Abstract
Patients with polycythemia vera (PV) have a JAK2 (a cytosolic tyrosine kinase) mutation and an increased risk of vascular thrombosis related to red blood cell (RBC) mass and platelet activation. We investigated functional RBC abnormalities that could be involved in thrombosis. RBC adhesion to human umbilical vein endothelial cells (HUVECs) was measured by a radiometric technique and in a flow system by video microscopy, and adhesion molecule expression was determined using specific antibodies (against CD36, CD49d, ICAM-4, Lu/BCAM, CD147, and CD47) and flow cytometry in a group of 38 patients with PV and a group of 36 healthy volunteers. Adhesion of PV RBCs was 3.7-fold higher than that of normal RBCs (P < .001). Adhesion was inhibited when PV RBCs were incubated with anti-Lutheran blood group/basal cell adhesion molecule (Lu/BCAM) or when HUVECs were treated with anti-laminin α5 and to a lesser extent with anti-α3 integrin. Lu/BCAM was constitutively phosphorylated in PV RBCs. Transfection of K562 cells with JAK2 617V>F resulted in increased expression and phosphorylation of Lu/BCAM. Phosphorylation of Lu/BCAM increases RBC adhesion. Our results indicate that JAK2 mutation might be linked to Lu/BCAM modification and increased RBC adhesiveness, which may be a factor favoring thrombosis in PV.
Introduction
Polycythemia vera (PV) is a chronic disorder in which the clonal proliferation of multipotent hematopoietic cells results in an increase in the red cell mass.1 This expansion is associated with circulatory disturbances, mostly related to hyperviscosity. PV is the most common myeloproliferative syndrome,2 characterized by erythropoietin-independent erythroid colony formation in vitro, and was recently shown to be associated in most patients with a somatic point mutation of the JAK2 tyrosine kinase (JAK2 617V>F).3,4 In addition, another JAK2 exon 12 mutation (539K>L) was recently described in patients with PV and idiopathic erythrocytosis.5 Since the first description of PV in 1892 in a patient with thrombosis, several criteria have been used to characterize this disorder: determination of red cell mass, bone marrow culture, and, more recently, JAK2 mutation. In the present study, we included patients according to the classical PV criteria and excluded patients with essential thrombocythemia. Thromboembolism remains a major cause of mortality and morbidity in PV.6 However, the optimal management of patients with PV remains controversial.7 Phlebotomy and aspirin are still the first-line therapies, but despite red cell mass reduction the risk of thrombosis remains higher than in a population of sex- and age-matched patients. In addition, the use of antiplatelet agents can increase the risk of hemorrhage. Because adhesion of red blood cells (RBCs) to endothelium was shown to be correlated to vascular risk in sickle cell anemia8 and diabetes mellitus,9 we investigated whether RBCs from patients with PV had abnormal interactions with endothelium by measuring RBC adhesion to human umbilical vein endothelial cells (HUVECs) under static and flow conditions. We also evaluated adhesion molecule expression on RBCs using specific antibodies and flow cytometry. Blocking experiments indicated that PV RBC adhesion was mediated by CD239, or Lutheran blood group/basal cell adhesion molecule (Lu/BCAM), on the RBC side and laminin α5 on the endothelial side. It has been recently shown that the adhesion molecule Lu/BCAM, the unique erythroid receptor for laminin α5, is phosphorylated when sickle RBCs are stimulated by epinephrine, and that this leads to increased adhesion to laminin α5.10 Here we show that Lu/BCAM is constitutively phosphorylated in PV RBCs and that expression of recombinant JAK2 617V>F in K562 cells potentiated Lu/BCAM phosphorylation. It is postulated that adhesion of PV RBCs to laminin α5 is due, in part at least, to the constitutive phosphorylation and overexpression of Lu/BCAM
Patients, materials, and methods
The study was approved by the Internal Review Boards from participating institutions and the Institut National de la Transfusion Sanguine comité d'ethique, and informed consent was obtained in accordance with the Declaration of Helsinki.
A total of 38 patients (22 men and 16 women) with PV and 36 healthy volunteers (17 men and 19 women) were included in this study after RBC mass and plasma volume determination. The patients were seen as outpatients from the Nuclear Medicine Clinic, Hôpital Saint Louis, Paris, France, and were recruited between January 2003 and February 2005. The mean age of the male patients was 71 years (range, 38-86 years); the mean age of the female patients was 67 years (range, 32-88 years). In the nonpolycythemic group, the mean age for men was 58 years (range, 28-82 years) and for women was 51 years (range, 26-76 years). The diagnosis of PV was made according to the recently modified Polycythemia Vera Study Group (PVSG) criteria11 : male hemoglobin (Hb) levels above 18.5 g/L, female Hb levels above 16.5 g/L, platelet count above 400 × 109/L, neutrophil count above 10 × 109/L, palpable splenomegaly, low erythropoietin level, and spontaneous bone marrow growth.
JAK2 617V>F mutation was determined by amplification refractory mutation system (ARMS) assay in 10 patients because DNA from the other patients was not available. JAK2 617V>F mutation was observed in 8 of 10 patients with PV, consistent with previously published data using this technique.3,12 In the 2 patients negative in the ARMS assay, the JAK2 mutation was detected using real-time polymerase chain reaction (PCR).13 Seven additional patients tested by real-time PCR were found to be positive for the JAK2 617V>F mutation. The group of patients tested for adhesion and Lu/BCAM phosphorylation were positive for JAK2 mutation. The high incidence of JAK2 617V>F mutation in our patients is in accordance with the recently published results (97%)14 ; another JAK2 exon 12 mutation (539K>L) was reported in patients with PV and idiopathic erythrocytosis.3,5
Erythrocyte adhesion measurement
Static conditions.
Blood samples were tested the day after collection for all the patients and after storage for repeated experiments. Samples were stored at −196°C as previously described.15 Patients with PV were free of treatment when tested for adhesion to cultured HUVECs. Endothelial cells, isolated from human umbilical veins,16 were cultured in 35-mm plastic dishes in M199 medium supplemented with fetal calf serum (20%) until confluence. The cells were used after 1 or 2 passages. Cultures that did not achieve confluence or in which detachment of cells was seen were discarded. The washed RBCs (25% hematocrit, 3 × 1012 RBCs/L) in Hanks balanced salt solution (HBSS) plus 0.5% human serum albumin were labeled with chromium 51 (51Cr) and incubated with HUVECs for 30 minutes at 37°C. Nonadhering RBCs were removed by 6 successive washes with HBSS, and the remaining adherent RBCs were lysed with distilled water. Radioactivity in the lysates was measured in a gamma counter9 and expressed as a percentage of the radioactivity in the sample originally added. From the known number of RBCs added and the known area of the dish, adhesion was then calculated as RBCs per square millimeter (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article).
Flow conditions.
Adhesion of RBCs to HUVECs was determined according to a previously described technique.17 Briefly, HUVECs were cultured in gelatin (2%)-coated glass capillaries (microslides; Camlab, Cambridge, United Kingdom) for 24 hours. Microslides containing confluent HUVECs were mounted on the stage of a videomicroscope, and one end of the microslide was attached to a Harvard syringe pump (Harvard Apparatus, South Natic, MA), allowing control of the flow rate. The other end was connected to an electronic valve permitting switching between RBC suspension and cell-free buffer. RBC suspension (0.5% hematocrit in HBSS plus 0.5% human serum albumin) was perfused through the microslide at a flow rate equivalent to a wall shear stress of 0.03 Pa for 10 minutes followed by washout of nonadherent cells for 10 minutes. The wall shear stress was then increased stepwise (from 0.07-1.00 Pa) every 10 minutes. At each stage, adherent RBCs were videorecorded in 10 consecutive fields of known dimensions, along the center line of the microslide. RBC suspensions were perfused at different wall shear stresses (0.07-1.00 Pa) for 10 minutes, followed by washout at the same stress and video recording. Adherent RBCs were counted using a computerized image analysis system (Optimas G. S., Silver Spring, MD; Media Cybernetics, Bethesda, MD; R&D Vision, Paris, France) averaged per field and expressed as the number of RBCs per square millimeter. Images were acquired using Microscope Laborlux (16 × 0.4 objective) (Leica Microsystem, Rueil, Malmaison, France) and Jai 2.40 camera (Jai, Toshiya shiba, Japan).
Expression of adhesion molecules on RBCs
The expression of the following adhesion molecules—CD36, CD49d, CD147, CD239 (Lu/BCAM), CD242 (ICAM-4), and CD47—was assessed using corresponding specific monoclonal antibodies (mAbs) and flow cytometry (FACScan flow cytometer; Becton Dickinson, San Jose, CA). CD36 and CD49d antibodies were from Beckman-Coulter (Miami, FL). CD147 antibody (clone TRA-1-85) was a generous gift of P. Goodfellow (University of Cambridge, United Kingdom). Anti-CD239 (clone F241) was produced in our institute (Institut National de la Transfusion Sanguine) in collaboration with D. Blanchard (Etablissement Francais du Sang [EFS], Nantes, France), and CD242 antibody (clone BS56) was a gift from Dr H. H. Sonneborn (Biotest AG, Dreieich, Germany). CD59 antibody (clone 2/24) from Chemicon (Temecula, CA) was used as a control. The results were expressed as specific antibody-binding capacity (SABC).18 The SABC per erythroid cell19 was calculated from a calibration curve obtained with Qifikit calibration beads, used according to the manufacturer's instructions (Dako, Denmark).
Adhesion assay with antibodies or recombinant proteins
To investigate the receptors supporting adhesion, RBCs were incubated with the following antibodies for 30 minutes at 37°C before assay: antibodies against Lu/BCAM (polyclonal anti-Lub, 50-100 μL; Biorad, Marnes La Coquette, France); mAb anti-Lu (5-20 μg/mL, clone F241); mAb against ICAM-4 (5-20μg/mL, clone BS56),20 and mAb against CD59 (negative control). Alternatively, HUVECs were treated with mAb against the laminin α5 chain, (5-20 μg/mL, clone 4C7; Chemicon)21 or mAbs (5-20 μg/mL) against the following integrins: α2β1 (clone P1E6; Covance, Berkeley, CA), α3 (clone ASC-1; Chemicon), α5 (clone 5H10-27; BD Biosciences, Le Pont de Claix, France), α622 (clone G0H3; BD Biosciences), β123 (clone mAb 13; BD Biosciences), and αVβ3 (clone LM609; Chemicon). Isotype-matched mouse Ig (IgG1 or IgG2a) were used as controls, as appropriate. Because we previously observed that adhesion of diabetic erythrocytes bearing advanced glycation end (AGE) products to HUVECs was mediated through a specific receptor for AGE (RAGE),24 we used a rabbit polyclonal anti-RAGE antibody (50-100 μg/mL) in control experiments.25 A subset of 10 patients was tested in static conditions, selected to be representative of the whole group according to their levels of adhesion. A total of 6 of the 10 patients were also tested with antibodies under conditions of flow.
Immunofluorescence microscopy
HUVECs (2 × 105 cells) were cultured on 12-mm diameter, 0.4-μM pore Costar Transwell polycarbonate filters (Corning Costar, Acton, MA) for 5 days. Cells were then fixed for 20 minutes with 4% paraformaldehyde, permeabilized for 10 minutes with 0.5% Triton X-100, and incubated with anti-laminin α5 chain (mAb 4C7, 1:40) or matched mouse IgG2a for 1 hour at room temperature. Filters were washed with phosphate-buffered saline (PBS)-0.5% bovine serum albumin (BSA) and incubated with an Alexa fluor 488-conjugated antimouse antibody (1:200) for 1 hour at room temperature. Filters were cut out, mounted with Prolong antifade (Molecular Probes, Eugene, OR) on microscope slides and examined by confocal microscopy using a Nikon EC-1 system equipped with a 63/1.4 objective (Nikon, Melville, NY).28
Erythrocyte phosphorylation assay
Phospholabeling of RBCs was performed on normal and PV RBCs as previously described10 : RBCs (200 μL) were preincubated in 1.8 mL buffer A (150 mM NaCl, 20 mM Tris-HCl [pH 7.5], 10 mM KCl, 1 mM MgCl2, 100 μg/mL streptomycin, and 25 μg/mL chloramphenicol) for 4 hours at 35°C to deplete endogenous ATP stores, then centrifuged at 750g for 3 minutes and resuspended in 1.8 mL buffer A containing 11.1 MBq (300 μCi) of orthophosphate 32P (25 mM) for 14 hours at 35°C. In some experiments, RBCs were stimulated by forskolin (200 μM) for 20 minutes at 35°C in the presence of 1 μM okadaic acid (phosphatase inhibitor) and then washed twice with cold PBS. Finally, RBCs were lysed with buffer A supplemented with 1% Triton X-100, 0.2% BSA, and phosphatase and protease inhibitors.
Lu/BCAM glycoprotein (Lu/BCAMgp) was immunoprecipitated from lysates using anti-Lu/BCAM (mAb F241) and protein A-sepharose CL4B beads overnight at 4°C after a preclearing step of 3 hours with goat serum and sepharose beads. After elution with Laemmli buffer (1 ×) at 100°C, SDS-PAGE, and transfer to nitrocellulose membranes, phosphorylated proteins were detected using a FujiFilm BAS-1800 II PhosphorImager (Fuji, Tokyo, Japan). Total Lu proteins were then revealed by Western blot using rabbit anti-Lu antibody 602 specifically directed against the cytoplasmic domain of the Lu/BCAM long isoform.10
K562 cells-Lu/BCAM phosphorylation assay
Transfected human K562 erythroleukemic cells expressing recombinant human Lu/BCAM (long isoform Lu/BCAMgp),27 under the control of a CMV promoter, were used in Lu/BCAMgp phosphorylation assays. Cells were transiently transfected with the MIGR plasmid expressing either JAK2-wild-type (WT) or JAK2-617V>F4 (a generous gift of Dr W. Vainchenker and J.-L. Villeval, INSERM U790, Institut Gustave Roussy, Villejuif, France) and grown for 2 days. Expression of JAK2 was tested in cell lysates by Western blot using a rabbit anti-JAK2 antibody (Chemicon). Cells (5 × 106) were washed 3 times with phosphate-free medium and incubated in the same medium for 2 hours at 37°C and 5% CO2, then after additional washes they were suspended in 2 mL phosphate-free medium supplemented with 7.4 MBq (200 μCi) of orthophosphate 32P and 1 μM okadaic acid. After 2 hours of incubation at 37°C, cells were washed twice with cold PBS, then lysed for 45 minutes at 4°C with lysing buffer (1% Triton-X100, 150 mM NaCl, 20 mM Tris-HCl [pH 8], 5 mM EDTA, 0.2% BSA) supplemented with phosphatase and protease inhibitors. Lu/BCAMgp was immunoprecipitated as described for RBCs. Phosphorylation was quantified using Multi Gauge V3.0 software (Raytest, Straubenhardt, Germany). Lu/BCAMgp phosphorylation in the presence of Jak2-617V>F was determined taking into account the percentage of green fluorescent protein-positive (GFP+) cells. Total immunoprecipitated Lu/BCAMgp was quantified by Western blot using a biotinylated anti-Lu antibody (R&D systems, Minneapolis, MN) and Quantity One software (Biorad).
Statistical analysis
Results are presented either as the mean plus or minus one standard error of the mean (SEM), or percentiles (10%-90%, 25%-75%, and external values). Statistical significance was determined using a 1-way analysis of variance (ANOVA) followed by the parametric Dunnett test.
Results
RBC adhesion
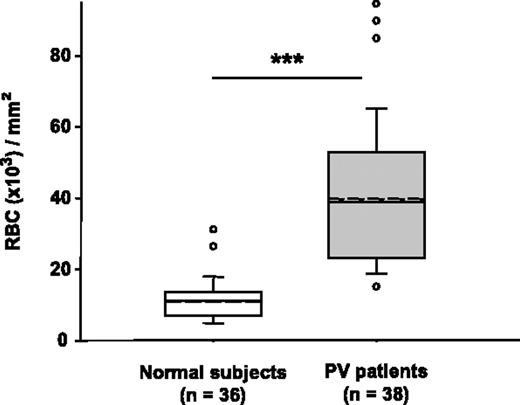
Adhesion of PV RBCs to HUVECs, studied under static conditions, was significantly increased compared with that of normal RBCs. The mean number of adhering PV RBCs was 3.7-fold higher compared with the adhesion of normal RBCs (Figure 1). To test whether the increase in adhesion was due to polycythemia or was specific to PV, we performed adhesion assays using RBCs from 5 patients who suffered from polycythemia secondary to pulmonary disease. RBC adhesion was similar to normal controls (10.3 ± 0.8 versus 10.9 ± 0.7 RBCs × 103/mm2, respectively). We evaluated whether RBC adhesion was altered by storage. We compared the adhesiveness of RBCs from 10 patients with PV and 10 healthy volunteers before and after storage at −196°C in glycerol (19% final concentration), and found no significant effects of storage (patients with PV: 45 ± 0.8 RBCs × 103/mm2 before and 44 ± 1.2 RBCs × 103/mm2 after storage; healthy volunteers: 10 ± 0.4 RBCs × 103/mm2 before and 11 ± 0.6 RBCs × 103/mm2 after storage).
RBCs from patients with PV adhere in greater numbers to HUVECs than RBCs from healthy volunteers under static conditions. RBC adhesion was measured using 51Cr-labeled RBCs. The nonadherent RBCs were removed by successive washes,6 and the radioactivity was measured in each wash and in the remaining RBCs attached to the HUVECs. The number of adherent RBCs was calculated. The unbroken line within the box represents the median; the dotted line represents the mean. The vertical lines extending beyond the boxes indicate the 25% and 75% percentiles, while the horizontal bars outside the boxes represent the 10% and 90% percentiles. ○ indicates the values outside this range. The RBC adhesion was significantly higher in the group of patients with PV compared with healthy volunteers (***P < .001).
RBCs from patients with PV adhere in greater numbers to HUVECs than RBCs from healthy volunteers under static conditions. RBC adhesion was measured using 51Cr-labeled RBCs. The nonadherent RBCs were removed by successive washes,6 and the radioactivity was measured in each wash and in the remaining RBCs attached to the HUVECs. The number of adherent RBCs was calculated. The unbroken line within the box represents the median; the dotted line represents the mean. The vertical lines extending beyond the boxes indicate the 25% and 75% percentiles, while the horizontal bars outside the boxes represent the 10% and 90% percentiles. ○ indicates the values outside this range. The RBC adhesion was significantly higher in the group of patients with PV compared with healthy volunteers (***P < .001).
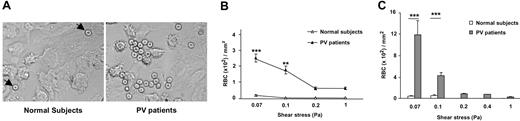
Two types of flow-based adhesion assays were used: (1) a test of the strength of RBC attachment to HUVECs, by allowing RBCs to adhere at low wall shear stress (0.03 Pa) and then analyzing washout with increasing stress (0.07-1.00 Pa); and (2) a test of their efficiency of attachment at increasing shear stress levels (0.07-1.00 Pa). In the first case, RBCs from patients with PV adhered in greater numbers at 0.07 Pa (Figure 2A) and were much more resistant to washout than control RBCs (patients with PV, 2.5 ± 0.25 RBCs × 102/mm2; healthy volunteers, 0.15 ± 0.05 RBCs × 102/mm2 at 0.07 Pa; Figure 2B). The number of RBCs remaining adherent after washout at the highest shear stress (1.00 Pa) was 0.6 ± 0.06 RBCs × 102/mm2 for the patients with PV and less than 0.001 RBCs × 102/mm2 for the control group (Figure 2B). Because 20 of 38 patients were tested under flow conditions the day after collection, and the others were tested after storage, we compared the values obtained before and after storage in 6 patients and 6 healthy volunteers in all the conditions. No significant difference was observed. For example, at 0.07 Pa, PV RBC adhesion was 1.9 ± 0.5 RBCs × 102/mm2 before storage and 2.1 ± 0.4 RBCs × 102/mm2 after storage; for healthy volunteers, values were 0.16 ± 0.05 RBCs × 102/mm2 and 0.14 ± 0.06 RBCs × 102/mm2, respectively.
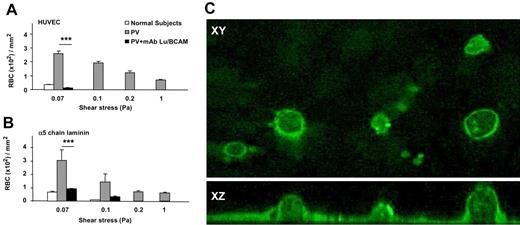
RBCs from patients with PV adhere more efficiently to HUVECs than RBCs from healthy volunteers under flow conditions and are resistant to wash-off by increasing shear stress. (A) A typical microscopic image shows that PV RBCs adhere to a higher extent to HUVECs than healthy volunteer RBCs after inflow at 0.03 Pa and washout (1.00 Pa). (B) Number of RBCs remaining adherent to HUVECs in microslides after inflow at 0.03 Pa and washout at increasing shear stress. (C) Number of RBCs adherent to HUVECs after inflow at different wall shear stresses **P < .01; ***P < .001 for PV RBCs compared with normal RBCs. Bars denote SEM.
RBCs from patients with PV adhere more efficiently to HUVECs than RBCs from healthy volunteers under flow conditions and are resistant to wash-off by increasing shear stress. (A) A typical microscopic image shows that PV RBCs adhere to a higher extent to HUVECs than healthy volunteer RBCs after inflow at 0.03 Pa and washout (1.00 Pa). (B) Number of RBCs remaining adherent to HUVECs in microslides after inflow at 0.03 Pa and washout at increasing shear stress. (C) Number of RBCs adherent to HUVECs after inflow at different wall shear stresses **P < .01; ***P < .001 for PV RBCs compared with normal RBCs. Bars denote SEM.
When RBCs were perfused at increasing flow rates, equivalent to a wall shear stress of 0.07 to 1.00 Pa, RBCs from patients with PV adhered more efficiently than controls (Figure 2C). The differences between normal RBCs and RBCs from patients with PV were greater when measured under flow condition than under static condition, which suggests that the results are relevant to in vivo circulatory conditions.
Expression of adhesion molecules on RBCs and HUVECs
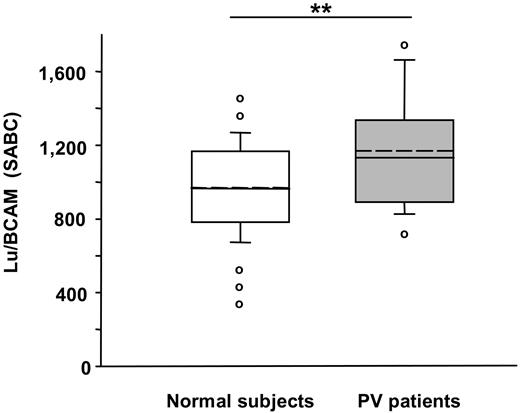
The expression of several adhesion molecules was tested by flow cytometry in PV and control RBCs. CD36 (glycoprotein IV, GP IV) and CD147 (basigin destain) were similarly expressed on normal and PV RBCs (CD36: 64 ± 4 and 61 ± 4 SABC; and CD147: 3214 ± 280 and 3563 ± 202 SABC, respectively). The expression of Lu/BCAM was significantly higher in the group of patients with PV than in the control group (Figure 3). Lu/BCAM SABC and the number of adhering PV RBCs were significantly correlated (r = 0.463, .01 < P > .001). ICAM-4 was expressed at similar levels on RBCs of patients with PV and healthy volunteers (1048 ± 186 and 1185 ± 306 SABC, respectively) of the same RhD blood type.
RBCs from patients with PV show greater expression of Lu/BCAM. Using specific antibodies and flow cytometry, we measured Lu/BCAM expression on RBCs from 16 healthy volunteers and 23 patients with PV; Lu/BCAM showed higher expression in patients with PV (**P < .01). The unbroken line within the box represents the median; the dotted line represents the mean. The vertical lines extending beyond the boxes indicate the 25% and 75% percentiles, while the horizontal bars outside the boxes represent the 10% and 90% percentiles. ○ indicates the values outside this range.
RBCs from patients with PV show greater expression of Lu/BCAM. Using specific antibodies and flow cytometry, we measured Lu/BCAM expression on RBCs from 16 healthy volunteers and 23 patients with PV; Lu/BCAM showed higher expression in patients with PV (**P < .01). The unbroken line within the box represents the median; the dotted line represents the mean. The vertical lines extending beyond the boxes indicate the 25% and 75% percentiles, while the horizontal bars outside the boxes represent the 10% and 90% percentiles. ○ indicates the values outside this range.
In the PV patient group, Lu/BCAM expression and reticulocyte count were not significantly correlated, which indicated that, besides the increase in reticulocytes, another factor was responsible for the Lu/BCAM enhancement. HUVECs expressed several integrins that may be involved in the adhesion process at significant but varying levels: CD49b (29 900 ± 100 SABC), CD49c (50 800 ± 120 SABC), CD49e (155 000 ± 600 SABC), CD49f (6900 ± 300 SABC), CD51 (49 200 ± 800 SABC), CD29 (200 500 ± 700 SABC), and CD61 (32 900 ± 110 SABC).
Blocking experiments and adhesion to laminin α5 chain
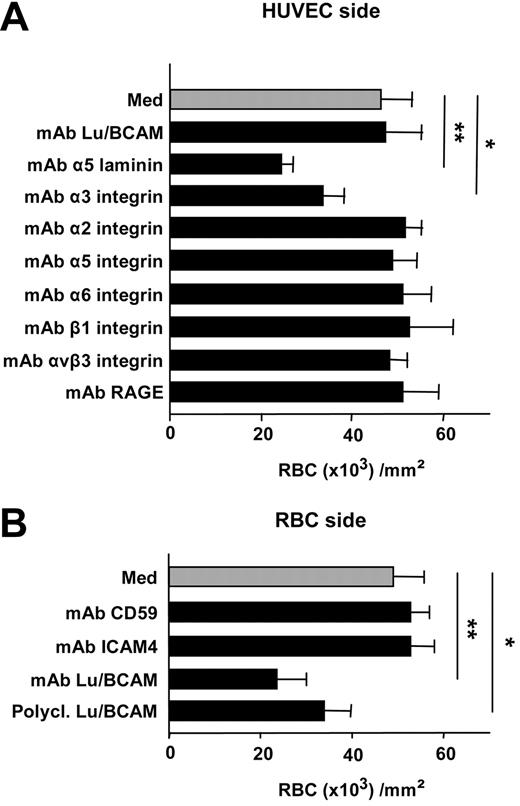
Under static conditions, incubation of HUVECs with anti-laminin α5 chain (mAb 4C7) inhibited RBC adhesion (47% ± 5% reduction; P = .001), while anti-Lu/BCAM antibody (F241) had no effect (Figure 4A). When incubated with HUVECs, mAbs against α2, α5, α6, or β1, or αvβ3 integrins did not alter RBC adhesion, while anti-α3 integrin significantly reduced the adhesion by 27% (± 7%; P < .05). Mouse Ig isotype-matched controls or anti-RAGE did not modify RBC adhesion (Figure 4A). Incubation of PV RBCs with anti-Lu/BCAM antibodies (mAb or polyclonal) reduced the adhesion by 52% (± 12%) and 31% (± 7%), respectively, while anti-ICAM-4 (mAb BS56) or anti-CD59 (clone 2/24) used as control had no effect (Figure 4B). When anti-Lu/BCAM and anti-α3 integrin or anti-laminin α5 and anti-α3 integrin were added simultaneously either to HUVECs or to RBCs, no significant modification of the inhibition was observed compared with anti-Lu/BCAM or anti-α3 integrin alone (anti-Lu/BCAM and anti-α3 integrin, 45% ± 6% inhibition; anti-α3 integrin and anti-α5 chain laminin, 32% ± 4% inhibition). Furthermore, addition of soluble recombinant Lu/BCAM (100 μg/mL) to HUVECs inhibited RBC adhesion (39% ± 0.5% reduction), while addition of recombinant VCAM-1 (100 μg/mL) to RBCs or ICAM-4 (100 μg/mL) to HUVECs did not significantly alter the PV RBC adhesion (9% ± 4% and 4% ± 1% reduction, respectively; n = 6, P value nonsignificant).
Effects of antibodies against different adhesion molecules on adhesion of PV RBCs to HUVECs under static conditions. (A) When incubated with HUVECs, neither anti-Lu/BCAM anti-α2, anti-α5, α6, β1, αvβ3 integrins, nor anti-RAGE antibodies modified adhesion of RBCs (n = 10). In contrast, anti-α5 chain laminin and to a lesser extent anti-α3 integrins significantly reduced adhesion (**P < .01; *P < .05; n = 10). (B) Monoclonal or polyclonal anti-Lu/BCAM incubated with RBCs inhibited the adhesion of HUVECs measured in static conditions (**P < .01; *:P < .05, respectively; n = 10), while anti-ICAM-4 or control mAb anti-CD59 had no significant effect on adhesion (n = 5). Bars denote SEM.
Effects of antibodies against different adhesion molecules on adhesion of PV RBCs to HUVECs under static conditions. (A) When incubated with HUVECs, neither anti-Lu/BCAM anti-α2, anti-α5, α6, β1, αvβ3 integrins, nor anti-RAGE antibodies modified adhesion of RBCs (n = 10). In contrast, anti-α5 chain laminin and to a lesser extent anti-α3 integrins significantly reduced adhesion (**P < .01; *P < .05; n = 10). (B) Monoclonal or polyclonal anti-Lu/BCAM incubated with RBCs inhibited the adhesion of HUVECs measured in static conditions (**P < .01; *:P < .05, respectively; n = 10), while anti-ICAM-4 or control mAb anti-CD59 had no significant effect on adhesion (n = 5). Bars denote SEM.
In flow experiments, treatment of RBCs with mAb against Lu/BCAM (F241) abolished RBC adhesion to HUVECs at 0.07 Pa and higher (Figure 5A). To investigate the potential interaction between erythroid Lu/BCAM and endothelial laminin α5, suggested by these data, adhesion assays were performed replacing HUVECs with laminin-10/11-coated microslides (human α5 chain laminin, 1 μg/cm2; Sigma, St Louis, MO). The adhesion of PV RBCs to coated laminin 10/11 was significantly higher than the adhesion of control RBCs (Figure 5B). In addition, incubation of PV RBCs with anti-Lu/BCAM mAb reduced RBC adhesion to laminin, which is consistent with reports that Lu/BCAM is the erythroid counterreceptor for laminin α5 chain10,29 (Figure 5B).
Inhibition of PV RBC adhesion to HUVECs or α5 chain laminin by mAb against Lu/BCAM measured under flow conditions. (A) Anti-Lu/BCAM inhibited the adhesion of PV RBCs to HUVECs at different shear stresses, and the inhibition at 0.07 Pa is highly significant (***P < .001; n = 6). (B) When HUVECs were replaced by α5 chain laminin, a known ligand of Lu/BCAM, anti-Lu/BCAM inhibited PV RBC adhesion (***P < .001; n = 6). (C) α5 chain laminin expression by HUVECs visualized by immunofluorescence and confocal microscopy. Top panel x-y horizontal plane and bottom panel x-z vertical section (magnification, × 100 000) exhibited the presence of α5 chain laminin at the cell membrane on apical and basal surfaces. No staining was visible with an isotype-matched mouse IgG2a. Bars denote SEM.
Inhibition of PV RBC adhesion to HUVECs or α5 chain laminin by mAb against Lu/BCAM measured under flow conditions. (A) Anti-Lu/BCAM inhibited the adhesion of PV RBCs to HUVECs at different shear stresses, and the inhibition at 0.07 Pa is highly significant (***P < .001; n = 6). (B) When HUVECs were replaced by α5 chain laminin, a known ligand of Lu/BCAM, anti-Lu/BCAM inhibited PV RBC adhesion (***P < .001; n = 6). (C) α5 chain laminin expression by HUVECs visualized by immunofluorescence and confocal microscopy. Top panel x-y horizontal plane and bottom panel x-z vertical section (magnification, × 100 000) exhibited the presence of α5 chain laminin at the cell membrane on apical and basal surfaces. No staining was visible with an isotype-matched mouse IgG2a. Bars denote SEM.
VLA-4 (CD49d) expressed on reticulocytes (but not on mature RBCs) is known to bind to endothelial VCAM-1.30 Because recombinant VCAM-1 had no significant inhibitory effect on adhesion when incubated with PV RBCs, it is unlikely that VLA-4 was a major factor responsible for the increase in PV RBC adhesion. Nevertheless, because the reticulocyte count in the total RBC population was higher in patients with PV compared with healthy volunteers (100 ± 4.1 × 109/L and 80 ± 2.1 × 109/L, respectively; P < .05), we analyzed the percentage of reticulocytes in the adherent RBC population. The percentages of reticulocytes in the adhering and the nonadhering PV RBC populations were similar (1.2% ± 0.1% and 1.5% ± 0.1%, respectively). As a control, we determined the reticulocyte count before and after storage at −196°C, and it did not change (108 ± 2 × 109/L and 101 ± 4 × 109/L, respectively, in patients with PV). VCAM-1 expression was up-regulated by TNFα (5 ng/mL). Mean fluorescence intensities were 9.9 ± 4 arbitrary units [AU] of fluorescence versus 307 ± 16 AU of fluorescence before and after TNFα stimulation, respectively). Thus did not modify the adhesion of PV RBCs under static conditions (45.2 ± 7.3 RBCs × 103/mm2 and 47.5 ± 3.3 RBCs × 103/mm2, respectively). After TNFα stimulation of HUVECs, the adhesion of PV RBCs, when measured in flow system, was slightly but not significantly increased either at 0.07 Pa or 0.2 Pa (20% ± 10% and 16% ± 10% increase, respectively).
Immunostaining of HUVECs by anti-laminin α5 antibody
Because laminin is generally expressed in basal lamina, we explored the expression of laminin α5 on HUVECs with immunofluorescence and confocal microscopy using a specific anti-laminin α5 (mAb 4C7). We found that the laminin α5 chain was not only present on the HUVEC basal side, but also covered the total cell surface, including the apical pole (Figure 5C). No staining was found with an isotype-matched mouse IgG2a. These results indicated that laminin α5 is present on the HUVEC membrane and could be accessible to RBCs in both our static and dynamic experimental assays.
Phosphorylation of Lu/BCAM in RBCs
Lu/BCAM phosphorylation has been shown to be essential for increased Lu/BCAM adhesiveness to laminin in sickle cell (SS) RBCs.10 Hines and collaborators31 first showed that the physiologic stress mediator epinephrine or forskolin increased Lu/BCAM-mediated adhesion of sickle RBCs to laminin α5 via a signaling pathway-dependent cAMP and protein kinase A (PKA). Our group next demonstrated that PKA-mediated phosphorylation of the Lu/BCAM long isoform increased adhesion to laminin in sickle RBCs, and we suggested that enhanced adhesion of stimulated sickle RBCs to laminin was mediated by a PKA-phosphorylated serine of Lu/BCAM.10
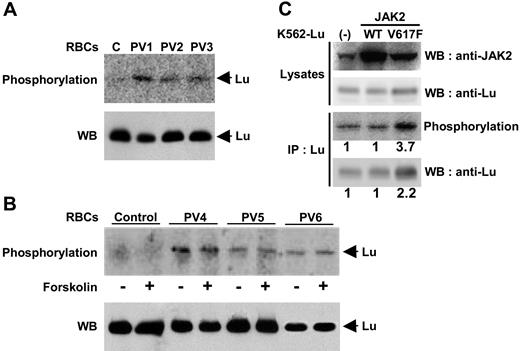
Here, we confirm that the Lu/BCAM long isoform was not phosphorylated in normal RBCs in the absence or presence of the exogenous stimulus forskolin (Figure 6A,B). In contrast, the Lu/BCAM long isoform expressed in RBCs from 6 patients with PV was phosphorylated (Figure 6A,B) when the cells were taken straight from the blood. This process was not augmented by forskolin treatment (Figure 6B), in contrast to RBCs from patients with sickle cell anemia or for transfected cells.10 Thus, the Lu/BCAM long isoform was constitutively phosphorylated in PV RBCs.
Phosphorylation of Lu/BCAM in RBCs and K562 cells. Normal or PV RBCs were incubated with 32P orthophosphate in the absence (A) or presence (B) of forskolin, and Lu/BCAM was immunoprecipitated using mAb against Lu/BCAM. After elution, SDS-PAGE, and transfer to nitrocellulose membrane, the phosphorylated proteins were detected using a PhosphorImager. As previously reported, no phosphorylation was detected in the normal RBCs, while in the 6 patients with PV, the Lu/BCAM long isoform was phosphorylated in the absence of any stimulation. Western blot (WB) using anti-Lu rabbit antibody 602 showed that equivalent amounts of Lu protein were immunoprecipitated for each patient in the absence or presence of forskolin in panel B. (C) Phosphorylation of recombinant Lu/BCAM gp in K562 cells. Recombinant JAK2 WT or 617V>F were transiently expressed in K562-Lu/BCAM cells and detected by Western blot (WB; top row). Expression of recombinant Lu/BCAM was tested in cell lysates by Western blot (second row). Phosphorylation of Lu/BCAMgp was analyzed after immunoprecipitation (IP; third panel), and total immunoprecipitated Lu/BCAM proteins were revealed by Western blot (bottom row). Results are representative of 3 independent experiments. The phosphorylation in K562 cells tranfected with 617V>F was 3.7-fold (± 1.1-fold) higher, while Lu/BCAM protein was doubled (2.2-fold ± 0.3-fold) compared with K562 cells transfected with WT JAK2.
Phosphorylation of Lu/BCAM in RBCs and K562 cells. Normal or PV RBCs were incubated with 32P orthophosphate in the absence (A) or presence (B) of forskolin, and Lu/BCAM was immunoprecipitated using mAb against Lu/BCAM. After elution, SDS-PAGE, and transfer to nitrocellulose membrane, the phosphorylated proteins were detected using a PhosphorImager. As previously reported, no phosphorylation was detected in the normal RBCs, while in the 6 patients with PV, the Lu/BCAM long isoform was phosphorylated in the absence of any stimulation. Western blot (WB) using anti-Lu rabbit antibody 602 showed that equivalent amounts of Lu protein were immunoprecipitated for each patient in the absence or presence of forskolin in panel B. (C) Phosphorylation of recombinant Lu/BCAM gp in K562 cells. Recombinant JAK2 WT or 617V>F were transiently expressed in K562-Lu/BCAM cells and detected by Western blot (WB; top row). Expression of recombinant Lu/BCAM was tested in cell lysates by Western blot (second row). Phosphorylation of Lu/BCAMgp was analyzed after immunoprecipitation (IP; third panel), and total immunoprecipitated Lu/BCAM proteins were revealed by Western blot (bottom row). Results are representative of 3 independent experiments. The phosphorylation in K562 cells tranfected with 617V>F was 3.7-fold (± 1.1-fold) higher, while Lu/BCAM protein was doubled (2.2-fold ± 0.3-fold) compared with K562 cells transfected with WT JAK2.
Expression of JAK2 617V>F and Lu/BCAM phosphorylation in transfected K562 cells
The phosphorylation state of Lu/BCAM was next analyzed in the presence of the activating mutation 617V>F of JAK2. Erythroleukemic K562 cells, expressing the long isoform of recombinant Lu/BCAM, were transiently transfected with the MIGR plasmid expressing JAK2 WT or JAK2 617V>F. The percentage of cells transfected by MIGR was tested by flow cytometry, as this plasmid also encodes GFP. Western blotting revealed endogenous JAK2 in native cells as well as recombinant JAK2 WT and 617V>Fin transfected cells (Figure 6C; top row). Recombinant JAK2 617V>F was functional because it enhanced the expression of recombinant Lu/BCAM, which is under the control of a CMV promoter (Figure 6C; second row). Phosphorylation of Lu/BCAM was analyzed and quantified in all cell types (Figure 6C; third row). Lu/BCAM phosphorylation was increased by 3.7-fold (± 1.1-fold) in the presence of JAK2 617V>F, while no difference was detected in the cells expressing JAK2 WT compared with that of native cells. This increase was not solely due to the overexpression of recombinant Lu/BCAM after activation of the CMV promoter. Lu/BCAM was 2.2-fold (± 0.3-fold) greater in the cells expressing JAK2 617V>F compared with those expressing endogenous or recombinant wild-type JAK2 (Figure 6C; bottom row). This result demonstrated an enhanced phosphorylation of Lu/BCAMgp in the presence of constitutively active JAK2 617V>F in K562 cells.
Discussion
This is the first description of an increase in the adhesion of RBCs from patients with PV to HUVECs measured under static or flow conditions. Increased adhesiveness of RBCs has been previously described in sickle cell anemia,8 malaria,32 and diabetes mellitus.9 In sickle cell anemia, the molecular bases for RBC adhesion to endothelium were interactions between VLA-4 and VCAM-1, and between ICAM-4 and αvβ3 integrin.10,33 In diabetes, interaction between RBCs advanced glycation end products and RAGE-supported adhesion.24 In PV, Lu/BCAM was overexpressed on RBCs, and this increase was similar to that reported in sickle cell anemia.10 The Lutheran blood group and BCAM are both carried by 2 glycoprotein isoforms, Lu and Lu(v13), of the immunoglobulin superfamily. The Lu/BCAM protein is the receptor for the laminin α5 chain (present in laminin 10/11 isoforms).27,34,35 Lu/BCAM proteins have been also recognized as laminin α5 receptors in kidney epithelial cells, smooth muscle cells, and endothelial cell lines.36-38 The specific cytoplasmic domain of Lu/BCAMgp includes serine phosphorylation sites, which is consistent with a receptor signaling function.10
The increased adhesion of PV RBCs to HUVECs was mediated by erythroid Lu/BCAM and endothelial laminin α5. The inhibition of RBC adhesion by anti-Lu/BCAM antibody under flow conditions was more pronounced than under static conditions. In vivo, shear stresses in the postcapillary venule vary from 0.1 to 0.5 Pa. In our studies, adhesion of PV RBCs could be established in this range, and a proportion of adherent cells could withstand such forces. These data suggest existence of a high-affinity adhesive interaction that could occur in the microcirculation. Laminins are major structural elements of all basal lamina, forming self-assembling networks. Here, confocal microscopy showed that laminin α5 was not only expressed in the subendothelium of HUVECs, but totally covered the cell membrane and was thus accessible to RBC Lu/BCAM at the apical surface. In addition to the laminin α5 chain, integrin α3β1 might be involved in the adhesive process because anti-α3 partially inhibited adhesion of PV RBCs. Anti-β1 did not inhibit adhesion, but in HUVECs, β1 integrin can be associated with α2, α5, and α6 integrins as well as α3 integrin; consequently, a higher number of β1 molecules are expressed (2 × 105 SABC) compared with α3 (5 × 104 SABC), which may explain the difference between the anti-α3 and anti-β1 inhibitory activity. The interaction of the laminin α5 chain with α3β1 and α6β1 has been shown in several cell types, including endothelial cells.39
JAK2 mutations found in patients with PV3,4 may be responsible not only for myeloproliferation and increased blood cell production but also for functional abnormalities. Lu/BCAM is expressed after band 3 during erythropoiesis and first appears on early orthochromatic erythroblasts.18,40 Phosphorylations are essential steps during erythropoiesis as exemplified by JAK2 mutation, and these may amplify the adhesiveness of PV RBCs. We recently showed that adhesion of erythroid cells expressing the Lu/BCAM long isoform to laminin α5 increased after phosphorylation by PKA.10 In the present study, we observed that Lu/BCAMgp was spontaneously phosphorylated in RBCs from patients with PV. Activating the PKA signaling pathway did not increase phosphorylation of PV RBC Lu/BCAM in contrast to sickle RBCs.31 Lu/BCAM phosphorylation could account for the increased adhesion of PV RBCs to laminin α5 expressed at the HUVEC surface in the absence of stimulation.
In experiments using K562 cells transfected either with JAK2 WT or JAK2 617V>F, we demonstrated that the presence of JAK2 617V>F increased Lu/BCAM expression and to a larger extent Lu/BCAM phosphorylation. These results strongly suggest a relationship between JAK2 mutation and Lu/BCAM phosphorylation. However, from these experiments we cannot conclude a direct link between JAK2 mutation and Lu/BCAM phosphorylation, and additional investigations should be performed to further explore the cascade of cellular events.
The clonality of the disease could explain why the excessive adhesiveness was not restricted to reticulocytes but included other circulating RBCs. Thrombotic complications are frequent in patients with PV, and in particular cerebrovascular events. In the Budd-Chiari syndrome, postsinusoidal obstruction may occur outside the liver in the hepatic veins; this is frequently observed in patients with PV in whom no other thrombosis is detectable. The variability in the expression of adhesion molecules along the vascular tree and differences in flow conditions might explain why the occurrence of thrombosis is not random. The number of patients investigated in the present study does not allow us to determine whether or not Lu/BCAM overexpression and/or phosphorylation is an index of thrombotic risk in patients with PV, but JAK2 617V>F mutation has been reported as a risk factor for thrombosis.41
In summary, we have described for the first time that PV RBCs exhibit increased adhesion to endothelial cells. Lu/BCAM proteins, which are overexpressed and constitutively phosphorylated in PV RBCs, appear to cause this enhanced adhesion by binding to laminin α5 present on the endothelial cells.
The online version of this article contains a data supplement.
A portion of this work was presented at the Annual Congress and Exposition of the American Society of Hematology, Atlanta, GA, 2005.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Eliane Vera from Centre National de Référence des Groupes Sanguins (CNRGS) for blood sample management and the staff of the Department of Obstetrics of the Center Hospitalier Robert Ballanger for providing the umbilical cords for HUVEC preparation. We are also grateful to Dr W. Vainchenker and Dr J-L. Villeval (INSERM U790, Institut Gustave Roussy, Villejuif, France) for providing the plasmid expressing either JAK2 WT or JAK2 617V>F and Dr B. Cassinat (Unite de Biologie Cellulaire, Hôpital Saint Louis, Paris, France) for JAK2 genotyping by real-time PCR.
This work was supported by Institut National de la Transfusion Sanguine, Université Paris 7, and INSERM U665.
Authorship
Contribution: M.-P.W. performed the cell culture and the adhesion experiments and participated in each phase of the work; W.E.N. was in charge of all the molecular typing and phosphorylation experiments and participated in each phase of the work; P.G. did all the red blood cell typing and quantification; J.-D.R. recruited the patients and collected the clinical information; J.-P.C. provided anti-ICAM-4 antibody and recombinant ICAM-4, and was associated with the scientific discussion; Y.C. actively participated in the investigation of the relationship between JAK2 mutation and Lu/BCAM phosphorylation; C.L.V.K. was associated with the scientific discussion and the follow-up of the project; and J.-L.W. designed the research, analyzed the results, and actively participated in the preparation of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jean-Luc Wautier, Laboratoire de Biologie Vasculaire et Cellulaire, INTS, 6 rue Alexandre Cabanel, 75739 Paris Cedex 15, France; e-mail: jlwautier@ints.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal