A malarial parasite protein stabilizes red cell membrane and inhibits further parasite invasion.
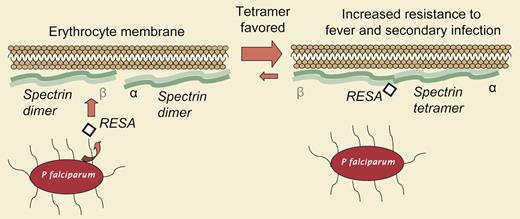
The parasite Plasmodium falciparum is the cause for the most prevalent form of malaria, a disease that claims several million lives annually. No vaccine yet exists and postinfection treatments are far less effective than might be hoped for with a deeper understanding of host-parasite interactions. The parasite invades individual erythrocytes in a very active process of adhesion and membrane deformation, and then the parasite sets in motion a molecular reprogramming of the red cell to maximize the infection. It already appears clear, for example, that the parasite protein, PfEMP1, inserts itself in the erythrocyte membrane and promotes adhesion of invaded red cells to blood vessels, leading to plaques and microinfarctions within the capillaries of the host. Hundreds of other parasite-secreted proteins seem to interact with the host erythrocyte, but the recent landmark publication of the complete P falciparum genome only strengthens the motivation for detailed studies of parasite protein interactions. In this issue of Blood, Pei and colleagues have now identified the binding site between the parasite's RESA (ring-infected erythrocyte surface antigen) protein and the major erythrocyte membrane protein spectrin (see figure). Spectrin assembles as an α-β heterotetramer into a cytoskeletal network at the inner surface of the red cell and is key to membrane resilience and stability; red cells in spectrin-deficient mice fragment in the circulation and are cleared within hours rather than the usual months. Pei and colleagues show that the parasite has evolved to recognize this important structural role of spectrin, and has tuned it to the parasite's advantage.
A malarial protein stabilizes spectrin and inhibits erythrocyte invasion by additional parasites.
A malarial protein stabilizes spectrin and inhibits erythrocyte invasion by additional parasites.
Pei and colleagues use recombinant peptides to map amino acids 663 through 770 of the 115-kilodalton (kDa) RESA protein into binding the sixteenth repeat of β-spectrin, a repeat known to reside at the tetramer interface of spectrin. By surface-plasmon resonance, binding stoichiometry is shown to be 1:1 with a submicromolar dissociation constant. Importantly, non-denaturing gel electrophoresis on extracted erythrocyte spectrin showed that treatment with the RESA peptide shifted the spectrin dimer–tetramer ratio in favor of the more membrane-stabilizing tetramer. Consistent with this, erythrocyte ghosts that had been lysed and resealed to entrap the RESA peptide were found to possess greater membrane stability when exposed to fluid shear using an ektacytometer. Finally, the same RESA-containing ghosts also proved more resistant to P falciparum invasion.
These results, in combination with a previous report linking RESA with increased erythrocyte thermostability, clearly demonstrate that binding of RESA to spectrin increases the overall stability of the spectrin tetramer and endows enhanced erythrocyte resistance to mechanical as well as thermal stress. This increased stability might well protect the parasite under the feverish conditions that accompany infection, but the authors also provide clear evidence that the increased membrane stability mechanically inhibits reinfection of erythrocytes already harboring parasites, thus maximizing the spread of the infection. New targets of opportunity in malaria might well arise from such detailed insight, but the findings also illustrate the broad importance of the spectrin cytoskeleton and modifications to it in red-cell pathophysiology.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal