versus wt. (B) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals. #P < .001, recipients of IFNγ−/− versus wt donor grafts and IFNγR−/− versus wt. **P < .01 and *P < .05, recipients of wt grafts versus IFNγR−/− donor grafts. Data expressed as means ± standard error (SE). (C) Semiquantitative histopathology and representative images (100 ×) of lung 14 days after BMT in recipients of wt (n = 7), IFNγ−/− (n = 8), and IFNγ−/− TCD grafts (n = 4). Data expressed as means ± SE of individual animals. ***P < .001, wt versus IFNγ−/− grafts. (D) TNFα and IFNγ levels in sera of recipients of wt (n = 10), IFNγ−/− (n = 10), and IFNγ−/− TCD (n = 4) grafts 7 days after SCT. Data expressed as means ± SE of individual animals. ND indicates not detected. #P < .001, wt versus IFNγ−/− grafts. (E) Donor CD4 and CD8 T-cell expansion and proportions of apoptosis within spleen of recipients of wt (n = 8) and IFNγ−/− (n = 9) grafts 14 days after SCT. Data expressed as means ± SE of individual animals. #P < .001, **P < .01.
In order to understand the cause of rapid mortality of recipients of IFNγ−/− allografts, we performed semiquantitative pathology of GVHD target organs at day 14 after BMT. Surprisingly, these animals had developed severe interstitial pneumonitis characteristic of IPS (Figure 1C). By contrast, GVHD in the liver, gastrointestinal tract and skin was equivalent or less severe in recipients of IFNγ−/− relative to wt allografts (data not shown). Thus, the early mortality in the absence of donor-derived IFNγ−/− was the result of severe IPS. Interestingly, this IPS developed in the context of lower systemic levels of TNFα, absent IFNγ−/− (Figure 1D) and impaired donor T-cell expansion that occurred despite reduced levels of apoptosis (Figure 1E).
In order to investigate which donor cells are required to be signaled by IFNγ in the pathogenic signaling cascade, we carried out mixing experiments where the non–T-cell compartment of the donor graft was wt or IFNγR−/− with the addition of either wt or IFNγR−/− purified T cells. Recipient groups that received IFNγR−/− T cells had enhanced survival and lower clinical scores after transplantation compared with the 2 recipient groups that received wt T cells (survival: 90% vs 45%; P < .04), regardless of the nature of the non-T-cell compartment (Figure 2A,B). We next studied the direct effect of IFNγ signaling through the donor T cell on subsequent expansion and differentiation. The expansion of CD8 donor T cells lacking the IFNγR was reduced after SCT, confirming that the ability of IFNγ to promote donor T-cell expansion is at least partly the result of direct effects on the T cell itself (Figure 2C). Cytokine analysis of sera also revealed significantly less IFNγ and conversely significantly greater IL-5 levels in the groups that received IFNγR−/− T cells (Figure 2D), consistent with Th2 differentiation. Of interest, TNFα levels were similar even in the complete absence of the IFNγR on the donor graft (Figure 2D), suggesting that the previously described priming effects of IFNγ22 is not a dominant pathway through donor cells. Furthermore, this initial systemic TNFα generation played an important role in the initial development of GVHD induced by IFNγR−/− donor cells since blockade with TNFR:Fc significantly reduced the severity of early GVHD (Figure S1A, available on the Blood website; see the Supplemental Materials link at the top of the online article).
The detrimental effects of IFNγ are mediated through the donor T cell. TCD splenocytes from G-CSF–mobilized wt or IFNγR−/− donors were added to purified wt or IFNγR−/− T cells (n = 10 per group) and transplanted into irradiated (1100 rad) B6D2F1 recipients. TCD splenocytes from wt donors were transplanted into B6D2F1 recipients (n = 3) as non-GVHD controls. (A) Survival determined by Kaplan-Meier analysis. *P < .05 recipients of wt TCD splenocytes plus wt T cells and IFNγR−/− TCD splenocytes plus wt T-cell grafts versus wt TCD splenocytes plus IFNγR−/− T cells and IFNγR−/− TCD splenocytes plus IFNγR−/− T cells. (B) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals **P < .01 recipients of wt TCD splenocytes plus wt T cells and IFNγR−/− TCD splenocytes plus wt T-cell grafts versus wt TCD splenocytes plus IFNγR−/− T cells and IFNγR−/− TCD splenocytes plus IFNγR−/− T cells. (C) Donor CD4+ and CD8+ T cells were quantified 12 days after SCT in B6D2F1 recipients of either wt TCD plus wt T cells (□; n = 6) or wt TCD plus IFNγR−/− T cells (■; n = 6). *P < .05. (D) Levels of TNFα, IFNγ, and IL-5 were determined by cytokine bead array from sera 7 days after transplant. Data expressed as means plus or minus SE of individual animals (n = 9-10 in allogeneic groups, n = 3 in TCD). *P < .05, #P < .001 versus relevant control group.
The detrimental effects of IFNγ are mediated through the donor T cell. TCD splenocytes from G-CSF–mobilized wt or IFNγR−/− donors were added to purified wt or IFNγR−/− T cells (n = 10 per group) and transplanted into irradiated (1100 rad) B6D2F1 recipients. TCD splenocytes from wt donors were transplanted into B6D2F1 recipients (n = 3) as non-GVHD controls. (A) Survival determined by Kaplan-Meier analysis. *P < .05 recipients of wt TCD splenocytes plus wt T cells and IFNγR−/− TCD splenocytes plus wt T-cell grafts versus wt TCD splenocytes plus IFNγR−/− T cells and IFNγR−/− TCD splenocytes plus IFNγR−/− T cells. (B) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals **P < .01 recipients of wt TCD splenocytes plus wt T cells and IFNγR−/− TCD splenocytes plus wt T-cell grafts versus wt TCD splenocytes plus IFNγR−/− T cells and IFNγR−/− TCD splenocytes plus IFNγR−/− T cells. (C) Donor CD4+ and CD8+ T cells were quantified 12 days after SCT in B6D2F1 recipients of either wt TCD plus wt T cells (□; n = 6) or wt TCD plus IFNγR−/− T cells (■; n = 6). *P < .05. (D) Levels of TNFα, IFNγ, and IL-5 were determined by cytokine bead array from sera 7 days after transplant. Data expressed as means plus or minus SE of individual animals (n = 9-10 in allogeneic groups, n = 3 in TCD). *P < .05, #P < .001 versus relevant control group.
Host responses to IFNγ dictate protection from GVHD
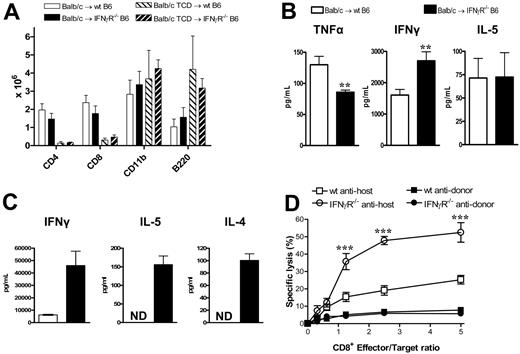
We next examined the effects of donor-derived IFNγ on recipient tissue and subsequent GVHD. Donor Balb/c mice were mobilized with G-CSF, and splenocytes were transplanted into lethally irradiated wt or IFNγR−/− recipients. As shown in Figure 3A,B, IFNγR−/− recipients of allogeneic grafts died within 11 days of transplantation with features of severe GVHD, while 60% of wt recipients survived long term. All IFNγR−/− recipients of TCD grafts survived long term, confirming the mortality in IFNγR−/− recipients was due to GVHD. Thus, the protective effects of donor-derived IFNγ are indeed mediated via effects on host tissue. In order to understand which target organs IFNγ was protecting from GVHD, we performed semiquantitative histopathology on SCT recipients 9 days after SCT. Consistent with our initial findings, IFNγR−/− recipients of allogeneic grafts were dying from severe IPS, characterized by an intense pulmonary cellular infiltrate of donor monocyte/macrophages and T cells (Figure 4A-C). This was an allogeneic phenomenon with all the features of IPS as it occurred in recipients of T-cell-replete but not TCD allografts (Figure 4A,B). IFNγR−/− recipients of allogeneic grafts also had higher levels of cutaneous GVHD, but surprisingly were protected from GVHD of the GI tract (Figure 4A). Thus, the response of host tissue to IFNγ is a critical determinant of injury within individual GVHD target organs.
The protective effects of IFNγ are mediated through host tissue. Balb/c donors were mobilized with G-CSF and splenocytes were transplanted into irradiated (1000 rad) wt (n = 15) or IFNγR−/− (n = 20) B6 recipients. TCD splenocytes were transplanted into wt (n = 6) or IFNγR−/− recipients (n = 6) as non-GVHD controls. Data pooled from 2 identical experiments. (A) Survival determined by Kaplan-Meier analysis. #P < .001, IFNγR−/− versus wt recipients. (B) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals. **P < .01, IFNγR−/− versus wt recipients. Data expressed as means ± SE.
The protective effects of IFNγ are mediated through host tissue. Balb/c donors were mobilized with G-CSF and splenocytes were transplanted into irradiated (1000 rad) wt (n = 15) or IFNγR−/− (n = 20) B6 recipients. TCD splenocytes were transplanted into wt (n = 6) or IFNγR−/− recipients (n = 6) as non-GVHD controls. Data pooled from 2 identical experiments. (A) Survival determined by Kaplan-Meier analysis. #P < .001, IFNγR−/− versus wt recipients. (B) GVHD clinical scores were determined as a measure of GVHD severity in surviving animals. **P < .01, IFNγR−/− versus wt recipients. Data expressed as means ± SE.
IFNγ prevents the development of idiopathic pneumonia syndrome. Tissue was collected at day 9 after transplantation from recipients of G-CSF–mobilized allogeneic or TCD grafts. (A) Semiquantitative histopathology was scored as described in “Materials and methods.” GI tract, *P < .03 (n = 4-5 per group); liver (n = 4-5 per group); skin **P < .01 (n = 11 in T-cell-replete and n = 4 in TCD groups); lung, **P < .01 (n = 6 per group). (B) Representative example of histological pulmonary tissue from wt and IFNγR−/− recipients of T-cell-replete and TCD grafts 9 days after transplantation (100 ×). (C) Donor (H-2Dd+) cellular infiltrate in pulmonary tissue was determined by flow cytometry. Significant increases in donor CD4+ T cells (*P < .02), CD8+ T cells, and F480+ macrophages (**P < .01) were seen in the lungs of IFNγR−/− recipients of T-cell-replete allogeneic grafts compared with wt recipients. Ly6G+ neutrophils were significantly decreased in IFNγR−/− recipients (**P < .01). Data represents mean plus or minus SE cells per right upper lobe of lung.
IFNγ prevents the development of idiopathic pneumonia syndrome. Tissue was collected at day 9 after transplantation from recipients of G-CSF–mobilized allogeneic or TCD grafts. (A) Semiquantitative histopathology was scored as described in “Materials and methods.” GI tract, *P < .03 (n = 4-5 per group); liver (n = 4-5 per group); skin **P < .01 (n = 11 in T-cell-replete and n = 4 in TCD groups); lung, **P < .01 (n = 6 per group). (B) Representative example of histological pulmonary tissue from wt and IFNγR−/− recipients of T-cell-replete and TCD grafts 9 days after transplantation (100 ×). (C) Donor (H-2Dd+) cellular infiltrate in pulmonary tissue was determined by flow cytometry. Significant increases in donor CD4+ T cells (*P < .02), CD8+ T cells, and F480+ macrophages (**P < .01) were seen in the lungs of IFNγR−/− recipients of T-cell-replete allogeneic grafts compared with wt recipients. Ly6G+ neutrophils were significantly decreased in IFNγR−/− recipients (**P < .01). Data represents mean plus or minus SE cells per right upper lobe of lung.
Donor T cells from IFNγR−/− recipients show a marked hyperresponsiveness to alloantigen
We next studied the effect of IFNγ signaling through recipient tissue on donor T-cell function. At nine days after transplantation, the absolute numbers of T cells, B cells, and mononuclear cells in the spleen were not different between wt and IFNγR−/− recipients (Figure 5A). Thus, the previously demonstrated expansion of these cells in the lung was an organ-specific phenomenon reflecting increased trafficking and/or expansion within the local lung environment. IFNγ levels within serum were raised in IFNγR−/− recipients compared with wt recipients, but IL-5 levels were similar (Figure 5B). Interestingly, despite the increased GVHD mortality, TNFα levels were decreased in the IFNγR−/− recipients, consistent with the low levels of GVHD in the GI tract. Donor CD4 and CD8 T cells were next sort-purified from wt or IFNγR−/− recipients 9 days after transplantation and restimulated with host DCs or used as effectors in chromium assays. As shown in Figure 5C, donor CD4 T cells from IFNγR−/− recipients displayed hyperresponsiveness to alloantigen with marked increases in cytokine production, while donor CD8 T cells displayed increased cytotoxicity against host-type targets (Figure 5D). Thus, the inability of host tissue to respond to IFNγ results in heightened donor T-cell effector responses, confirming that IFNγ acts to suppress donor T-cell responses during GVHD via effects on host tissue.
Donor T cells from IFNγR−/− recipients are hyperresponsive to alloantigens. Splenocytes from G-CSF–mobilized Balb/c donors were transplanted into irradiated (1000 rad) wt or IFNγR−/− B6 recipients. Analyses were performed 9 days after transplantation. (A) Absolute numbers of donor splenic T cells, CD11b+ monocytes/neutrophils, and B cells (n = 4-5 in allogeneic T-cell-replete and n = 3 in TCD groups). (B) IFNγ, IL-5, and TNFα determined in sera by cytokine bead array. **P < .01 (n = 10-17). (C) Purified donor (H-2Dd+) CD4+ T cells were sort-purified (> 95%) from wt or IFNγR−/− recipients and restimulated with purified DCs. IL-5 and IL-4 were measured in supernatants by cytokine bead arrays, while IFNγ was measured by ELISA. Data are means plus or minus SE of triplicate wells and is 1 of 3 representative experiments is shown. ND indicates not detected. (D) Purified donor CD8+ (H-2Dd+) T cells were sort-purified (> 95%) from wt or IFNγR−/− recipients and used as effectors in 51Cr-release CTL assays against host-type EL4 targets and donor-type A20 targets. Data represent means plus or minus SE of triplicate wells combined from 2 replicate experiments. ***P < .001, wt antihost versus IFNγR−/− antihost.
Donor T cells from IFNγR−/− recipients are hyperresponsive to alloantigens. Splenocytes from G-CSF–mobilized Balb/c donors were transplanted into irradiated (1000 rad) wt or IFNγR−/− B6 recipients. Analyses were performed 9 days after transplantation. (A) Absolute numbers of donor splenic T cells, CD11b+ monocytes/neutrophils, and B cells (n = 4-5 in allogeneic T-cell-replete and n = 3 in TCD groups). (B) IFNγ, IL-5, and TNFα determined in sera by cytokine bead array. **P < .01 (n = 10-17). (C) Purified donor (H-2Dd+) CD4+ T cells were sort-purified (> 95%) from wt or IFNγR−/− recipients and restimulated with purified DCs. IL-5 and IL-4 were measured in supernatants by cytokine bead arrays, while IFNγ was measured by ELISA. Data are means plus or minus SE of triplicate wells and is 1 of 3 representative experiments is shown. ND indicates not detected. (D) Purified donor CD8+ (H-2Dd+) T cells were sort-purified (> 95%) from wt or IFNγR−/− recipients and used as effectors in 51Cr-release CTL assays against host-type EL4 targets and donor-type A20 targets. Data represent means plus or minus SE of triplicate wells combined from 2 replicate experiments. ***P < .001, wt antihost versus IFNγR−/− antihost.
IFNγ signaling through nonhematopoietic cells controls GVHD target tissue injury
Donor-derived IFNγ could conceivably be acting at 2 host sites to modulate GVHD. First, it may act on host APCs early after SCT to, in this case, inhibit their stimulatory capacity and reduce donor T-cell effector function. Secondly, IFNγ may act on GVHD target tissue to provide direct protection from pulmonary GVHD or induction of GVHD or the GI tract, respectively. To dissect the level at which modulation of GVHD was occurring, we generated mixed bone marrow chimeras in which wt or IFNγR−/− bone marrow was transplanted into either wt or IFNγR−/− recipient mice as described in “Materials and methods.” At 3 months after transplantation, we undertook flow cytometric analysis to study the turn over of APCs (macrophages and DCs) in the lung in these chimeras. As shown in Figure 6A, pulmonary macrophages and DCs were of donor origin (CD45.2+) in these chimeras. Thus, we could indeed use these animals as secondary transplant recipients to dissect the role of the IFNγR on host lung APCs versus lung parenchymal tissue in the development of IPS. When these mixed chimeras were used as recipients, histologic analysis 9 days after transplantation (Figure 6B-C) confirmed the development of IPS in IFNγR−/− → IFNγR−/− and wt → IFNγR−/− chimeras recipients. In contrast, neither IFNγR−/− → wt or wt → wt chimeric recipients developed IPS, confirming that the protection from IPS provided by IFNγ was via signaling through nonhematopoietic cells (ie, lung parenchyma). Conversely, the induction of GVHD of the GI tract by IFNγ required signaling through nonhematopoietic cells since only IFNγR−/− → wt and wt → wt chimeric recipients developed gastrointestinal GVHD.
IFNγ differentially controls IPS and GVHD of the GI tract by direct signaling through nonhematopoietic host tissue. (A) Mixed chimeric mice were generated by transplanting wt or IFNγR−/− bone marrow into irradiated (1000 rad) wt or IFNγR−/− recipients as described in “Materials and methods.” After 3 months, these chimeric mice were phenotyped using CD45.1/CD45.2 disparity to ensure APCs (F4/80+ macrophages, CD19+ B cells, and CD11c+ DCs) had fully reconstituted as donor within lung tissue. The mixed chimeric B6 mice were then used as recipients (conditioned with 950 rad total body irradiation and given transplants of G-CSF–mobilized Balb/c donor splenocytes). In some cases, TCD splenocytes were transplanted as non-GVHD controls. (B) Representative lung (top) or GI tract (bottom) in the recipient chimeras (100 ×). (C) Semiquantitative histologic analysis of transplant chimera recipients as described in “Materials and methods.” Data represent mean plus or minus SE of individual animals combined from 2 replicate experiments. n = 6-15 in T-cell–replete groups and n = 4-7 in TCD groups. ***P < .001, wt → wt and IFNγR−/− → wt versus wt → IFNγR−/− and IFNγR−/− → IFNγR−/− as shown. ND indicates no pathology detected.
IFNγ differentially controls IPS and GVHD of the GI tract by direct signaling through nonhematopoietic host tissue. (A) Mixed chimeric mice were generated by transplanting wt or IFNγR−/− bone marrow into irradiated (1000 rad) wt or IFNγR−/− recipients as described in “Materials and methods.” After 3 months, these chimeric mice were phenotyped using CD45.1/CD45.2 disparity to ensure APCs (F4/80+ macrophages, CD19+ B cells, and CD11c+ DCs) had fully reconstituted as donor within lung tissue. The mixed chimeric B6 mice were then used as recipients (conditioned with 950 rad total body irradiation and given transplants of G-CSF–mobilized Balb/c donor splenocytes). In some cases, TCD splenocytes were transplanted as non-GVHD controls. (B) Representative lung (top) or GI tract (bottom) in the recipient chimeras (100 ×). (C) Semiquantitative histologic analysis of transplant chimera recipients as described in “Materials and methods.” Data represent mean plus or minus SE of individual animals combined from 2 replicate experiments. n = 6-15 in T-cell–replete groups and n = 4-7 in TCD groups. ***P < .001, wt → wt and IFNγR−/− → wt versus wt → IFNγR−/− and IFNγR−/− → IFNγR−/− as shown. ND indicates no pathology detected.
IFNγ-dependent protection from IPS is not via the nitric oxide or indoleamine 2,3 dioxygenase pathways
IFNγ may conceivably be acting on lung parenchymal tissue to prevent IPS by inducing the secretion of immunosuppressive molecules and/or inhibiting donor cell migration across pulmonary endothelium into the lung. A recent study demonstrated that endogenous nitric oxide (NO) inhibits the development IPS23 and this molecule is also known to be an IFNγ-dependent negative regulator of T cell function. We therefore determined iNOS and eNOS mRNA transcript levels within lung tissue using real-time PCR analysis. Surprisingly, these studies demonstrated slightly increased iNOS and eNOS in the lung of IFNγR-/- compared with wt recipients, the opposite to that which would be expected if these molecules were providing IFNγ-dependent inhibition of donor alloreactivity (Figure 7A). Since these PCR results may reflect levels of iNOS and eNOS in infiltrating donor cells rather than recipient lung parenchyma, we also utilized iNOS−/− and eNOS−/− mice as SCT recipients. These knockout mice failed to develop any features of IPS, as would have been expected if the generation of NO from host tissue in response to IFNγ was preventing the development of IPS in wt recipients (Figure 7B). We next investigated IDO as an alternative IFNγ-dependent inhibitory pathway. IDO is also capable of inhibiting T-cell proliferation24 and has an inhibitory role in the rejection of lung allografts.25,26 Real-time PCR analysis from lung tissue after transplantation demonstrated a 10-fold higher expression of IDO in wt recipients compared with IFNγR−/− recipients. However, the inhibition of IDO in vivo with 1-methyl-tryptophan (1MT) during SCT did not result in the development of IPS (Figure 7A-B). In contrast, inhibition of IDO or the use of NOS−/− recipient mice did alter other parameters of GVHD (GI tract histopathology and TNFα levels; data not shown), confirming alternative effects of these molecules in GVHD. Thus, the absence of these IFNγ-dependent inhibitory molecules in host tissue did not result in the development of IPS, excluding their involvement in the IFNγ-dependent protective pathway.
Host-derived nitric oxide synthase and IDO do not contribute to IPS. (A) iNOS, eNOS, and IDO transcript levels were determined by real-time PCR in lungs from wt and IFNγR−/− recipients of allogeneic Balb/c grafts 3 days after SCT. Results normalized to β2 microglobulin and expressed as means ± SE of duplicates from individual animals (n = 3 per group). (B) Semiquantitative lung histology in wt and iNOS−/− recipients or wt and eNOS−/− recipients of Balb/c grafts 9 days after SCT (n = 8 per group). Non-GVHD controls received TCD grafts (n = 4). A cohort of wt recipients of allogeneic T-cell–replete grafts were implanted with slow release pellets containing the IDO inhibitor 1-MT or vehicle as described in “Materials and methods” (n = 9 per group). Non-GVHD controls received TCD grafts in conjunction with slow release pellets containing 1-MT (n = 3). ND indicates no pathology detected. Data expressed as means plus or minus SE of individual animals, combined from 2 experiments. (C) Representative hematoxylin and eosin–stained images of lung venules from wt and IFNγR−/− recipients given transplants of T-cell–replete grafts demonstrating leukocyte adhesion and migration across the endothelium in the IFNγR−/− recipients (400 ×). (D) Quantitative analysis of leukocyte adhesion and transmigration within lung venules in wt and IFNγR−/− recipients of T-cell–replete or TCD grafts (n = 5-6 per group). Data are means plus or minus SE of individual animals where the mean number of adherent and transmigrating leukocytes was determined in 10 venules per lung. **P < .01, wt versus IFNγR−/− recipients. (E) Purified donor (H-2Dd+) CD4+ T cells were sort-purified (> 95%) from the lungs of wt (□) or IFNγR−/− (■) recipients and restimulated with purified allogeneic DCs. Proliferation was determined by 3H incorporation 60 hours later and IFNγ, IL-5, and IL-4 were measured in supernatants by cytokine bead array. Data are means plus or minus SE of triplicate wells and is 1 of 2 replicate experiments. ND indicates not detected.
Host-derived nitric oxide synthase and IDO do not contribute to IPS. (A) iNOS, eNOS, and IDO transcript levels were determined by real-time PCR in lungs from wt and IFNγR−/− recipients of allogeneic Balb/c grafts 3 days after SCT. Results normalized to β2 microglobulin and expressed as means ± SE of duplicates from individual animals (n = 3 per group). (B) Semiquantitative lung histology in wt and iNOS−/− recipients or wt and eNOS−/− recipients of Balb/c grafts 9 days after SCT (n = 8 per group). Non-GVHD controls received TCD grafts (n = 4). A cohort of wt recipients of allogeneic T-cell–replete grafts were implanted with slow release pellets containing the IDO inhibitor 1-MT or vehicle as described in “Materials and methods” (n = 9 per group). Non-GVHD controls received TCD grafts in conjunction with slow release pellets containing 1-MT (n = 3). ND indicates no pathology detected. Data expressed as means plus or minus SE of individual animals, combined from 2 experiments. (C) Representative hematoxylin and eosin–stained images of lung venules from wt and IFNγR−/− recipients given transplants of T-cell–replete grafts demonstrating leukocyte adhesion and migration across the endothelium in the IFNγR−/− recipients (400 ×). (D) Quantitative analysis of leukocyte adhesion and transmigration within lung venules in wt and IFNγR−/− recipients of T-cell–replete or TCD grafts (n = 5-6 per group). Data are means plus or minus SE of individual animals where the mean number of adherent and transmigrating leukocytes was determined in 10 venules per lung. **P < .01, wt versus IFNγR−/− recipients. (E) Purified donor (H-2Dd+) CD4+ T cells were sort-purified (> 95%) from the lungs of wt (□) or IFNγR−/− (■) recipients and restimulated with purified allogeneic DCs. Proliferation was determined by 3H incorporation 60 hours later and IFNγ, IL-5, and IL-4 were measured in supernatants by cytokine bead array. Data are means plus or minus SE of triplicate wells and is 1 of 2 replicate experiments. ND indicates not detected.
IFNγ inhibits donor cell migration into the lung and subsequent expansion
We next examined whether IFNγ was controlling leukocyte adhesion and/or migration into the lung. As shown in Figure 7C,D, recipients of IFNγR−/− allogeneic grafts demonstrated significantly higher levels of donor cell adhesion and migration across endothelium within pulmonary venules. Since IFNγ has previously been shown to inhibit leukocyte transendothelial migration through endothelial cell monolayers in a VCAM-1–dependent fashion,27,28 we examined VCAM-1 expression by immunohistochemistry within lung when IPS is evident. Interestingly, a subtle increase in endothelial VCAM-1 expression was seen in IFNγR−/− recipients (Figure S1B), although the full significance of this will require studies based on functional inhibition of VCAM-1 and/or VLA-4. In contrast, ICAM was highly expressed throughout lung without any differences between groups evident (data not shown). Interestingly, mRNA levels of HOX-1 were also elevated in IFNγR−/− recipients (Figure S1C), consistent with a response to the lung injury evident in these animals.29,30 We were unable to detect consistent increases in chemokines known to influence IPS, including those controlled by IFNγ,31-34 in cell lysates from lungs of allogeneic IFNγR−/− recipients (Figure S1D). These chemokines were also at identical levels in bronchoalveolar lavage fluid (data not shown). Finally, we analyzed the responses of donor cells within the lung of IFNγR−/− recipients to see if they were functionally hyperresponsive to host antigens. As shown in Figure 7E, donor CD4 T cells from these SCT recipients had up to 10-fold higher proliferative and cytokine responses to host antigens. A similar pattern of hyperresponsiveness was seen in donor CD8 T cells from the lungs of IFNγR−/− recipients with IPS (data not shown). Thus, IFNγ acts on lung parenchyma (ie, endothelia and epithelia) to inhibit donor cell migration into the lung and the functional responses of these cells to host antigens once there.
Discussion
IFNγ has been attributed with both pathogenic and protective effects in transplantation medicine although clear mechanisms to explain this paradox have not been defined. By using receptor deficient mice we demonstrate here that IFNγ indeed has direct pathogenic effects on donor T cells to promote Th1 differentiation and the GI parenchyma to directly promote GVHD. However, these effects are overwhelmed by protective effects mediated through the lung parenchyma to prevent the development of fulminant IPS. It should be noted that G-CSF-mobilized grafts were used in these studies to reflect the majority of current clinical practice. However, IPS also developed in an equivalent fashion in IFNγR−/− recipients of traditional bone marrow grafts supplemented with splenic T cells (data not shown).
Initial studies using IFNγ−/− donor mice in nonirradiated recipients demonstrated a delay in GVHD mortality in the absence of this cytokine associated with Th2 differentiation8,35 and impairment of Fas-dependent CTL function.9 Conversely, subsequent studies using irradiated recipient mice demonstrated enhanced acute GVHD following transplantation of IFNγ−/− grafts or IFNγ−/− neutralization10,11 and protection following the administration of IFNγ after BMT.36 This effect was associated with enhanced in vitro proliferation and IL-2 generation from donor T cells to alloantigen.11 Interestingly, IFNγ was subsequently shown to inhibit the capacity of donor CD8 T cells to expand after BMT and induce GVHD, but graft-versus-leukemia (GVL) effects were paradoxically enhanced.11 Together, these data confirmed the now established principle that BMT conditioning has important effects on the incidence and severity of GVHD,16,37 and that CD4 and CD8 T-cell effects may be differentially regulated after BMT.38
With the benefit of simultaneous data generated using both IFNγ−/− and IFNγR−/− mice, we have now established the mechanism of action of this cytokine in GVHD pathogenesis, which has yielded highly surprising and novel results. Importantly, the proposed concept that IFNγ inhibits GVHD by inhibition of donor T-cell function inferred from previous studies is not consistent with the data gained when IFNγR−/− donor cells are transplanted. Thus, SCT recipients receiving donor T cells unable to respond to IFNγ (lacking the cognate receptor) are protected from GVHD rather than experiencing more severe GVHD, as would be expected if IFNγ attenuated GVHD via effects on donor cells. Instead, IFNγ acts directly on donor T cells to drive Th1 expansion and differentiation after SCT.
The demonstration here that the protective/suppressive effects of IFNγ are instead all mediated through recipient tissue was unexpected. The differential effects of this cytokine on the incidence of GVHD within specific target organs was also surprising and clearly demonstrated that the rapid mortality in recipients lacking IFNγ signaling was due to the development of severe IPS in association with enhanced cutaneous GVHD. Given that IPS has previously been causally linked to proinflammatory cytokines (particularly nitric oxide and TNFα23,39-41 ), adhesion molecules,42,43 and chemokines known to be up-regulated by IFNγ,32-34 this represents a substantial paradox. We considered that IFNγ signaling of host tissue may have been regulating IPS by inhibiting host APC function. However, this scenario, in addition to effects on any other host cell of hematopoietic origin, was excluded by transplants using mixed IFNγR−/− chimeric recipients. Thus, the regulatory effect was instead entirely mediated by pulmonary parenchyma, which prevented donor leukocyte migration into the lung and subsequent expansion specifically within this organ. Intriguingly, unlike most pathways controlling cell migration across endothelium, some adhesion molecules such as VCAM-1 and platelet endothelial cell adhesion molecule (PECAM) have been demonstrated to be inhibited by IFNγ,27,44 and so represent candidates that may potentially be specifically up-regulated in the absence of IFNγ signaling. Endogenous nitric oxide has been reported to both inhibit and contribute to the development of IPS,23,45 perhaps suggesting alternative roles for this molecule in different phases of the disease process. Nitric oxide is also known to induce GVHD-associated T-cell suppression,46 and both iNOS and eNOS are highly active in type II alveolar cells and lung endothelium.47 In these studies both enzymes were up-regulated in lungs lacking the IFNγR after transplantation, consistent with the state of inflammation within these organs. The failure of iNOS−/− or eNOS−/− recipient mice to develop IPS excluded a protective effect within host tissue for these molecules, consistent with previous studies.45 Therefore, any influence of the nitric oxide pathway on IPS in this model is likely to be pathogenic and mediated by donor cells. Similarly, inhibition of IDO failed to influence the development of IPS in a meaningful timeframe, effectively excluding this as an IFNγ-dependent protective pathway. Thus, the molecular mechanisms by which IFNγ signaling in lung parenchyma prevent IPS are unclear, and we are currently using gene- and proteomic-based array techniques to identify differentially regulated candidate molecules.
The GI tract is known to be highly sensitive to damage by TNFα,37 and so the finding of low levels of GVHD in the GI tract of IFNγR−/− recipients in association with reduced levels of TNFα is perhaps not surprising. However, it has been previously proposed that the role of IFNγ in this pathway was to prime monocytes and macrophages so that they secrete pathogenic quantities of TNFα following LPS stimulation.22,37 Surprisingly, the studies here demonstrate that priming by IFNγ occurs predominantly via cells of host origin since TNFα levels were reduced when the host but not the donor lacked the IFNγR (Figures 2D, 5B). However, the pathogenic effect of IFNγ on GVHD of the GI tract was not mediated through host macrophages, or indeed any other cell of hematopoietic origin, since GI tract injury occurred only when the GI tract parenchyma itself expressed the IFNγR. The effects of IFNγ on the GI tract have been described, and primarily include crypt hypertrophy and villous atrophy.48,49 This study supports the notion that these direct effects are central to the primary pathogenesis of gut GVHD induced by IFNγ.
IPS is a life-threatening condition developing in 6% to 10% of transplant recipients and carries a high mortality. The pathophysiology of the disease remains unclear but is associated with the generation of proinflammatory cytokines and up-regulation of both adhesion molecules and chemokines. The unexpected role of IFNγ in preventing IPS despite its induction of a plethora of known pathogenic molecules suggests that this inhibitory pathway is immunodominant and likely to be of high biological relevance. Importantly, polymorphisms in both IFNγ50 and the IFNγR51 have been described in the clinic and the former at least is known to correlate with the severity of GVHD after BMT. It is therefore highly plausible that polymorphisms in the donor IFNγ and the recipient IFNγR alleles will predict those at high risk for the development of IPS after BMT. Finally, identification of the inhibitory molecular pathways downstream of IFNγ signaling within the lung parenchyma may yield novel therapeutic strategies that can be applied to patients at high risk of developing this devastating transplantation complication.
An Inside Blood analysis of this article appears at the front of this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported in part by grants from the Queensland Cancer Fund and National Health and Medical Research Council (NHMRC). C.R.E. and K.P.A.M. are NHMRC RD Wright Fellows. G.R.H. is an NHMRC Practitioner Fellow.
Authorship
Contribution: A.C.B. designed and performed research, analyzed the data, and contributed to manuscript preparation; T.B. designed and performed research; R.K. performed research; A.D.C. analyzed all histology; E.M., A.C.S., V.R., H.B., R.S., and N.R. performed research; O.S. performed chemokine analysis; S.R.M. performed the experimental design of chemokine analysis; C.R.E. provided intellectual input and experimental design; K.P.A.M. provided intellectual input, experimental design, and performed research; and G.R.H. provided intellectual input, experimental design, and contributed to manuscript preparation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Geoffrey R. Hill, Bone Marrow Transplantation Laboratory, Queensland Institute of Medical Research, 300 Herston Rd, QLD 4006, Australia; e-mail: geoff.hill@qimr.edu.au.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal