Abstract
Hereditary folate malabsorption (HFM) is a rare autosomal recessive disorder caused by impaired intestinal folate absorption and impaired folate transport into the central nervous system. Recent studies in 1 family revealed that the molecular basis for this disorder is a loss-of-function mutation in the PCFT gene encoding a proton-coupled folate transporter. The current study broadens the understanding of the spectrum of alterations in the PCFT gene associated with HFM in 5 additional patients. There was no racial, ethnic, or sex pattern. A total of 4 different homozygous mutations were detected in 4 patients; 2 heterozygous mutations were identified in the fifth patient. Mutations involved 4 of the 5 exons, all at highly conserved amino acid residues. A total of 4 of the mutated transporters resulted in a complete loss of transport function, primarily due to decreased protein stability and/or defects in membrane trafficking, while 2 of the mutated carriers manifested residual function. Folate transport at low pH was markedly impaired in transformed lymphocytes from 2 patients. These findings further substantiate the role that mutations in PCFT play in the pathogenesis of HFM and will make possible rapid diagnosis and treatment of this disorder in infants, and prenatal diagnosis in families that carry a mutated gene.
Introduction
Hereditary folate malabsorption (HFM; Online Mendelian Inheritance in Man [OMIM 229050]) is a rare autosomal recessive disorder caused by impaired intestinal folate absorption with folate deficiency characterized by anemia; hypoimmunoglobulinemia with recurrent infections, such as Pneumocystis carinii pneumonitis; and recurrent or chronic diarrhea. In many patients, neurologic abnormalities such as seizures or mental retardation emerge at some point in early childhood, which are attributed to impaired transport of folates into the central nervous system.1 When this disorder is diagnosed early, signs and symptoms of HFM can be obviated by parenteral administration of folates or with higher doses of folates by the oral route.1,2 If untreated, the disease is fatal and, if treatment is delayed, the neurologic deficits can become permanent.3,4 Hence, it is important that physicians are aware of this disorder and establish a diagnosis and institute treatment as early as possible in infancy. The clinical characteristics of HFM and its treatment were the subject of a recent comprehensive review.1
The molecular basis for HFM was recently shown, in 1 family, to be due to a mutation in a novel proton-coupled folate transporter (PCFT) that mediates intestinal folate absorption.5 PCFT has a low pH optimum that allows efficient transport of folates in the acid microclimate of the duodenum and jejunum,6 the major sites of folate absorption, where this transporter is highly expressed. This same gene was previously reported to be a heme carrier protein (HCP1) that mediates heme-iron absorption,7 but its major function appears to be folate transport.5
The objective of this paper is to extend the understanding of the spectrum of genomic alterations in the PCFT gene that are the basis for HFM along with an analysis of the stability, membrane trafficking, and functional properties of the mutants identified in 5 additional families with this disease. Two patients were the subject of case reports prior to the characterization of the underlying genetic defect.3,8 In 1 family, the genetic defect was traced through 3 generations. In 2 patients from the family previously reported, folate transport was assessed in transformed lymphocytes at low pH.5
Patients, materials, and methods
Patients
Patient P1 was a male child of 2 African-American parents who denied consanguinity. He presented at age 3 months with pancytopenia, a megaloblastic bone marrow, hypoimmunoglobulinemia, and Pneumocystis carinii pneumonia. He is mentally retarded, has a seizure disorder, and has been treated with parenteral 5-formylTHF (5-formyltetrahydrofolate). This patient was the subject of a previous case report.8 Patient P2 was also the subject of a prior case report,3 and was the ninth child (female) of Turkish parents who denied consanguinity. She presented at 5 months of age with a history of fever, diarrhea, and convulsions. She was anemic and leukopenic, with a megaloblastic bone marrow and hypoimmunoglobulinemia. Despite treatment with parenteral folate, she had chronic seizures and persistent neurologic defects, including hemiplegia and mental retardation. The third patient (P3-female) is of European ancestry and presented in infancy with a folate-responsive megaloblastic anemia, and a developmental delay in speech-receptive language and fine motor skills. The parents were second cousins. The fourth patient (P4-female) is an Arab child from Israel who presented at the age of 4 months with anemia, diarrhea, and failure to thrive. Another member of her family had been diagnosed with folate malabsorption.
The fifth patient, of Spanish/Brazilian/Mexican origin, (P5-male) presented in October 2005 at the age of 4 months with severe anemia. He subsequently developed Pneumocystis carinii pneumonia. The child had a sister who developed pancytopenia at age 3 months and died due to cytomegalovirus pneumonia. In the hospital, the patient's hemoglobin (Hb) fell to a low of 55 g/L (5.5 g/dL); macrocytes and hypersegmented neutrophils were noted, and the bone marrow was megaloblastic. There was a falling platelet count that reached a nadir of 44 × 109/L (44 000/mm3). The patient's serum folate was less than 0.91 nM (0.4 ng/mL) (nl [normal] > 6.3 nM [2.8 ng/mL]). Serum immunoglobulins were low: IgG was 1.35 g/L (134 mg/dL) (nl, 7.0-16 g/L [700-1600 mg/dL]); IgA, 0.13 g/L (13 mg/dL) (nl, 0.70-4.0 g/L [70-400 mg/dL]), and IgM, 0.08 g/L (8 mg/dL) (nl, 0.4-2.3 g/L [40-230 mg/dL]). The patient was treated with intravenous folate, then subsequently placed on oral 5-formylTHF. The pneumonia was treated successfully; the patient had a rapid onset of reticulocytosis, his hemogram normalized, and the pneumonia resolved. He was subsequently maintained on an oral dose of 10 mg 5-formylTHF twice daily. He is at present developing normally, with a Hb level of 125 g/L (12.5 g/dL) and a hematocrit (Hct) of 37%, white blood cell (WBC) count of 10.6 × 109/L (10.6/mm3), and platelet count of 371 × 109/L (371/mm3), with a blood folate level of 13.14 nM (5.8 ng/mL). The patient's serum iron (Fe) is 16.47 μM (92 μg/dL) (nl, 6.27-26.85 μM [35-150 μg/dL]); total iron binding capacity (TIBC), 56.56 μM (316 μg/dL) (nl, 44.75-80.55 μM [250-450 μg/dL]); and ferritin, 78 pM (34.7 ng/mL) (nl, 43-831 pM [19-370 ng/mL]). Blood was also obtained for analysis from the child's parents and grandparents.
Patients 6 (P6) and 7 (P7) were female siblings, diagnosed and treated in infancy, who were the subject of a previous case report1 ; studies from this laboratory established a mutation in PCFT as the basis for HFM.5 One of the siblings is on 200 mg of oral 5-formylTHF per day and currently has a Hb level of 139 g/L (13.9 g/dL), a Hct of 42.4%, WBC count of 8.4 × 109/L (8.4/mm3), platelet count of 297 × 109/L (297 K/mm3), and a serum ferritin of 121 pM (54 ng/mL) (nl, 22.5-236 pM [10-105 ng/mL]). The other sibling, on 150 mg oral 5-formylTHF per day, has a Hb count of 138 g/L (12.8 g/dL), a Hct of 39.5%, WBC count of 8.3 × 109/L (8.3/ mm3), platelet count of 189 × 109/L (189/mm3), serum Fe of 16.47 μM (92 μg/dL) (nl, 7.16-34.01 μM [40-190 μg/dL]), TIBC of 45.29 μM (253 μg/dL) (nl, 44.75-80.55 μM [250-400 μg/dL]), and ferritin of 218 pM (97 ng/mL). These patients, now at ages 6 and 9 years, have developed normally.
Cell lines and chemicals
HeLa cells were maintained in RPMI-1640 medium supplemented with 10% fetal bovine serum, 100 U/mL penicillin, and 100 μg/mL streptomycin. The natural 6S isomer of tritiated 5-methyltetrahydrofolate ([3H]5-methylTHF) was obtained from Moravek Biochemicals (Brea, CA); unlabeled (6S)5-methylTHF was purchased from Schircks Laboratories (Jona, Switzerland).
Identification of mutations in the PCFT gene
This study and the associated informed consent (obtained in accordance with the Declaration of Helsinki) were approved by Albert Einstein College of Medicine institutional review board (CCl no. 2006-279). Blood was obtained from patients with the clinical diagnosis of HFM (P1, P5, P6, and P7) and from their relatives (P5, P6, P7), and genomic DNA was extracted by the Gentra Systems purification kit (Minneapolis, MN). For 3 patients with HFM (P2, P3, and P4), genomic DNA was obtained from their skin fibroblasts. The primers and conditions for genomic polymerase chain reaction (PCR) were reported previously.5 PCFT DNA fragments were purified from agarose gel and sequenced on an ABI 3730 DNA analyzer (Applied Biosystems, Foster City, CA) in the Albert Einstein Cancer Center Genomics Facility. When required, the mutated regions were sequenced with both sense and antisense primers.
Site-directed mutagenesis and transient transfection
PCFT cDNA was cloned in pCDNA 3.1 (+), and mutations in the coding region were introduced by site-directed mutagenesis using PfuTurbo DNA polymerase (Stratagene, La Jolla, CA) as described previously.9 The entire PCFT coding region in the plasmid was sequenced to verify the presence of the mutation. Transient transfection was performed in HeLa cells using Lipofectamine 2000 Reagent (Invitrogen, Carlsbad, CA) according to the manufacturer's protocol. Transport studies and Northern and Western blot analyses were conducted 48 hours after transfection while immunofluorescence staining was performed 24 hours after transfection.
Transport of (6S)[3H]5-methylTHF
For 5-methylTHF uptake in transient transfectants, cells were washed twice with HBS [HEPES-buffered saline] (20 mM HEPES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 5 mM glucose [pH 7.4]) and incubated in this buffer at 37°C for 20 minutes. The buffer was then removed, and uptake was initiated by the addition of 0.5 ml of MBS or MES-buffered saline (MBS: 20 mM MES, 140 mM NaCl, 5 mM KCl, 2 mM MgCl2, 5 mM glucose [pH 5.5]) containing 0.5 μM [3H](6S)5-methylTHF. Uptake was terminated by injection of 5 ml ice-cold HBS, after which the vials were washed 3 times with this solution. Adherent cells were dissolved in 0.2 M NaOH (0.5 mL) by incubation at 65°C for 30 minutes; then, lysate (0.4 mL) was transferred to scintillation vials and radioactivity was determined; 10 μL was processed for protein determination (BCA; Pierce, Rockford, IL).
For 5-methylTHF uptake in Epstein-Barr virus (EBV)-transformed B-lymphoblastoid cells, lymphocytes were generated from blood (P6 and P7) at the Albert Einstein Human Genetics Cell Culture Core. The cells were harvested by centrifugation and washed once with HBS and twice with unbuffered saline (XBS; 160 mM NaCl, 5 mM KCl, 2 mM MgCl2, 5 mM glucose [pH 7.4]). The cells were resuspended into XBS at a high density. Uptake was initiated by addition of the cell suspension (50 μL) into 500 μL MBS (pH 5.5) that had been prewarmed at 37°C and which contained 0.5 μM [3H](6S)5-methylTHF. Uptake was stopped after 2 minutes by adding 5 mL ice-cold HBS; the cells were separated by centrifugation, washed 3 times with ice-cold HBS, and dissolved in 0.5 mL of 0.2 M NaOH for scintillation counting and protein determination as described in the previous paragraph. Some cells were exposed to [3H](6S)5-methylTHF for a few seconds before addition of ice-cold HBS. Tritium associated with these cells was considered bound to the cell surface; this was subtracted from total cell [3H](6S)5-methylTHF to determine uptake into the cells.
Northern blot analysis
Total RNA was extracted with TRIzol reagent (Invitrogen). RNA (20 μg) was resolved by electrophoresis on 1.0% formaldehyde-agarose gels. After transfer of RNA to Nytran N Nylon membranes (Whatman, Florham Park, NJ), the membrane was probed with a PCFT cDNA fragment (from 716 to 983 of NM 080669). The membrane was rehybridized with β-actin after stripping the PCFT probe.
Western blot analysis
Cells transiently transfected with PCFT and mock constructs were physically detached from the plates and resuspended in PBS. The cells were spun down and resuspended in a lysis buffer (0.1% SDS, 1% Triton X-100, 1 mM EDTA, 150 mM NaCl, 20 mM Tris [pH 7.4]) containing protease inhibitors (P2714; Sigma-Aldrich, St Louis, MO). Cells were sonicated on ice with 2- to 3-second bursts. Protein concentrations of total lysates were determined by the BCA Protein Assay (Pierce). After mixing the lysate with the sample loading buffer at room temperature, equal amounts of protein (20 μg) were separated by 12% SDS-PAGE, followed by blotting on a nitrocellulose membrane (Hybond-P; Amersham, Piscataway, NJ). The blot was probed with an antibody directed to the C-terminus of PCFT and processed as described previously.5
Detection of PCFT by immunofluorescence in cells
HeLa cells were grown on tissue-culture chamber slides. At 1 day after transient PCFT transfection, the cells were fixed with 4% paraformaldehyde for 30 minutes, followed by permeabilization with 0.2% Triton X-100 in PBS for 15 minutes. Cells were then blocked in PBS containing 2% BSA and 5% donkey serum for 30 minutes and probed with an anti-hPCFT antibody for 1 hour. Following a thorough wash with PBS, the slides were reprobed with FITC-conjugated swine anti-rabbit antibody (Dako, Carpinteria, CA) for 30 minutes. The slides were then washed and mounted on cover slips with mounting medium containing DAPI or PI (Vector Laboratories, Burlingame, CA) and examined for green fluorescence at the Albert Einstein Cancer Center Analytical Imaging Facility. The image was obtained on Olympus 1X70 inverted Epifluorescence Microscope (Olympus, Center Valley, PA) with a 60×/1.40 NA oil objective, recorded on a Sensicam QE cooled CCD Camera (Applied Scientific Instrumentation, Eugene, OR), acquired with software IP Lab 4.0 (BD Biosciences, Rockville, MD) and further processed with Photoshop CS (Adobe Systems, San Jose, CA).
Results
Identification of mutations in PCFT in patients with HFM
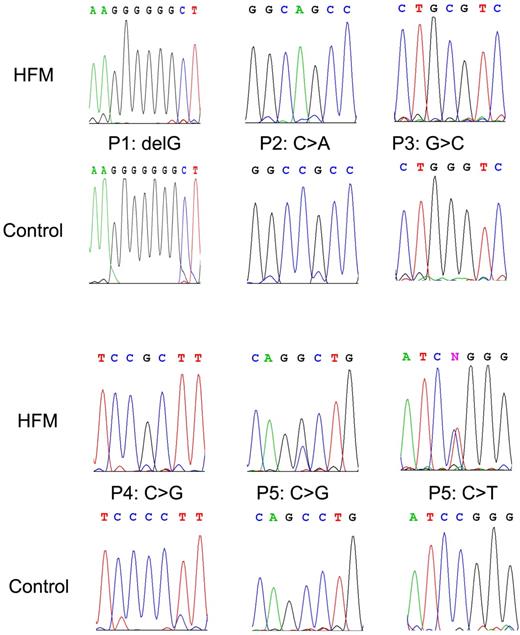
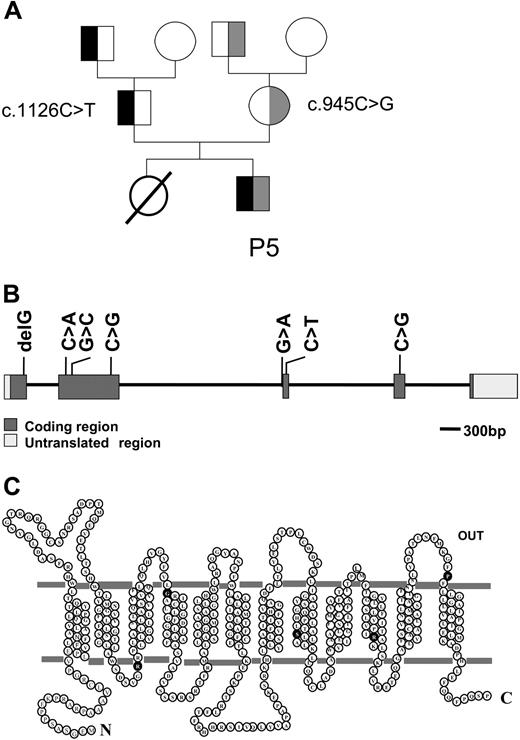
A single homozygous mutation was identified in patients P1, P2, P3, and P4, while 2 heterozygous mutations were identified in patient P5 (Figure 1). Except for a “G” deletion found in P1, all mutations were base substitutions. Whereas the deletion of a “G” resulted in a truncated protein that ends after 88 amino acids due to a frame shift at amino acid position 65, all base substitutions resulted in point mutations in the PCFT protein (Table 1). In patient P5, c.1126C > T was detected in the father and paternal grandfather, and c.954C > G was detected in the mother and maternal grandfather (Figure 2A). No PCFT mutations were found in the maternal and paternal grandmothers. Interestingly, arginine was involved in all point mutations at the protein level. Either arginine was replaced with serine (P2) or tryptophan (1 of the mutations from P5), or other amino acids were substituted with arginine (P3, P4, and the other mutation of P5; Table 1). All of the mutated residues are highly conserved across species. The locations of these mutations within the PCFT gene are indicated in Figure 2B. Amino acid residues affected by point mutations are depicted in the predicted topological structure of the transporter (Figure 2C). No other mutations/polymorphisms were detected in the coding region of PCFT in any of the patients studied.
Representative chromatograms of DNA sequence data. Top panels (HFM) represent the mutated DNA in patients with HFM, whereas the bottom panels (control) are corresponding wild-type sequences.
Representative chromatograms of DNA sequence data. Top panels (HFM) represent the mutated DNA in patients with HFM, whereas the bottom panels (control) are corresponding wild-type sequences.
PCFT mutations identified in patients with HFM and the resulting changes in protein composition*
| Patient . | Nucleotide change† . | Amino acid changes . |
|---|---|---|
| 1 | c.194delG‡ | p. G65AfsX25 |
| 2 | c.337C > A | p. R113S |
| 3 | c.439G > C | p. G147R |
| 4 | c.1274C > G | p. P425R |
| 5 | c.954C > G; c.1126C > T | p. S318R; p. R376W |
| 6, 7§ | c.1082-1G > A | p. Y362_G389del |
| Patient . | Nucleotide change† . | Amino acid changes . |
|---|---|---|
| 1 | c.194delG‡ | p. G65AfsX25 |
| 2 | c.337C > A | p. R113S |
| 3 | c.439G > C | p. G147R |
| 4 | c.1274C > G | p. P425R |
| 5 | c.954C > G; c.1126C > T | p. S318R; p. R376W |
| 6, 7§ | c.1082-1G > A | p. Y362_G389del |
The mutations are described according to the nomenclature derived by the Human Genome Variation Society (http://www.hgvs.org/mutnomen).
Genbank reference sequence, NM_080669. The cDNA is numbered from the initiation codon.
Since there is a span of seven Gs from position 188-194, the deleted G was arbitrarily assigned to the last G, or G194.
The PCFT mutation in this family was recently reported by this laboratory.5
Pedigree of a family with HFM; localization of PCFT mutations. (A) Pedigree of the family of patient P5. The black color indicates the c.1126C > T mutation, and the gray color indicates the c.954C > G mutation. (B) The genomic organization of the PCFT gene and the location of mutations detected in patients with HFM. (C) The location of amino acid substitutions (●) associated with mutations in PCFT gene in a topological structure predicted by online databases (ExPASy Proteomics Tools10 and Transport Classification Database11 ).
Pedigree of a family with HFM; localization of PCFT mutations. (A) Pedigree of the family of patient P5. The black color indicates the c.1126C > T mutation, and the gray color indicates the c.954C > G mutation. (B) The genomic organization of the PCFT gene and the location of mutations detected in patients with HFM. (C) The location of amino acid substitutions (●) associated with mutations in PCFT gene in a topological structure predicted by online databases (ExPASy Proteomics Tools10 and Transport Classification Database11 ).
Assessment of the transport function of PCFT mutants
Nucleotide changes corresponding to the mutant proteins were individually introduced into a PCFT expression vector by site-directed mutagenesis and then transiently transfected into HeLa cells. [3H](6S)5-methylTHF, the physiologic blood folate in humans, was used as the transport substrate. As indicated in Figure 3, while HeLa cells transfected with the wild-type transporter cDNA demonstrated high levels of 5-methylTHF uptake at pH 5.5, there was essentially no uptake detected in cells transfected with the cDNA of G65fsX25, R113S, R376W, and S318R. There was, however, low but statistically significant levels of residual transport activity (13% and 3.5% for the G147R and P425R mutants, respectively), compared with the wild-type transporter when the background activity of mock-transfected cells was subtracted.
[3H]5-methylTHF uptake in HeLa cells transiently transfected with the cDNA of PCFT mutants. Uptake of 0.5 μM [3H](6S)5-methylTHF was assessed at pH 5.5 and 37°C over 2 minutes; P values reflect differences in activities of the mutated carriers as compared with the mock transfectants. The data are the mean plus or minus SEM from 4 independent experiments.
[3H]5-methylTHF uptake in HeLa cells transiently transfected with the cDNA of PCFT mutants. Uptake of 0.5 μM [3H](6S)5-methylTHF was assessed at pH 5.5 and 37°C over 2 minutes; P values reflect differences in activities of the mutated carriers as compared with the mock transfectants. The data are the mean plus or minus SEM from 4 independent experiments.
Assessment of mRNA expression, protein expression, and membrane trafficking of PCFT mutants
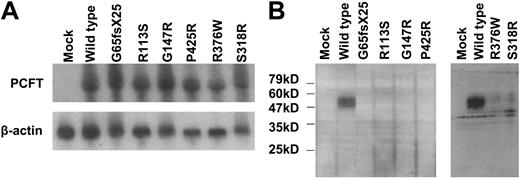
mRNA expression levels of mutated PCFT were analyzed by Northern blot analysis. As indicated in Figure 4A, the PCFT mRNA derived from the transient transfection was absent in the mock transfectant, and there was no significant difference between the level of wild-type PCFT mRNA and the level of any mutated PCFT mRNA detected. Expression of PCFT mutant proteins was analyzed by Western Blot. As indicated in Figure 4B, wild-type PCFT was readily detected in transient transfectants; however, none of the mutant proteins were seen. Hence, the mutant proteins were either not expressed in cells and/or were degraded during the sample processing steps due to poor stability. Of note is that the polyclonal antibody directed to human PCFT was not sufficiently sensitive to detect endogenous PCFT in HeLa cells as indicated in the “mock” lane. Even when the films were overexposed, protein could not be detected in the mock-transfected cells. However, low protein signals could be detected for R376W and S318R mutants, indicating that these transporters were expressed, but at a much lower level than the wild-type PCFT (data not shown).
Northern and Western blot analyses of PCFT mutants. (A) Northern blot analysis. Total RNA (20 μg) was fractionated on a formaldehyde-agarose gel, and the blot was successively probed with a PCFT fragment and β-actin. The images represent 1 of 2 independent experiments. (B) Western blot analysis. An equal amount of lysate (20 μg) was loaded on SDS gels, and the blots were probed with a peptide antibody directed to the C-terminus of PCFT. The numbers on the left indicate the molecular sizes of the protein ladder. The left and right panels represent 2 different analyzes each with mock and wild-type PCFT as controls. Each blot is representative of 3 separate experiments.
Northern and Western blot analyses of PCFT mutants. (A) Northern blot analysis. Total RNA (20 μg) was fractionated on a formaldehyde-agarose gel, and the blot was successively probed with a PCFT fragment and β-actin. The images represent 1 of 2 independent experiments. (B) Western blot analysis. An equal amount of lysate (20 μg) was loaded on SDS gels, and the blots were probed with a peptide antibody directed to the C-terminus of PCFT. The numbers on the left indicate the molecular sizes of the protein ladder. The left and right panels represent 2 different analyzes each with mock and wild-type PCFT as controls. Each blot is representative of 3 separate experiments.
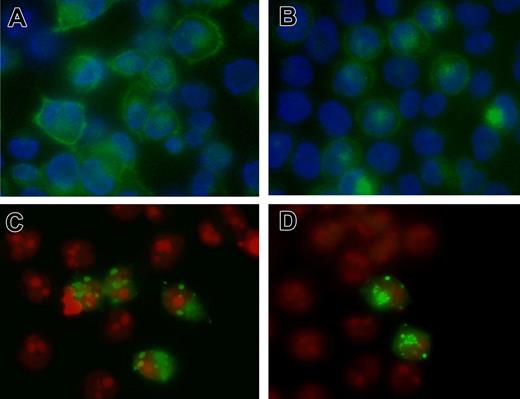
Membrane trafficking as well as protein expression was also examined by immunofluorescence staining. Again, there was no staining in the mock transfectants, indicating that the antibody did not detect endogenous PCFT in HeLa cells. However, when wild-type PCFT was overexpressed in HeLa cells, staining was detected primarily at the plasma membrane (Figure 5A). No staining was seen for the G65fsX25, R113S, and G147R mutants. Plasma membrane staining was seen for the P425 mutant (Figure 5B). Staining was also detected for the R376W (Figure 5C) and S318R (Figure 5D) mutants, but in a lower percentage of cells, and was primarily localized in intracellular vesicles.
Immunofluorescence staining of PCFT. HeLa cells were transiently transfected with cDNA of wild-type and PCFT mutants and labeled with a rabbit antibody targeted to human PCFT. (A) Wild-type PCFT. (B) P425R. (C) R376W. (D) S318R. Green fluorescence indicates localization of PCFT, while the blue fluorescence (panels A-B) or red fluorescence (panels C,D) shows nucleus counterstained by DAPI or PI, respectively. The image is a representative view from at least 3 experiments. The same antibody was used for immunohistochemical and Western blot (Figure 4B) analyses. The image was obtained on Olympus 1X70 inverted Epifluorescence Microscope (Olympus) with a 60×/1.40 oil objective, recorded on a Sensicam QE cooled CCD Camera (Applied Scientific Instrumentation), acquired with software IP Lab 4.0 (BD Biosciences) and further processed with Photoshop CS (Adobe Systems).
Immunofluorescence staining of PCFT. HeLa cells were transiently transfected with cDNA of wild-type and PCFT mutants and labeled with a rabbit antibody targeted to human PCFT. (A) Wild-type PCFT. (B) P425R. (C) R376W. (D) S318R. Green fluorescence indicates localization of PCFT, while the blue fluorescence (panels A-B) or red fluorescence (panels C,D) shows nucleus counterstained by DAPI or PI, respectively. The image is a representative view from at least 3 experiments. The same antibody was used for immunohistochemical and Western blot (Figure 4B) analyses. The image was obtained on Olympus 1X70 inverted Epifluorescence Microscope (Olympus) with a 60×/1.40 oil objective, recorded on a Sensicam QE cooled CCD Camera (Applied Scientific Instrumentation), acquired with software IP Lab 4.0 (BD Biosciences) and further processed with Photoshop CS (Adobe Systems).
It appeared that there was some discordance between function and protein expression level for 2 mutants. Complete loss of function was associated with the complete lack of protein expression for the mutants G65fsX25 and R113S and with defects in membrane trafficking, in addition to decreased protein expression, for mutants S318R and R376W. However, 13% of function was observed, but protein expression was not detected, for G147R, suggesting a small amount of transporter was expressed and active, but below the detection limit. On the other hand, P425 is expressed (Figure 5B), but manifested only 3.5% of function, consistent with a decrease in affinity of this mutant for folate and/or decreased mobility of this transporter and/or decreased accessibility of carrier to its folate substrates.
5-MethylTHF transport in lymphocytes derived from patients with HFM
Transformed lymphocytes were available for analysis of [3H](6S)5-methylTHF transport from the father, mother, and 2 affected daughters (P6 and P7) in our initial report.5 It can be seen that transport into the daughter's lymphocytes was one-fourth to one-fifth the rate of transport into the parent's lymphocytes at pH 5.5 (Figure 6). Since both parents carry 1 mutated nonfunctional allele, the transport rate in lymphocytes would be expected to be higher if both alleles were wild-type. Hence, the degree of loss of transport activity noted in the daughter's lymphocytes would be expected to be much greater when compared with transport in lymphocytes harboring 2 wild-type PCFT alleles.
[3H]5-methylTHF uptake at low pH in lymphocytes derived from patients P6 and P7 and their parents. Uptake of 0.5 μM [3H](6S)5-methylTHF at pH 5.5 was assessed over 2 minutes. F+/− and M+/− indicate cells derived from father and mother, respectively, who each carry 1 mutated PCFT allele. The data are the mean plus or minus SEM from 3 independent experiments.
[3H]5-methylTHF uptake at low pH in lymphocytes derived from patients P6 and P7 and their parents. Uptake of 0.5 μM [3H](6S)5-methylTHF at pH 5.5 was assessed over 2 minutes. F+/− and M+/− indicate cells derived from father and mother, respectively, who each carry 1 mutated PCFT allele. The data are the mean plus or minus SEM from 3 independent experiments.
Discussion
HFM was first described in 1961,12 and since then there have been 12 reported families with this disorder.1,4 The molecular basis for HFM in 1 family in which 2 children were affected (P6 and P7) was recently shown by this laboratory to be due to a mutation in a novel proton-coupled folate transporter, PCFT, a member of the superfamily of solute carriers (SLC46A1).5 In this family, the mutation occurred in an intron splice acceptor site, resulting in deletion of the third exon and a smaller transcript, a known splice-variant, that codes for a protein that is not functional. The current report extends the understanding of the genetic basis of HFM to 5 additional patients with this disease, from 5 different families, 3 of which represent cases not previously reported. There was no consistent racial, ethnic, or sex pattern in this group; families were of Latino, African-American, Turkish, Arabic, and European origin.
Defects in PCFT were identified in all patients with HFM examined, suggesting that this disease is due exclusively to a defect in this folate transporter. There did not appear to be any specific “hotspot” for mutations in the PCFT gene associated with HFM; mutations were found in 4 of the 5 exons. All the point mutations were at highly conserved residues and resulted in substitutions of amino acids with different charges. None of the mutations resulted in instability of mRNA, but all mutant transporters displayed decreased protein stability compared with the wild-type carrier, and 2 also exhibited defects in membrane trafficking. In 2 patients, there was some residual transport activity mediated by the mutant carrier, but there was insufficient information to relate this to the severity of the disease or the amount of folate required for treatment. In 2 sisters previously reported,1,5 a marked transport defect was demonstrated in their transformed lymphocytes at the low pH optimum of this carrier. This indicates that PFCT is expressed in immortalized lymphocytes and contributes to 5-methylTHF transport activity at low pH in these cells.
In addition to impaired intestinal folate absorption, patients with HFM have a defect in the transport of folates into the central nervous system (CNS). The cerebrospinal fluid (CSF)/blood folate ratio in healthy humans is 3:1. In HFM, CSF folate is very low or not detectable. Even when blood folate levels in patients with HFM are restored to, or above, the normal levels by folate supplementation, the CSF/blood folate ratio remains low.1 Transport of folates into the brain appears to be mediated, at least in part, by folate receptor-α,13 since autoantibodies to this receptor are associated with cerebral folate deficiency, a disorder in which blood folate levels are normal, but CSF folate is very low.14 The defect in transport of folates into the brain and CSF in HFM indicates that PCFT also plays a critical role in this process. This may be due to its requirement for folate receptor function. Hence, in the endocytic process mediated by folate receptor-α, folate bound to the receptor is internalized in vesicles that traffic, intact, within the cytoplasm, where the vesicles acidify and folate is released from the receptor. The mechanism of folate export from the vesicle has not been clarified, but may be mediated by PCFT driven by the transvesicular pH gradient as previously suggested by this5 and other laboratories.15,16 The dual function of proton-coupled intestinal absorption and lysosomal transport was observed for the divalent metal transport 1 (DMT1)17 and proposed for the amino acid transporter PAT1.18,19
The mechanism by which therapeutic doses of folates are absorbed in patients with mutated PCFT is uncertain. If the kinetic change in the mutated carrier is due to a decreased affinity for folates, and there is some retention of residual activity, as occurs with the G147R and P425R variants, high oral doses could achieve sufficient intestinal absorption to meet the requirements for this vitamin. Also, since reduced folate carrier (RFC) is expressed along the entire small intestine, with sufficient folate intake, some absorption may occur by this mechanism within the unfavorable acid environment of the upper small intestine. Alternatively, the delivery of high levels of folate to more distal alkaline areas of the small intestine could result in folate absorption via RFC, which operates most efficiently at physiologic pH. This notion is consistent with the observation that RFC expression in the intestine is up-regulated when mice are fed a folate-deficient diet.20,21 In this regard, the active isomers of 5-formyltetrahydrofolate (leucovorin) or 5-methylTHF (metafolin) would be the preferred forms of folate, since their affinity for RFC is more than 2 orders of magnitude greater than the affinity of folic acid for this transporter.22
PCFT was recently identified as a heme carrier protein (HCP1).7 However, the affinity of this transporter for hemin is at least 2 orders of magnitude lower than its affinity for folates and, while folate transport mediated by PCFT is both electrogenic and proton-dependent, uptake of hemin is not.7 Further, the anemia of HFM, along with the other signs and symptoms of this disorder, can be corrected by the administration of folate alone, rendering patients healthy, without any evidence of iron deficiency. Hence, it is unlikely that this transporter plays an important role in the intestinal absorption of iron or makes a significant contribution to iron homeostasis or the usage of iron by hematopoietic cells.
With the identification of the mechanism of intestinal folate absorption, it will be important to determine the prevalence of mutations or polymorphisms in PCFT that may account for variations in folate status among individuals. It does not appear that this gene is required for embryonic neural tube development, since patients with HFM have not been reported to have spina bifida. However, alterations in PCFT could contribute to abnormalities in the mother's folate status during pregnancy, especially when folate intake is low or marginal, so that defects in expression or function of this gene could, on this basis, be a contributing factor to neural tube defects in the developing embryo. In any event, the identification of mutations in PCFT as the basis for HFM will allow rapid diagnosis and treatment of this disease in infants, and prenatal diagnosis in families that carry a mutant gene.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Eugenia Tsai for her expert technical assistance.
This work was supported in part by the National Institutes of Health (grant no. CA-82621).
National Institutes of Health
Authorship
Contribution: R.Z. and I.D.G. contributed equally in the design and writing of this paper. S.H.M. performed the majority of laboratory studies to which A. S. and A.Q. contributed. C.S., J.J.M, and D.S.R. provided patient specimens. G.L.G. provided support for these studies.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: I. David Goldman, Departments of Medicine, and Molecular Pharmacology, Albert Einstein College of Medicine, 1300 Morris Park Ave, Bronx, NY, 10461; e-mail: igoldman@aecom.yu.edu.

![Figure 3. [3H]5-methylTHF uptake in HeLa cells transiently transfected with the cDNA of PCFT mutants. Uptake of 0.5 μM [3H](6S)5-methylTHF was assessed at pH 5.5 and 37°C over 2 minutes; P values reflect differences in activities of the mutated carriers as compared with the mock transfectants. The data are the mean plus or minus SEM from 4 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2007-02-077099/5/m_zh80150705390003.jpeg?Expires=1769088459&Signature=MdxUjA2SP~ege94iSzyG-sglyfrXe3BmKnydhqrONDIv8ZapqJu2C10nYETWAMfxVdphZGitb0d6P92~Pos8VktAouHh~px50mcMnBqjNV7oJ5ZC4Txj7a3xOtVzMulFBR-DgFCUW4G~hHPtB5pNWMs~rdgYoDCFfafdmAWuxr6QZUaZH7M-0io-haEsvnZmztEq7M0arT8rY8nqBOUPnnmf~gO~vexTUXkstNvrVgtErTGOkSFkuIjkq52YY4AjSdbMay1cc~Rsj~Wi5Db5FuBkQB4RmohNKmfnYzytUhtwHAceu9JmQDtHQldnN6yLuSsfjkW8f1w0Gk5IqtSiDA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 6. [3H]5-methylTHF uptake at low pH in lymphocytes derived from patients P6 and P7 and their parents. Uptake of 0.5 μM [3H](6S)5-methylTHF at pH 5.5 was assessed over 2 minutes. F+/− and M+/− indicate cells derived from father and mother, respectively, who each carry 1 mutated PCFT allele. The data are the mean plus or minus SEM from 3 independent experiments.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2007-02-077099/5/m_zh80150705390006.jpeg?Expires=1769088459&Signature=TgDpgXvNA8he2H0mYgiwCA-E4MjluDXZ6jZe4pMCmIw8ALHXUxoCRkrFuvdASEICJW6eqgbA8gAHcEfm~CiKgmoHU9x9ZGNAGtnBLClXMuwO5yI-VJwhpwmMq5vVRbDDgLlPjzgVAWU9cRiXLvjt29gnee-jXP--Y9nCl~D59GrvkvI3WKzEJizLMLMoRbsxkppt7sz-JEt0AOStkCwnxcgg5cysNRWHKpKdVKjOUXwFmxBiCN2EAXFQLOVa0OXUzppxmbfIilX83DcaIw144wL2DeGk7ytPUbp6SzBUqE7-1s7SSLw1MxV-nG-Db2kpasOpbAvH-8bv1Oxx4SEVqQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)