In this issue of Blood, Löffler and colleagues report the regulation of microRNA-21 by IL-6, through a Stat3-dependent mechanism, which results in the repression of apoptosis in multiple myeloma cells.
Originally identified in Caehorhabditis elegans as regulators of development, microRNAs (miRNAs) are conserved across species and have important regulatory roles in everything from development to disease pathogenesis. With the identification of more than 300 miRNAs in humans1 regulating an estimated 30% of the genes in the human genome,2 miRNAs represent an important regulatory mechanism that, until recently, was unappreciated. While work continues in identifying the targets of miRNAs, since the short 18-to-22-nucleotide RNAs can theoretically bind to many target mRNAs, there have not been many reports on the regulation of the miRNAs themselves.
The IL-6 pathway, through Stat3 activation, is an important pathway for many carcinomas, including both multiple myeloma and prostate cancer (reviewed by Hodge et al3 ). Stat3 coordinates the expression of many genes, including several antiapoptotic proteins. The transcriptional regulation of these genes contributes to the survival of cancer cells, but it has been recently demonstrated that there is a second independent survival pathway linked to Stat3 activation.4
The report by Löffler and colleagues defines a regulatory pathway beginning with an exogenous signal and culminating in the up-regulation of an miRNA, resulting in the inhibition of apoptosis. Using an informatics approach, they identify 2 phylogenetically conserved STAT3 binding sites in the upstream region of microRNA-21 (miR-21). Through a chromatin immunoprecipitation (ChIP) assay, they demonstrate the binding of Stat3 to an upstream enhancer following IL-6 treatment of multiple myeloma cell lines. They further show that either mutation of the Stat3 binding sites in the enhancer of miR-21 or the siRNA knockdown of Stat3 abrogates induction of miR-21 by IL-6. Moreover, ectopic miR-21 expression is sufficient to sustain growth of IL-6-dependent cell lines in the absence of IL-6. This represents one of the first descriptions of miRNA regulation in humans and identifies miR-21 as an important regulator of cancer-cell survival.
How does miR-21 expression result in an antiapoptotic signal? Although Löffler and colleagues do not explore this, tropomyosin 1 (TPM1), a tumor suppressor gene, was recently identified as a target for miR-21 in breast cancer cells.5 Although TPM1, which associates with actin and stabilizes microfilament structures, provides an attractive model for the ability of miR-21 expression to contribute to breast cancer etiology, mirRNAs have multiple predicted targets. Indeed, Zhu et al5 identified many proteins regulated by miR-21 and therefore the exact mechanism of how miR-21 inhibits apoptosis and/or promotes proliferation warrants further investigation. Nevertheless, in describing the regulation of miR-21, Löffler and colleagues have defined an additional mechanism of how IL-6 represses apoptosis of multiple meyloma, thereby expanding the list of potential therapeutic targets (see figure).
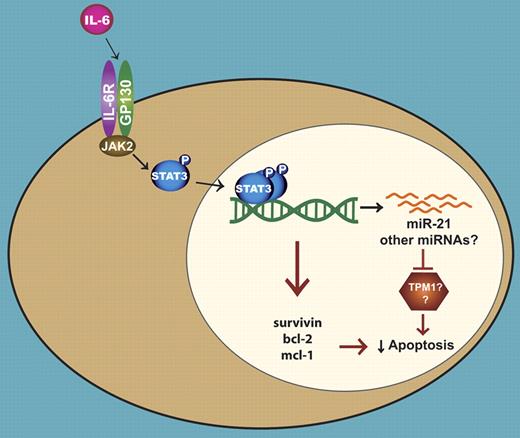
An illustration depicting the role of miR-21 in the IL-6 induced survival of multiple myeloma cells. IL-6 leads to the activation of Stat3, which then dimerizes and binds to its cognate sites located in the regulatory regions of genes. The Stat3-mediated transcription of several antiapoptotic genes contributes to the survival of myeloma cells. In a separate mechanism, Stat3 directs the expression of miR-21, resulting in the suppression of apoptosis possibly through the inhibition of TPM1 and/or other proteins.
An illustration depicting the role of miR-21 in the IL-6 induced survival of multiple myeloma cells. IL-6 leads to the activation of Stat3, which then dimerizes and binds to its cognate sites located in the regulatory regions of genes. The Stat3-mediated transcription of several antiapoptotic genes contributes to the survival of myeloma cells. In a separate mechanism, Stat3 directs the expression of miR-21, resulting in the suppression of apoptosis possibly through the inhibition of TPM1 and/or other proteins.
Conflict-of-interest disclosure: This publication has been funded in part with Federal funds from the National Cancer Institute, National Institutes of Health, under contract No. N01-CO-12400. This research was supported in part by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute. ■
The content of this publication does not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products, or organizations imply endorsement by the US Government.
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal