Abstract
Leukemia cell motility and transendothelial migration into extramedullary sites are regulated by angiogenic factors and are considered unfavorable prognostic factors in acute leukemias. We have studied cross talk among (1) the vascular endothelial growth factor receptor-1, FLT-1; (2) the human eag-related gene 1 (hERG1) K+ channels; and (3) integrin receptors in acute myeloid leukemia (AML) cells. FLT-1, hERG1, and the β1 integrin were found to form a macromolecular signaling complex. The latter mostly recruited the hERG1B isoform of hERG1 channels, and its assembly was necessary for FLT-1 signaling activation and AML cell migration. Both effects were inhibited when hERG1 channels were specifically blocked. A FLT-1/hERG1/β1 complex was also observed in primary AML blasts, obtained from a population of human patients. The co-expression of FLT-1 and hERG1 conferred a pro-migratory phenotype to AML blasts. Such a phenotype was also observed in vivo. The hERG1-positive blasts were more efficient in invading the peripheral circulation and the extramedullary sites after engraftment into immunodeficient mice. Moreover, hERG1 expression in leukemia patients correlated with a higher probability of relapse and shorter survival periods. We conclude that in AML, hERG1 channels mediate the FLT-1–dependent cell migration and invasion, and hence confer a greater malignancy.
Introduction
The persistence of leukemia cells outside the bone marrow (BM) microenvironment and, in particular, their migration from the peripheral blood (PB) into extramedullary organs are considered unfavorable prognostic factors in acute leukemias.1–3 Leukemia cell migration into extramedullary sites may also reduce responsiveness to induction chemotherapy.1–3 Therefore, there is considerable interest in deciphering the molecular mechanisms that regulate leukemia cell motility and transendothelial migration, and, consequently, determining how leukemia cells exit from the BM and infiltrate extramedullary organs. A crucial role is thought to be played by angiogenic factors and angiogenesis-related signals, especially those centered on the vascular endothelial growth factor (VEGF) and its receptors (VEGFRs).4–6 Some lines of evidence suggest that VEGF/VEGFRs binding exerts an autocrine regulatory effect on the leukemic cell population, in addition to the paracrine effect of VEGF on the endothelium.6 Among VEGFRs, both VEGFR-1 (FLT-1) and VEGFR-2 (KDR) are tyrosine kinase receptors. KDR is expressed in endothelial cells. It transduces angiogenic signals5,6 and enhances acute myeloid leukemia (AML) cell survival.7,8 Little is known about the role of FLT-1 in acute leukemia progression. Recent studies suggest that it induces proliferation of AML cell lines and regulates the localization of immature malignant precursors within the BM in myelodysplastic syndromes.9 Studies of multiple myeloma reinforce the idea that FLT-1 regulates the migration of malignant hemopoietic cells.6,10 Fragoso et al11 have recently demonstrated that FLT-1 activation stimulates cell migration of acute lymphoblastic leukemia cells. In addition, FLT-1 neutralization in vivo with specific antibodies impairs leukemia cell exit from the BM and prolongs survival of injected mice.11
A peculiar aspect of VEGFR-mediated signaling is epitomized by their interaction with the integrin family of adhesion receptors.12–14 The αvβ3 integrin associates with KDR in endothelial cells,13 whereas the β1 subunit forms a functional complex with VEGFR-3 in chondrocytes.15 Moreover, soluble FLT-1 secreted by endothelial cells binds to α5β1 integrin.16 Finally, a membrane complex containing PKC, β1 integrin, and FLT-1 has been shown to assemble in multiple myeloma cells.17 Increasing evidence shows that the formation of protein complexes within the plasma membrane exerts a synergic effect on intracellular signaling.18 These complexes often include ion channels and adhesion receptors, and especially K+ channels and integrins.19 In particular, we have recently shown that one such complex is formed in a variety of mammalian cell lines (including the preosteoblastic leukemic cell line FLG.29.1) by β1 integrin and the voltage-gated human eag-related gene 1 (hERG1) K+ channel. In this case, the ion channel activity modulates the downstream signaling elicited by integrin activation.20,21 hERG1 is typically expressed in excitable cells, such as neurons and cardiac myocytes, where it regulates resting potential and action potential repolarization.22 However, hERG1 is also aberrantly expressed in a variety of neoplastic cell types,23,24 where it plays a part in cell proliferation, invasion, and VEGF secretion.25–29
For the purposes of the present article, it is important to bear in mind that hERG1 is found in a broad range of human leukemic cell lines and in primary human AML,25 as well as in chronic lymphocytic leukemias.26 Leukemia cells express a truncated form of hERG1 that, although lacking most of the N-terminus, produces functional hERG currents. This isoform is referred to as hERG1B to distinguish it from the full-length hERG1A.27 hERG1 activity is necessary for leukemia cells to progress beyond the G1/S boundary.25 Consistent with these results, the clonogenic potential of AML circulating blasts is reduced by hERG1-specific blockers.25
We sought to determine whether FLT-1, hERG1 channels, and integrins are associated in AML cells, and how this may regulate leukemia cell invasiveness. In addition, by inoculating AML blasts into nonobese diabetic-severe combined immunodeficient (NOD-SCID) mice, we found that hERG1 channel activity regulates leukemia blast mobilization from BM spaces into extramedullary sites in vivo. Suggested clinical implications of this observation are also reported.
Patients, materials, and methods
Cell culture and stimulation
The human cell lines FLG 29.1, K562, NB4, KG1, HL60, and TF1 were maintained in RPMI 1640 medium supplemented with 10% fetal bovine serum (complete medium; Hyclone, Milan, Italy) at 37°C in 5% CO2. For signaling experiments, cells were deprived of serum for 12 to 14 hours, in RPMI medium, before stimulation with either VEGF165 (100 ng/mL), placental growth factor (PlGF; 50 ng/mL), or TS2/16 antibody (20 μg/mL), or diluted in RPMI medium and applied for 30 minutes at 37°C.
Treatment with K+ channel inhibitors
We tested the following K+ channel inhibitors: (1) Way 123 398 (Way) and E4031 (these compounds specifically block hERG1 channels when applied to cells maintained in standard extracellular saline solutions, at concentrations of approximately 1 μM22,28 ); (2) tetra-ethyl-ammonium, a wide-range inhibitor of voltage-dependent K+ channels that does not affect hERG1 at the concentration used (5 mM); and (3) charybdotoxin (C. toxin), an inhibitor of intermediate-conductance Ca2+-activated K+ channels and voltage-dependent Kv 1.3 channels. Way was a kind gift from Dr W. Spinelli (Wyeth-Ayerst Research, Princeton, NJ); E4031 and tetra-ethyl-ammonium were from Sigma (St. Louis, MO); C. toxin was from Alomone Labs (Jerusalem, Israel). All inhibitors were dissolved in phosphate-buffered saline, except C. toxin, which was dissolved in ethanol. Cells were preincubated with the various K+ channels inhibitors, in RPMI medium, for 15 minutes at 37°C, and then stimulated as needed.
Migration assay
Cell migration was measured by a Boyden chamber assay. The 2 compartments of the chamber (NeuroProbe, Gaithersburg, MD) were separated by a porous polycarbonate membrane (pore diameter 8 μm), previously coated with fibronectin (FN) (50 μg/mL) or bovine serum albumin (BSA; 250 μg/mL). Starved cells (106/mL) were added to the upper well, in the presence or absence of inhibitors, whereas VEGF165 (100 ng/mL) or PlGF (50 ng/mL) were added to the lower well. After 18 hours of incubation, migrated cells were collected from the lower compartment, spun down, and counted using a hemocytometer. Only live cells, as determined by Trypan blue exclusion, were considered in the quantification. Experiments were performed in triplicate, and results are shown as the number of migrating cells/mL.
Silencing of Akt by small interfering RNAs
FLG 29.1 cells were transiently transfected with purified duplex small interfering (si) RNAs for Akt and with a scrambled control purchased from Qiagen (Valencia, CA), using the Transmessenger kit (Qiagen). After 54 hours, cells were transferred into a Boyden chamber (for cell migration assay) or into 96-well plates for viability (WST) assay, and incubated for further 18 hours. A parallel sample (treated with siRNAs for a total of 72 hours) was processed for the evaluation of Akt expression.
Cell viability assay
To assess cell viability and proliferation, the cell viability assay (WST; Roche Diagnostics, Mennheim, Germany) was used. Cells were seeded at 105/well in 96-well plates, and incubated for 18 hours. At the end of incubation, the WST reagent was added, and absorbance was measured at 450 nm.
VEGF secretion
AML cells were seeded into 24-well cell clusters at 2 × 105 cells/mL in serum-free medium. After a 48-hour incubation, the medium was collected and used for VEGF measurement using the DuoSet ELISA Development System (R&D Systems, Wiesbaden, Germany). Cells were subsequently recovered and counted to normalize VEGF secretion data.
Immunocytochemistry
Immunocytochemistry (ICC) was performed as described.25 Images were acquired on a Leica DM 4000B microscope with a Leica DFC 320 photocamera (Leica Microsystems, Milan, Italy) (PL Fluotar 40×/0.70, PL Fluotar 100×/1.30 OIL objective).
Preparation of AML blasts and normal CD34+ cells
AML blasts and CD34+ cells were prepared from PB samples and stimulated as described.25
Molecular biologic methods
RNA extraction and RT-PCR.
Total RNA was extracted, and reverse-transcription-polymerase chain reaction (RT-PCR) for herg1 and gapdh was performed.25 For flt1 and kdr amplification, primers and conditions were as in Dias et al.7 In some samples only the amplification of herg1 and gapdh was performed, due to the amount of RNA extracted from the primary sample.
RT Quantitative PCR.
Herg1a and herg1b mRNA were quantified by RT quantitative PCR (RQ-PCR), with the ABI PRISM 7700 Sequence Detection System and the SYBR Green Master Mix Kit (Applied Biosystems, Foster City, CA). The β-glucuronidase (GUS) gene was used as standard reference. The relative expression of herg1a and herg1b was calculated by using a comparative threshold cycle method. The primer sequences were as follows: herg1a sense, 5′-GTGGAAATCGCCTTCTACCG-3′; antisense, 5′-GCCCCATCCTCGTTCTTCA-3′; and herg1b sense, 5′-GCGCATCTCCAGCCTCGTG-3′; antisense, 5′-ACGTCGGCGCCCAGGGACA-3′.
Patients
PB samples were obtained from 61 patients diagnosed with AML. They represent a group of consecutive patients with high PB blast counts, treated either at the Hematology Unit in Florence or at the Department of Internal Medicine in Turin. In each case, diagnosis was based on morphologic, immunophenotypic, and molecular analysis. All samples were obtained with signed informed consent, in accordance with the Declaration of Helsinki, by using a protocol approved by the Comitato Etico Azienda Ospedaliera Universitaria di Careggi, Firenze, Italy. Patient details are summarized in Table 1. Patients were treated by standard induction chemotherapy with daunorubicin-aracytine. Daunorubicin was administered at 60 mg/m2 on days 1, 2, and 3, in association with aracytine as a continuous infusion for 7 days at 100 or 200 mg/m2, for patients older than or younger than 60 years of age, respectively. Complete remission and relapse were evaluated by standard hematologic parameters.
Characteristics of AML patients at diagnosis
| Reference number . | Age . | Sex . | FAB . | WBC count . | % Blast . | herg1 . | flt1 . | kdr . |
|---|---|---|---|---|---|---|---|---|
| A62 | ND | F | M0 | 70 000 | 70 | − | − | − |
| A65 | ND | M | M0 | 180 000 | 85 | + | + | + |
| A17 | 68 | M | M1 | 78 780 | 86 | + | + | − |
| A21 | 74 | M | M1 | 32 000 | 76 | + | + | − |
| A26 | 64 | F | M1 | 100 000 | 96 | + | + | − |
| A38 | ND | M | M1 | 125 000 | 90 | + | + | − |
| A44 | 70 | F | M1 | 32 000 | 98 | + | ND | ND |
| A47 | ND | M | M1 | 12 100 | 90 | − | ND | ND |
| A50 | ND | F | M1 | 232 000 | 96 | + | + | + |
| A54 | ND | F | M1 | 200 000 | 100 | − | + | + |
| A61 | ND | F | M1 | 200 000 | 90 | + | + | + |
| A2 | 56 | M | M2 | ND | 64 | + | + | − |
| A6 | 63 | M | M2 | 22 690 | 96 | + | + | + |
| A9 | 70 | M | M2 | 101 080 | 100 | + | + | − |
| A10 | 28 | M | M2 | ND | ND | − | + | − |
| A15 | 63 | M | M2 | 14 250 | 32 | + | + | − |
| A16 | 31 | M | M2 | 116 000 | 64 | − | − | + |
| A19 | 55 | M | M2 | 18 560 | 80 | + | + | − |
| A25 | 65 | M | M2 | ND | ND | + | + | − |
| A27 | 31 | M | M2 | 16 000 | 62 | + | ND | ND |
| A33 | 58 | M | M2 | ND | ND | + | − | − |
| A34 | 42 | M | M2 | ND | ND | + | + | − |
| A36 | 74 | M | M2 | ND | ND | − | − | + |
| A46 | 57 | F | M2 | 150 000 | 85 | + | − | ND |
| A53 | ND | M | M2 | 30 000 | 70 | + | + | ND |
| A55 | ND | F | M2 | 170 000 | 99 | + | − | |
| A57 | ND | F | M2 | 87 000 | 74 | + | ND | ND |
| A60 | ND | M | M2 | 78 300 | 84 | − | + | + |
| A63 | ND | M | M2 | 70 000 | 80 | + | + | + |
| A11 | 46 | M | M3 | 10 490 | 92 | − | + | − |
| A14 | 23 | M | M3 | 80 190 | 96 | − | − | − |
| A29 | 58 | M | M3 | 13 000 | 64 | + | + | − |
| A1 | 72 | M | M4 | 19 200 | 22 | − | + | − |
| A4 | 73 | M | M4 | 14 200 | ND | + | − | − |
| A12 | 79 | M | M4 | 19 860 | 45 | + | − | − |
| A18 | 61 | F | M4 | 94 160 | 90 | − | − | − |
| A20 | 68 | M | M4 | 60 260 | 82 | − | − | − |
| A23 | 48 | M | M4 | 63 000 | 20 | + | − | − |
| A24 | 53 | M | M4 | 80 000 | 100 | − | ND | ND |
| A31 | 49 | M | M4 | 69 000 | 40 | + | + | − |
| A32 | 70 | M | M4 | 22 000 | 97 | + | + | − |
| A37 | ND | F | M4 | 150 000 | 80 | + | − | − |
| A41 | ND | M | M4 | 73 000 | 96 | − | ND | ND |
| A42 | ND | M | M4 | 27 800 | 90 | + | + | − |
| A45 | 54 | M | M4 | 107 000 | 50 | + | + | − |
| A48 | 19 | F | M4 | 135 000 | 98 | + | + | + |
| A49 | ND | M | M4 | 52 000 | 70 | + | + | + |
| A59 | ND | F | M4 | 300 000 | 98 | + | + | − |
| A3 | 51 | F | M5 | 21 460 | 74 | − | ND | ND |
| A13 | 56 | M | M5 | 64 800 | 58 | − | + | − |
| A35 | ND | M | M5 | ND | ND | − | − | ND |
| A52 | ND | M | M5 | 60 000 | 84 | − | + | − |
| A56 | ND | F | M5 | 90 000 | 80 | + | + | + |
| A58 | ND | F | M5 | 210 000 | 70 | + | + | − |
| A39 | ND | F | M6 | ND | ND | + | + | − |
| A40 | ND | M | M6 | 20 000 | 85 | + | + | − |
| A64 | ND | M | M6 | 170 000 | 34 | + | + | + |
| A22 | 53 | F | M7 | 14 000 | ND | − | ND | ND |
| A51 | ND | F | M7 | 65 000 | 80 | + | + | ND |
| A7 | 71 | M | ND | 36 590 | 58 | + | + | + |
| A8 | 77 | M | ND | 306 000 | 98 | + | − | + |
| Reference number . | Age . | Sex . | FAB . | WBC count . | % Blast . | herg1 . | flt1 . | kdr . |
|---|---|---|---|---|---|---|---|---|
| A62 | ND | F | M0 | 70 000 | 70 | − | − | − |
| A65 | ND | M | M0 | 180 000 | 85 | + | + | + |
| A17 | 68 | M | M1 | 78 780 | 86 | + | + | − |
| A21 | 74 | M | M1 | 32 000 | 76 | + | + | − |
| A26 | 64 | F | M1 | 100 000 | 96 | + | + | − |
| A38 | ND | M | M1 | 125 000 | 90 | + | + | − |
| A44 | 70 | F | M1 | 32 000 | 98 | + | ND | ND |
| A47 | ND | M | M1 | 12 100 | 90 | − | ND | ND |
| A50 | ND | F | M1 | 232 000 | 96 | + | + | + |
| A54 | ND | F | M1 | 200 000 | 100 | − | + | + |
| A61 | ND | F | M1 | 200 000 | 90 | + | + | + |
| A2 | 56 | M | M2 | ND | 64 | + | + | − |
| A6 | 63 | M | M2 | 22 690 | 96 | + | + | + |
| A9 | 70 | M | M2 | 101 080 | 100 | + | + | − |
| A10 | 28 | M | M2 | ND | ND | − | + | − |
| A15 | 63 | M | M2 | 14 250 | 32 | + | + | − |
| A16 | 31 | M | M2 | 116 000 | 64 | − | − | + |
| A19 | 55 | M | M2 | 18 560 | 80 | + | + | − |
| A25 | 65 | M | M2 | ND | ND | + | + | − |
| A27 | 31 | M | M2 | 16 000 | 62 | + | ND | ND |
| A33 | 58 | M | M2 | ND | ND | + | − | − |
| A34 | 42 | M | M2 | ND | ND | + | + | − |
| A36 | 74 | M | M2 | ND | ND | − | − | + |
| A46 | 57 | F | M2 | 150 000 | 85 | + | − | ND |
| A53 | ND | M | M2 | 30 000 | 70 | + | + | ND |
| A55 | ND | F | M2 | 170 000 | 99 | + | − | |
| A57 | ND | F | M2 | 87 000 | 74 | + | ND | ND |
| A60 | ND | M | M2 | 78 300 | 84 | − | + | + |
| A63 | ND | M | M2 | 70 000 | 80 | + | + | + |
| A11 | 46 | M | M3 | 10 490 | 92 | − | + | − |
| A14 | 23 | M | M3 | 80 190 | 96 | − | − | − |
| A29 | 58 | M | M3 | 13 000 | 64 | + | + | − |
| A1 | 72 | M | M4 | 19 200 | 22 | − | + | − |
| A4 | 73 | M | M4 | 14 200 | ND | + | − | − |
| A12 | 79 | M | M4 | 19 860 | 45 | + | − | − |
| A18 | 61 | F | M4 | 94 160 | 90 | − | − | − |
| A20 | 68 | M | M4 | 60 260 | 82 | − | − | − |
| A23 | 48 | M | M4 | 63 000 | 20 | + | − | − |
| A24 | 53 | M | M4 | 80 000 | 100 | − | ND | ND |
| A31 | 49 | M | M4 | 69 000 | 40 | + | + | − |
| A32 | 70 | M | M4 | 22 000 | 97 | + | + | − |
| A37 | ND | F | M4 | 150 000 | 80 | + | − | − |
| A41 | ND | M | M4 | 73 000 | 96 | − | ND | ND |
| A42 | ND | M | M4 | 27 800 | 90 | + | + | − |
| A45 | 54 | M | M4 | 107 000 | 50 | + | + | − |
| A48 | 19 | F | M4 | 135 000 | 98 | + | + | + |
| A49 | ND | M | M4 | 52 000 | 70 | + | + | + |
| A59 | ND | F | M4 | 300 000 | 98 | + | + | − |
| A3 | 51 | F | M5 | 21 460 | 74 | − | ND | ND |
| A13 | 56 | M | M5 | 64 800 | 58 | − | + | − |
| A35 | ND | M | M5 | ND | ND | − | − | ND |
| A52 | ND | M | M5 | 60 000 | 84 | − | + | − |
| A56 | ND | F | M5 | 90 000 | 80 | + | + | + |
| A58 | ND | F | M5 | 210 000 | 70 | + | + | − |
| A39 | ND | F | M6 | ND | ND | + | + | − |
| A40 | ND | M | M6 | 20 000 | 85 | + | + | − |
| A64 | ND | M | M6 | 170 000 | 34 | + | + | + |
| A22 | 53 | F | M7 | 14 000 | ND | − | ND | ND |
| A51 | ND | F | M7 | 65 000 | 80 | + | + | ND |
| A7 | 71 | M | ND | 36 590 | 58 | + | + | + |
| A8 | 77 | M | ND | 306 000 | 98 | + | − | + |
Diagnosis was based on morphological, immunophenotypic, and molecular analysis. herg1, flt1, and kdr expression was evaluated by RT-PCR.
ND indicates not determined; WBC, white blood cell.
Additional information is provided in Document S1 (available on the Blood website; see the Supplemental Materials link at the top of the online article).
Results
AML cells express hERG1 and FLT-1
As cellular models, we have used several human AML cell lines of different French-American-British (FAB) phenotypes (Figure 1) and K562 cells (ie, cells derived from the acute phase of a chronic myeloid leukemia). First, we tested the expression of herg1 and assayed hERG1 protein by RT-PCR and Western blot (WB), respectively. All the AML cell lines we examined expressed herg1 (Figure 1A) and contained the corresponding hERG1 protein (Figure 1B). In the WB lanes (Figure 1B), the bands of different molecular weights represent differently glycosylated forms of the hERG1A and hERG1B proteins. In particular, the hERG1A and hERG1B bands with the higher molecular weight represent proteins with the highest level of glycosylation, which is typical of the forms expressed on the plasma membrane.21,27 We conclude that hERG1A and hERG1B are both expressed in AML cell lines, albeit with a prevalence of the latter, and that both isoforms are properly expressed onto the plasma membrane.
hERG1 and VEGF receptors expression and activity in human acute myeloid leukemia cell lines. (A) RT-PCR analysis of herg1 (top panel, 575-bp band), flt1 (second panel, 550-bp band), kdr (third panel, 660-bp band) and gapdh (bottom panel, 138-bp band) transcripts in AML cell lines. Lane ST, molecular weight standard (100 bp; New England Biolabs); lane C+, SH-SY5Y cell line for herg1, HUVEC cell line for flt1 and kdr. (B) hERG1 protein(s) expression on AML cell lines. Cell lysates from AML cell lines, cultured in the presence of serum, were blotted and probed with the anti-pan hERG1 antibody. The top bands, weighing 135 to 150 kDa, refer to the hERG1A isoform; the bottom bands, weighing 75 to 100 kDa, refer to the hERG1B isoform. Reprobing of the membrane with antitubulin antibody is reported in the bottom panel. (C) FLT-1 protein expression in AML cell lines. Cell lysates from cells grown in the presence of serum were blotted and probed with the anti FLT-1 antibody. The arrow indicates the 180 kDa band corresponding to FLT-1. Reprobing of the membrane with antitubulin antibody is reported in the bottom panel. (D) Effect of VEGF165 addition on FLT-1 tyrosine phosphorylation. Proteins extracted from AML cell lines, treated with BSA (250μg/mL) or VEGF165 (100 ng/mL) for 30 minutes, were immunoprecipitated using anti FLT-1 antibody and the blot revealed using anti p-Tyr antibody. Reprobing the membrane with anti FLT-1 antibody is reported in the bottom panel. Preliminary experiments showed that VEGF165 triggered FLT-1 p-Tyr within 15 minutes, reaching a maximum after 30 minutes[b]. IP indicates immunoprecipitation.
hERG1 and VEGF receptors expression and activity in human acute myeloid leukemia cell lines. (A) RT-PCR analysis of herg1 (top panel, 575-bp band), flt1 (second panel, 550-bp band), kdr (third panel, 660-bp band) and gapdh (bottom panel, 138-bp band) transcripts in AML cell lines. Lane ST, molecular weight standard (100 bp; New England Biolabs); lane C+, SH-SY5Y cell line for herg1, HUVEC cell line for flt1 and kdr. (B) hERG1 protein(s) expression on AML cell lines. Cell lysates from AML cell lines, cultured in the presence of serum, were blotted and probed with the anti-pan hERG1 antibody. The top bands, weighing 135 to 150 kDa, refer to the hERG1A isoform; the bottom bands, weighing 75 to 100 kDa, refer to the hERG1B isoform. Reprobing of the membrane with antitubulin antibody is reported in the bottom panel. (C) FLT-1 protein expression in AML cell lines. Cell lysates from cells grown in the presence of serum were blotted and probed with the anti FLT-1 antibody. The arrow indicates the 180 kDa band corresponding to FLT-1. Reprobing of the membrane with antitubulin antibody is reported in the bottom panel. (D) Effect of VEGF165 addition on FLT-1 tyrosine phosphorylation. Proteins extracted from AML cell lines, treated with BSA (250μg/mL) or VEGF165 (100 ng/mL) for 30 minutes, were immunoprecipitated using anti FLT-1 antibody and the blot revealed using anti p-Tyr antibody. Reprobing the membrane with anti FLT-1 antibody is reported in the bottom panel. Preliminary experiments showed that VEGF165 triggered FLT-1 p-Tyr within 15 minutes, reaching a maximum after 30 minutes[b]. IP indicates immunoprecipitation.
We subsequently investigated the expression of transcripts for VEGFRs. All of our cell lines expressed flt1, but not kdr (Figure 1A). The proper translation of flt1 was confirmed by WB (Figure 1C, arrow). To verify that the FLT-1 receptor was functional, we assessed the level of tyrosine phosphorylation (p-Tyr) after VEGF165 treatment. Serum-starved cells were incubated with VEGF165 for 30 minutes, and FLT-1 was subsequently immunoprecipitated and probed with anti p-Tyr antibody. It turned out that VEGF165 always triggered the p-Tyr of FLT-1 (Figure 1D). A variable level of p-Tyr on the FLT-1 receptor was also observed in the absence of VEGF (see below), a finding that can be attributed to autocrine VEGF production by leukemia cells (see below). Nevertheless, p-Tyr was significantly increased by VEGF165 to levels similar to those previously reported in myeloma and chronic lymphocytic leukemia cells.32,33 These data are summarized in Table 2.
Summary of hERG1 and VEGF receptor expression in AML cell lines of different FAB phenotypes
| Cell line . | FAB . | hERG1 . | FLT-1 . | KDR . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCR . | WB . | |||||||||
| 1A . | 1B . | PCR . | WB . | p-Tyr level . | PCR . | WB . | p-Tyr . | |||
| FLG 29.1 | M5 | + | + | +++ | + | + | 3.6 | − | − | n.d. |
| K562 | acute phase of CML | + | + | +++ | + | ++ | 2.2 | − | − | n.d. |
| NB4 | M3 | + | ++ | +++ | + | + | 2.9 | − | − | n.d. |
| KG1 | M1 | + | ++ | ++ | + | + | 2.4 | − | − | n.d. |
| TF1 | M6 | + | + | ++ | + | + | 1.9 | − | − | n.d. |
| Cell line . | FAB . | hERG1 . | FLT-1 . | KDR . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| PCR . | WB . | |||||||||
| 1A . | 1B . | PCR . | WB . | p-Tyr level . | PCR . | WB . | p-Tyr . | |||
| FLG 29.1 | M5 | + | + | +++ | + | + | 3.6 | − | − | n.d. |
| K562 | acute phase of CML | + | + | +++ | + | ++ | 2.2 | − | − | n.d. |
| NB4 | M3 | + | ++ | +++ | + | + | 2.9 | − | − | n.d. |
| KG1 | M1 | + | ++ | ++ | + | + | 2.4 | − | − | n.d. |
| TF1 | M6 | + | + | ++ | + | + | 1.9 | − | − | n.d. |
PCR results are expressed as −, not expressed; +, expressed. WB results are expressed as −, not expressed; +, low level of expression; ++, medium level of expression; +++, high level of expression; n.d., not determined. A densitometric analysis of FLT-1 tyrosine phosphorylation in AML cell lines stimulated with BSA or VEGF was performed as described in ′Patients, materials, and methods′; data are reported in the ′FLT-1: p-Tyr level′ lane and are expressed as n-fold increase in VEGF-treated cells compared with BSA-treated cells.
Formation of a FLT-1/hERG1/β1 complex in AML cell lines
To test whether FLT-1 associated with hERG1, we performed co-immunoprecipitation experiments on lysates from AML cell lines cultured in the presence of serum. After immunoprecipitating FLT-1, WB tests with an anti pan-hERG1 antibody always revealed co-immunoprecipitation with the ion channel, although the intensity of the effect was different in the different cell lines (Figure 2A). The molecular weight of the hERG1 bands observed after WB suggests that the complex with FLT-1 was predominantly formed by the hERG1B isoform. This was confirmed by treating the blot with a specific anti hERG1B antibody. Subsequent application of an anti β1 integrin antibody revealed that β1 also co-immunoprecipitates with FLT-1 and hERG1B. We concluded that FLT-1, hERG1B, and β1 integrin co-immunoprecipitate in serum-cultured leukemia cells, thereby suggesting the formation of a macromolecular FLT-1/hERG1/β1 membrane complex.
Physical association between FLT-1 receptor, hERG1, and β1 integrin subunit in AML cell lines. (A) Co-immunoprecipitation of FLT-1 and hERG1B in AML cell lines. Cell lysates from AML cell lines cultured in the presence of serum were immunoprecipitated with anti FLT-1 antibody; blots were probed with anti-pan hERG1 antibody (top), anti-hERG1B antibody (upper middle), anti-β1 antibody (lower middle), and anti-FLT-1 antibody (bottom). The pan hERG1 antibody recognizes both hERG1A and hERG1B isoforms.27 Note that with the anti-hERG1B antibody only the higher molecular weight bands are visible, whereas the lower bands can be detected only after long exposure of the membrane (as reported in Lee et al33 ). Bands relative to the hERG1B protein are indicated by the arrows in the top and middle panels. (B) Co-immunoprecipitation of FLT-1, hERG1B, and β1 integrin after addition of VEGF, PlGF, and β1 activating antibody in FLG 29.1 cells. Cells were treated for 30 minutes with BSA (250μg/mL), VEGF165 (100 ng/mL), PlGF (50 ng/mL), the β1 activating antibody TS2/16 (20 μg/mL) or both VEGF165 and TS2/16. Proteins were extracted and immunoprecipitated using anti FLT-1 antibody; blot was sequentially revealed using anti-hERG1B antibody (top), anti-pan hERG1 antibody (upper middle), anti-β1 antibody (lower middle), or with anti FLT-1 antibody (bottom). Inset (left), Densitometric analysis of the amount of hERG1B protein co-immunoprecipitated with FLT-1 after stimulation. The analysis was performed as described in “Materials and methods”; data are the means (± standard error of the mean [SEM]) of 3 separate experiments. *Statistically significant differences between samples are indicated by the horizontal bars. VEGF-treated cells vs BSA-treated cells, Student t test, P = .03; PlGF-treated cells vs BSA-treated cells, Student t test, P = .026; TS2/16-treated cells vs BSA-treated cells, Student t test not significant (NS); VEGF plus TS2/16-treated cells vs BSA-treated cells, Student t test, P = .03; VEGF plus TS2/16-treated cells vs VEGF/PlGF-treated cells, Student t test, NS. Inset (right), densitometric analysis of the amount of β1 integrin co-immunoprecipitated with FLT-1 after stimulation. VEGF plus TS2/16-treated cells vs TS2/16–treated cells, Student t test, P = .03. (C) Co-immunoprecipitation of FLT-1, hERG1B and β1 integrin in AML cell lines after addition of VEGF and β1 activating antibody. AML cell lines were stimulated, and proteins were extracted and immunoprecipitated as noted. Blots were probed with anti pan hERG1 antibody (top), anti-β1A antibody (middle), and anti-FLT-1 antibody (bottom). Inset, Densitometric analysis of the amount of hERG1B protein co-immunoprecipitated with FLT-1 after stimulation with BSA or VEGF plus TS2/16. The analysis was performed as described in Document S1; data are the means (± SEM) of 3 separate experiments. *Statistically significant differences between samples comprised in the horizontal bars, P < .05, Student t test.
Physical association between FLT-1 receptor, hERG1, and β1 integrin subunit in AML cell lines. (A) Co-immunoprecipitation of FLT-1 and hERG1B in AML cell lines. Cell lysates from AML cell lines cultured in the presence of serum were immunoprecipitated with anti FLT-1 antibody; blots were probed with anti-pan hERG1 antibody (top), anti-hERG1B antibody (upper middle), anti-β1 antibody (lower middle), and anti-FLT-1 antibody (bottom). The pan hERG1 antibody recognizes both hERG1A and hERG1B isoforms.27 Note that with the anti-hERG1B antibody only the higher molecular weight bands are visible, whereas the lower bands can be detected only after long exposure of the membrane (as reported in Lee et al33 ). Bands relative to the hERG1B protein are indicated by the arrows in the top and middle panels. (B) Co-immunoprecipitation of FLT-1, hERG1B, and β1 integrin after addition of VEGF, PlGF, and β1 activating antibody in FLG 29.1 cells. Cells were treated for 30 minutes with BSA (250μg/mL), VEGF165 (100 ng/mL), PlGF (50 ng/mL), the β1 activating antibody TS2/16 (20 μg/mL) or both VEGF165 and TS2/16. Proteins were extracted and immunoprecipitated using anti FLT-1 antibody; blot was sequentially revealed using anti-hERG1B antibody (top), anti-pan hERG1 antibody (upper middle), anti-β1 antibody (lower middle), or with anti FLT-1 antibody (bottom). Inset (left), Densitometric analysis of the amount of hERG1B protein co-immunoprecipitated with FLT-1 after stimulation. The analysis was performed as described in “Materials and methods”; data are the means (± standard error of the mean [SEM]) of 3 separate experiments. *Statistically significant differences between samples are indicated by the horizontal bars. VEGF-treated cells vs BSA-treated cells, Student t test, P = .03; PlGF-treated cells vs BSA-treated cells, Student t test, P = .026; TS2/16-treated cells vs BSA-treated cells, Student t test not significant (NS); VEGF plus TS2/16-treated cells vs BSA-treated cells, Student t test, P = .03; VEGF plus TS2/16-treated cells vs VEGF/PlGF-treated cells, Student t test, NS. Inset (right), densitometric analysis of the amount of β1 integrin co-immunoprecipitated with FLT-1 after stimulation. VEGF plus TS2/16-treated cells vs TS2/16–treated cells, Student t test, P = .03. (C) Co-immunoprecipitation of FLT-1, hERG1B and β1 integrin in AML cell lines after addition of VEGF and β1 activating antibody. AML cell lines were stimulated, and proteins were extracted and immunoprecipitated as noted. Blots were probed with anti pan hERG1 antibody (top), anti-β1A antibody (middle), and anti-FLT-1 antibody (bottom). Inset, Densitometric analysis of the amount of hERG1B protein co-immunoprecipitated with FLT-1 after stimulation with BSA or VEGF plus TS2/16. The analysis was performed as described in Document S1; data are the means (± SEM) of 3 separate experiments. *Statistically significant differences between samples comprised in the horizontal bars, P < .05, Student t test.
Effect of VEGF/PlGF and of integrin stimulation on the FLT-1/hERG1/β1 complex
We next studied whether the assembly of the multiprotein complex was affected by VEGF165 addition, concomitant or not with β1 integrin activation. This point was first assessed in FLG 29.1, a leukemia cell line in which we had previously shown β1/hERG1 association.34 Co-immunoprecipitation experiments were performed on serum-starved cells, after addition of either BSA (control) or VEGF165. After precipitating FLT-1, the blot was treated with an antibody specific for hERG1B. The hERG1B-specific bands were present on both controls and cells stimulated with VEGF165, and the signal was increased after stimulation with VEGF165 (Figure 2B, top panel). The same effect was observed when leukemia cells were treated with the specific FLT-1 ligand, PlGF6 (Figure 2B, right). The exclusive presence of hERG1B (compared with hERG1A) was confirmed by reprobing the membranes with anti-pan hERG1 antibody.
Next, we tested the effects produced on FLT-1/hERG1 assembly by application of a β1 integrin activating antibody (TS2/16), either in the absence or in the presence of VEGF165 (or BSA for control). As it turned out (Figure 2B, uppermost left panel), addition of TS2/16 scarcely altered the level of FLT-1/hERG1B association obtained after treatment with either BSA or VEGF165. Moreover, probing our blots with anti β1 integrin antibody showed that β1 integrin was not recruited to the complex by VEGF165 stimulation in the absence of TS2/16. On the contrary, TS2/16 addition induced the association of the integrin subunit in the FLT-1/hERG1 complex when VEGF165 was absent. Finally, simultaneous stimulation with VEGF165 and TS2/16 produced further increase of the level of integrin association with the FLT-1/hERG1 complex. Our results are better appreciated by observing the corresponding densitometric analyses (Figure 2B, graph).
Similar results were found with the other AML cell lines (Figure 2C). VEGF165 addition in the presence of integrin stimulation always induced the formation of a FLT-1/hERG1/β1 complex. It is particularly noteworthy that β1 integrin was associated with the FLT-1/hERG1 complex even when cells were cultured in the presence of serum with no supplementary stimulation (Figure 2A). We assume this effect is caused by simultaneous stimulation of both VEGFRs and integrins by serum.13
Functional role of the FLT-1/hERG1/β1 complex in leukemic cell lines
To understand the physiologic importance of these results, we asked: (1) does FLT-1/hERG1/β1 complex formation affect FLT-1 activation (ie, its p-Tyr); (2) what is the effect of altering hERG1 channel activity on downstream signaling; and (3) does this complex affect AML cell motility, based on the fact that VEGF165 binding to FLT-1 is known to stimulate migration of several normal and leukemic hemopoietic cells.7,32,35–39
Effect of protein complex formation on FLT-1 activation.
FLT-1 p-Tyr was increased in FLG 29.1 cells by the addition of either VEGF165, PlGF or the β1 activating antibody TS2/16, compared with controls (Figure 3A). Densitometric analysis showed that stimulation with VEGF plus TS2/16 increased FLT-1 p-Tyr by 3.7-fold (Figure 3A, graph). Integrin activation thus appears to be necessary for full activation of FLT-1 by VEGF.
Modulation of FLT-1 tyrosine phosphorylation in AML cell lines. (A) Effect of various treatments on FLT-1 tyrosine phosphorylation in FLG 29.1 cells. Serum-starved FLG 29.1 cells were stimulated with BSA (250 μg/mL), VEGF165 (100 ng/mL), TS2/16 antibody (20μg/mL), or both VEGF165 andTS2/16, as well as with PlGF (50 ng/mL), for 30 minutes. Total cell lysates were immunoprecipitated with anti-FLT-1 antibody. Immunoprecipitates were fractionated on SDS-PAGE and subjected to WB analysis with the anti p-Tyr antibody (top). The same blot was reprobed with anti-FLT-1 antibody (bottom). Inset, Densitometric analysis of data obtained treating the cells as in panel A. The analysis was performed as described in “Materials and methods”; data are means (± SEM) of 3 separate experiments. *Statistically significant differences between samples indicated by the horizontal bars. VEGF-treated cells vs BSA-treated cells, Student t test, P = .01; TS2/16-treated cells vs BSA-treated cells, Student t test, P = .02; VEGF plus TS2/16-treated cells vs BSA-treated cells, Student t test, P = .01; PlGF-treated cells vs BSA-treated cells, Student t test, P = .023. (B) Effect of various ion channel blockers on FLT-1 tyrosine phosphorylation in FLG 29.1 cells. Serum-starved FLG 29.1 cells were stimulated with VEGF165 andTS2/16 for 30 minutes, in the absence or presence of inhibitors of different potassium channels: Way (1 μM) or E4031 (1 μM; both specific inhibitors of hERG1 K+ channels); tetra-ethyl-ammonium (5 mM); a wide inhibitor of K+ channels, proven not to affect hERG1 channels at 5 mM concentration); C. toxin (1 μM; an inhibitor of Ca2+-dependent K+ channels that are known to be expressed in leukemia cells). Total cell lysates were treated as in panel A. Inset, Densitometric analysis of data obtained treating the cells as in panel B. The analysis was performed as described in Document S1; data are means (± SEM) of 3 separate experiments. *Statistically significant differences between samples are indicated by the horizontal bars. VEGF plus TS2/16-treated cells vs VEGF plus TS2/16 plus Way-treated cells, Student t test, P = .03 and VEGF plus TS2/16-treated cells vs VEGF plus TS2/16 plus E4031-treated cells, Student t test, P = .04. (C) Effect of VEGF plus TS2/16 treatment on FLT-1 tyrosine phosphorylation on various AML cell lines (left). Serum-starved AML cells were stimulated with BSA (250 μg/mL) or VEGF165 (100 ng/mL) plus TS2/16 antibody (20 μg/mL) for 30 minutes. Cell lysates, IPs, and WBs were performed as described in panel A. Effect of hERG inhibitors on FLT-1 tyrosine phosphorylation of AML cell lines (right). Culture conditions, cell stimulation, IPs, and WBs were performed in the same conditions as in panel A. Inset, Densitometric analysis of FLT-1 phosphorylation in AML cell lines stimulated with BSA or VEGF plus TS2/16. The analysis was performed as described in Document S1; data are means (± SEM) of 3 separate experiments. VEGF plus TS2/16-treated cells vs BSA-treated cells, Student t test, P < .05 for all the cell lines tested. (D) Effects of VEGF165, TS2/16, and Way on MAPK (left) and Akt phosphorylation in FLG 29.1 leukemia cells. Cell lysates were obtained from FLG 29.1 cells treated with BSA, VEGF165, TS2/16, or VEGF plus TS2/16, the latter either in the absence or in the presence of 1 μM Way for 30 minutes. Proteins were blotted and probed with the anti p-MAPK antibody (right) or the anti phospho Akt antibodies (Document S1). Reprobing the membranes with anti ERKs antibody or anti-Akt antibody is reported in the bottom panels.
Modulation of FLT-1 tyrosine phosphorylation in AML cell lines. (A) Effect of various treatments on FLT-1 tyrosine phosphorylation in FLG 29.1 cells. Serum-starved FLG 29.1 cells were stimulated with BSA (250 μg/mL), VEGF165 (100 ng/mL), TS2/16 antibody (20μg/mL), or both VEGF165 andTS2/16, as well as with PlGF (50 ng/mL), for 30 minutes. Total cell lysates were immunoprecipitated with anti-FLT-1 antibody. Immunoprecipitates were fractionated on SDS-PAGE and subjected to WB analysis with the anti p-Tyr antibody (top). The same blot was reprobed with anti-FLT-1 antibody (bottom). Inset, Densitometric analysis of data obtained treating the cells as in panel A. The analysis was performed as described in “Materials and methods”; data are means (± SEM) of 3 separate experiments. *Statistically significant differences between samples indicated by the horizontal bars. VEGF-treated cells vs BSA-treated cells, Student t test, P = .01; TS2/16-treated cells vs BSA-treated cells, Student t test, P = .02; VEGF plus TS2/16-treated cells vs BSA-treated cells, Student t test, P = .01; PlGF-treated cells vs BSA-treated cells, Student t test, P = .023. (B) Effect of various ion channel blockers on FLT-1 tyrosine phosphorylation in FLG 29.1 cells. Serum-starved FLG 29.1 cells were stimulated with VEGF165 andTS2/16 for 30 minutes, in the absence or presence of inhibitors of different potassium channels: Way (1 μM) or E4031 (1 μM; both specific inhibitors of hERG1 K+ channels); tetra-ethyl-ammonium (5 mM); a wide inhibitor of K+ channels, proven not to affect hERG1 channels at 5 mM concentration); C. toxin (1 μM; an inhibitor of Ca2+-dependent K+ channels that are known to be expressed in leukemia cells). Total cell lysates were treated as in panel A. Inset, Densitometric analysis of data obtained treating the cells as in panel B. The analysis was performed as described in Document S1; data are means (± SEM) of 3 separate experiments. *Statistically significant differences between samples are indicated by the horizontal bars. VEGF plus TS2/16-treated cells vs VEGF plus TS2/16 plus Way-treated cells, Student t test, P = .03 and VEGF plus TS2/16-treated cells vs VEGF plus TS2/16 plus E4031-treated cells, Student t test, P = .04. (C) Effect of VEGF plus TS2/16 treatment on FLT-1 tyrosine phosphorylation on various AML cell lines (left). Serum-starved AML cells were stimulated with BSA (250 μg/mL) or VEGF165 (100 ng/mL) plus TS2/16 antibody (20 μg/mL) for 30 minutes. Cell lysates, IPs, and WBs were performed as described in panel A. Effect of hERG inhibitors on FLT-1 tyrosine phosphorylation of AML cell lines (right). Culture conditions, cell stimulation, IPs, and WBs were performed in the same conditions as in panel A. Inset, Densitometric analysis of FLT-1 phosphorylation in AML cell lines stimulated with BSA or VEGF plus TS2/16. The analysis was performed as described in Document S1; data are means (± SEM) of 3 separate experiments. VEGF plus TS2/16-treated cells vs BSA-treated cells, Student t test, P < .05 for all the cell lines tested. (D) Effects of VEGF165, TS2/16, and Way on MAPK (left) and Akt phosphorylation in FLG 29.1 leukemia cells. Cell lysates were obtained from FLG 29.1 cells treated with BSA, VEGF165, TS2/16, or VEGF plus TS2/16, the latter either in the absence or in the presence of 1 μM Way for 30 minutes. Proteins were blotted and probed with the anti p-MAPK antibody (right) or the anti phospho Akt antibodies (Document S1). Reprobing the membranes with anti ERKs antibody or anti-Akt antibody is reported in the bottom panels.
Effect of hERG1 activity on FLT-1 activation and downstream signaling.
In FLG 29.1 cells, the level of FLT-1 p-Tyr produced in the presence of VEGF165 plus TS2/16 was considerably decreased by inhibiting hERG1. The latter effect was obtained by using either Way or E4031 (Figure 3B). The inhibition of FLT-1 p-Tyr was more than 60% (Figure 3B, inset), whereas no effect was observed by treating the cells with other K+ channel blockers (tetra-ethyl-ammonium and C. toxin)40–44 (Figure 3B).
The stimulation of FLT-1 p-Tyr by VEGF plus TS2/16 occurred in all of our AML cell lines (Figure 3C), and the effect was reduced by addition of 1 μM Way. The results obtained with NB4 and KG1 cells are reported in Figure 3C (right panel).
Finally, we analyzed the effect of VEGF plus TS2/16 on intracellular signaling pathways switched on by FLT-1 activation.6,12 Figure 3D shows that VEGF plus TS2/16 stimulated phosphorylation of both the mitogen-activated protein kinases (MAPK) and of Akt, the kinase downstream to the phosphatidylinositol-3-kinase (PI3K). Application of 1 μM Way strongly decreased the effect. The levels of total extracellular-signal-related kinases (ERK) 1 and ERK2 and of Akt remained unchanged throughout the experiments (Figure 3D, bottom panels).
Role of the FLT-1/hERG1/β1 complex in leukemia cell migration.
By using a Boyden chamber assay, we observed that VEGF165 increased the number of migrating FLG 29.1 cells, and the effect was potentiated by the concomitant stimulation of integrins (VEGF + FN; Figure 4A). The same occurred after addition of the specific FLT-1 ligand PlGF. This result stresses the specific role of the VEGFR-1 in leukemia cell migration. The cell migration induced by VEGF plus FN was inhibited by Way and E4031, but not by tetra-ethyl-ammonium and C. toxin. In this case, however, a full inhibition was only obtained with higher Way concentrations (40 μM), necessary to produce full and sustained channel block when cells are incubated in protein-containing media for prolonged times.28 Leukemia cell migration was also inhibited by applying an antibody against the FN receptor (anti-FN-R), which had been previously shown to block β1-containing integrins.20 The effect was similar to that produced by 1 μM Way. When the anti-FN-R and 1 μM Way were applied together, their effects cooperated to almost completely block leukemia cell migration, an effect similar to the one exerted by 40 μM Way. We also tested which of the signaling pathways switched on by FLT-1 activation regulated leukemia cell migration. The PI3K inhibitor, LY, strongly reduced FLG 29.1 cell migration. An inhibitory effect was also obtained with Akt-specific small interfering RNAs (Akt-siRNAs). Such Akt-siRNAs decreased Akt expression of FLG 29.1 cells by 50%, in our experimental conditions (Figure 4A, inset). Neither LY nor Akt-siRNAs affected FLG 29.1 cell survival/proliferation, as measured by the WST assay (Figure 4B). However, leukemia cell survival/proliferation was strongly reduced by the MAPK inhibitor, PD.
Cell migration in response to FLT-1and integrin stimulation of human AML cell lines: role of hERG1 channels. (A) Analysis of cell migration in FLG 29.1 cells. Cells were allowed to migrate through BSA-coated filters (□) and on FN-coated filters in the presence of VEGF165 (100 ng/mL), or PlGF (50 ng/mL). Cell migration was carried out at 37°C and 5% CO2 for 18 hours as detailed in “Patients, materials, and methods; Migration assay.” Values are reported as number of migrated cells/mL and represent means of 4 experiments, each performed in triplicate. Results shown are means (± SEM). The following inhibitors were used, at the final concentrations reported in parentheses: the hERG1 specific blocker Way (1 and 40 μM); the specific hERG1 blocker E4031 (1μM); tetra-ethyl-ammonium (used at 5 mM, a concentration known to block voltage-dependent K+ channels other than hERG1); C. toxin, a blocker of Ca2+-dependent K+ channels (1 μM); the anti FN-R antibody, known to block all the β1 containing integrins20 (1:50); the MAPK inhibitor LY (10 μM); the PI3K inhibitor PD (30 μM). When needed, cells were pretreated with ion channel inhibitors at 37°C for 15 minutes as reported in “Patients, materials, and methods; Treatment with channels inhibitors.” FLG 29.1 were also treated for 48 hours with Akt-siRNA and migration was assessed for a further 18 hours. Control scrambled siRNAs were used as reported in “Patients, materials and methods; Silencing of Akt by small interfering RNAs.” *Statistically significant differences between samples are indicated by the horizontal bars and are P < .05, Student t test. Inset, WB analysis of Akt expression levels in cells treated with Akt-siRNA or scrambled-siRNA. (B) Effects of various treatments on FLG 29.1 cell proliferation/survival. Cells, treated as in panel A, were incubated in 96-well cell culture plates for 18 hours. At the end of incubation, the WST reagent was added, and absorbance was measured. Data are reported as percentage of the control and represent mean (± SEM) of 3 different experiments, each performed in triplicate. (C) Effect of VEGF and integrin stimulation, as well as of hERG1 blockers on migration of various AML cell lines. Cell migration and treatments were performed as in panel A. Results shown are means (± SEM). *Statistically significant differences between samples are indicated by the horizontal bars and are P < .05, Student t test. The correlation between the amount of leukemia cells stimulated to migrate by VEGF plus FN (normalized on the amount of migrated cells in control conditions) and the amount of FLT-1/hERG1/β1 complex in cells stimulated by VEGF plus FN (normalized on the amount of the complex in cells treated with BSA, taken from Figure 2C) was determined by regression analysis (P = .02).
Cell migration in response to FLT-1and integrin stimulation of human AML cell lines: role of hERG1 channels. (A) Analysis of cell migration in FLG 29.1 cells. Cells were allowed to migrate through BSA-coated filters (□) and on FN-coated filters in the presence of VEGF165 (100 ng/mL), or PlGF (50 ng/mL). Cell migration was carried out at 37°C and 5% CO2 for 18 hours as detailed in “Patients, materials, and methods; Migration assay.” Values are reported as number of migrated cells/mL and represent means of 4 experiments, each performed in triplicate. Results shown are means (± SEM). The following inhibitors were used, at the final concentrations reported in parentheses: the hERG1 specific blocker Way (1 and 40 μM); the specific hERG1 blocker E4031 (1μM); tetra-ethyl-ammonium (used at 5 mM, a concentration known to block voltage-dependent K+ channels other than hERG1); C. toxin, a blocker of Ca2+-dependent K+ channels (1 μM); the anti FN-R antibody, known to block all the β1 containing integrins20 (1:50); the MAPK inhibitor LY (10 μM); the PI3K inhibitor PD (30 μM). When needed, cells were pretreated with ion channel inhibitors at 37°C for 15 minutes as reported in “Patients, materials, and methods; Treatment with channels inhibitors.” FLG 29.1 were also treated for 48 hours with Akt-siRNA and migration was assessed for a further 18 hours. Control scrambled siRNAs were used as reported in “Patients, materials and methods; Silencing of Akt by small interfering RNAs.” *Statistically significant differences between samples are indicated by the horizontal bars and are P < .05, Student t test. Inset, WB analysis of Akt expression levels in cells treated with Akt-siRNA or scrambled-siRNA. (B) Effects of various treatments on FLG 29.1 cell proliferation/survival. Cells, treated as in panel A, were incubated in 96-well cell culture plates for 18 hours. At the end of incubation, the WST reagent was added, and absorbance was measured. Data are reported as percentage of the control and represent mean (± SEM) of 3 different experiments, each performed in triplicate. (C) Effect of VEGF and integrin stimulation, as well as of hERG1 blockers on migration of various AML cell lines. Cell migration and treatments were performed as in panel A. Results shown are means (± SEM). *Statistically significant differences between samples are indicated by the horizontal bars and are P < .05, Student t test. The correlation between the amount of leukemia cells stimulated to migrate by VEGF plus FN (normalized on the amount of migrated cells in control conditions) and the amount of FLT-1/hERG1/β1 complex in cells stimulated by VEGF plus FN (normalized on the amount of the complex in cells treated with BSA, taken from Figure 2C) was determined by regression analysis (P = .02).
Expression of herg1, flt-1 and kdr transcripts in peripheral blasts from AML patients. (A) Representative RT-PCR of herg1 (upper panel, 575-bp band), flt-1 (upper middle panel, 550-bp band), kdr (lower middle panel, 660-bp band), and gapdh (bottom panel, 138-bp band) transcripts in primary AML patients. RNA extracted from blasts of AML patients was retrotranscribed and amplified for herg1, flt-1, kdr, and gapdh using primers described in “Patients, materials, and methods; Molecular biologic methods.” For case reference numbers, see Table 1. (B) Immunocytochemistry detection of hERG1 and FLT-1 protein on AML blasts. Cells from a representative AML example relating to case A49 were immunostained with an anti-pan hERG1 antibody (left) and anti FLT-1 antibody (right) as reported in “Patients, materials, and methods; Immunohistochemistry” (100× magnification). Images were acquired on a Leica DM 4000B microscope with a Leica DFC 320 photocamera (Leica Microsystems) (PL Fluotar 40×/0.70, PL Fluotar 100×/1.30 OIL objective). To determine BM angiogenesis, BM sections were stained with anti-CD34 antibodies. Vascular morphometric parameters were quantified following the procedure used by Korkolopoulou et al,30 with Leica DC Viewer software.
Expression of herg1, flt-1 and kdr transcripts in peripheral blasts from AML patients. (A) Representative RT-PCR of herg1 (upper panel, 575-bp band), flt-1 (upper middle panel, 550-bp band), kdr (lower middle panel, 660-bp band), and gapdh (bottom panel, 138-bp band) transcripts in primary AML patients. RNA extracted from blasts of AML patients was retrotranscribed and amplified for herg1, flt-1, kdr, and gapdh using primers described in “Patients, materials, and methods; Molecular biologic methods.” For case reference numbers, see Table 1. (B) Immunocytochemistry detection of hERG1 and FLT-1 protein on AML blasts. Cells from a representative AML example relating to case A49 were immunostained with an anti-pan hERG1 antibody (left) and anti FLT-1 antibody (right) as reported in “Patients, materials, and methods; Immunohistochemistry” (100× magnification). Images were acquired on a Leica DM 4000B microscope with a Leica DFC 320 photocamera (Leica Microsystems) (PL Fluotar 40×/0.70, PL Fluotar 100×/1.30 OIL objective). To determine BM angiogenesis, BM sections were stained with anti-CD34 antibodies. Vascular morphometric parameters were quantified following the procedure used by Korkolopoulou et al,30 with Leica DC Viewer software.
With regard to the other AML lines, cell migration was stimulated by VEGF plus FN, with an average increment of more than 2-fold, compared with control (BSA). Once again, the effect was significantly reduced in the presence of 1 μM Way (Figure 4C). A positive correlation was found between the number of leukemia cells stimulated to migrate by VEGF plus FN (relative to BSA), and the density of the FLT-1/hERG1/β1 complex assembled in the same conditions (Figure 4).
Expression of hERG1 gene(s), VEGFRs, and FLT-1/hERG1/β1 complex in primary leukemia blasts
We hypothesized that the aforementioned mechanism might operate in primary AML blasts to induce a significant leukemia cell migration. To assess this possibility, we first determined the expression of herg1 and VEGFRs (flt-1 and kdr) transcripts in mononuclear cells obtained from the PB of 61 patients affected by de novo AML. For herg1 detection, we used primers capable of amplifying both the herg1a and the herg1b transcripts. A representative example of the RT-PCR results is reported in Figure 5A. Herg1 mRNA was expressed in the majority of the AML samples (69% of the cases, 42/61), irrespective of the FAB type. In some of the samples, a more quantitative estimate of herg1 transcripts was obtained by applying RQ-PCR, with primers specific for either herg1a or herg1b. Results are shown in Table 3, where data relative to some of the AML cell lines used throughout this study are also reported. The level of herg1a/b transcripts in PB mononuclear cells was taken as 1. When compared with PB mononuclear cells, 81% of our AML cases showed increased transcription of herg1a, and increased transcription of herg1b in 72% of cases. The samples examined by RQ-PCR and reported in Table 3 correspond to some of the samples shown in Figure 5A and analyzed by conventional RT-PCR. Their comparison shows concordance between the level of the transcripts and the intensity of the PCR bands.
Real-time PCR analysis and VEGF secretion on 12 primary AML samples and AML cell lines
| Case . | FAB . | hERG1 . | VEGF (pg/ml) . | |
|---|---|---|---|---|
| 1A . | 1B . | |||
| PBMNC | / | 1.00 | 1.00 | n.d. |
| A17 | M1 | 18.00 | 68.10 | 175 ± 4 |
| A21 | M1 | 10.40 | 7.94 | 85 ± 7 |
| A50 | M1 | 190.00 | 70.60 | 148 ± 14 |
| A16 | M2 | 0.43 | 0.70 | 66 ± 13 |
| A25 | M2 | 3.28 | 0.55 | 71 ± 13 |
| A60 | M2 | 0.05 | 0.35 | 59 ± 2 |
| A57 | M2 | 9.40 | 238.00 | 124 ± 11 |
| A29 | M3 | 4.22 | 562000.00 | 144 ± 12 |
| A4 | M4 | 107.00 | 20.10 | 183 ± 6 |
| A48 | M4 | 12.20 | 0.92 | 125 ± 6 |
| A64 | M6 | 6.98 | 30.20 | 85 ± 4 |
| A1 | M4 | 0.85 | 0.44 | 77 ± 2 |
| FLG | M5 | 280.00 | 6840.00 | 358 ± 24 |
| FLG+Way | M5 | n.d. | n.d. | 237 ± 16 |
| K562 | Acute phase of CML | 10.10 | 480.00 | 99 ± 8 |
| NB4 | M3 | 27.80 | 660.00 | 253 ± 14 |
| HL60 | M3 | 13.70 | 94.70 | 132 ± 8 |
| hERG1-HL60 | M3 | 224.00 | 98.10 | 292 ± 21 |
| Case . | FAB . | hERG1 . | VEGF (pg/ml) . | |
|---|---|---|---|---|
| 1A . | 1B . | |||
| PBMNC | / | 1.00 | 1.00 | n.d. |
| A17 | M1 | 18.00 | 68.10 | 175 ± 4 |
| A21 | M1 | 10.40 | 7.94 | 85 ± 7 |
| A50 | M1 | 190.00 | 70.60 | 148 ± 14 |
| A16 | M2 | 0.43 | 0.70 | 66 ± 13 |
| A25 | M2 | 3.28 | 0.55 | 71 ± 13 |
| A60 | M2 | 0.05 | 0.35 | 59 ± 2 |
| A57 | M2 | 9.40 | 238.00 | 124 ± 11 |
| A29 | M3 | 4.22 | 562000.00 | 144 ± 12 |
| A4 | M4 | 107.00 | 20.10 | 183 ± 6 |
| A48 | M4 | 12.20 | 0.92 | 125 ± 6 |
| A64 | M6 | 6.98 | 30.20 | 85 ± 4 |
| A1 | M4 | 0.85 | 0.44 | 77 ± 2 |
| FLG | M5 | 280.00 | 6840.00 | 358 ± 24 |
| FLG+Way | M5 | n.d. | n.d. | 237 ± 16 |
| K562 | Acute phase of CML | 10.10 | 480.00 | 99 ± 8 |
| NB4 | M3 | 27.80 | 660.00 | 253 ± 14 |
| HL60 | M3 | 13.70 | 94.70 | 132 ± 8 |
| hERG1-HL60 | M3 | 224.00 | 98.10 | 292 ± 21 |
The numbers represent the transcript levels of herg1a and herg1b in primary leukemia samples and AML cell lines, standardized to the levels in normal PB mononuclear cells from 6 donors. VEGF was measured by enzyme-linked immunosorbent assay (ELISA) test on the supernatants of cells cultured in serum free medium for 48 hours (“Patients, materials, and methods”). FLG 29.1 cells were treated or not with 40 μM Way throughout the entire time of the experiment as in Masi et al.28 hERG1-HL60 cells are HL60 cells stably transfected with the herg1 cDNA (“Patients, materials, and methods” and the legend for Figure 7). For case reference numbers in, see Table 1. A significant correlation emerged between herg1a expression and the amount of secreted VEGF in both primary AML blasts and AML cell lines (regression analysis, P = .002).
Flt1 transcript expression was detected in 71% (38/55) of patients, whereas only 31% (15/47) expressed kdr. These results agree with those reported by others.7,11 Features of AML patients are summarized in Table 1. Statistical analysis showed a significant correlation between herg1 and flt1 gene expression in AML blast (P = .04; Fisher exact test). Such a correlation was also observed at the protein level, because both hERG1 and FLT-1 were detected by ICC in the same AML cases. A representative example relating to case A49 is reported in Figure 5B.
Finally, we determined the amount of VEGF secreted by the AML blasts. The amounts of VEGF secreted by some of our AML cell lines are also reported (Table 3). VEGF secretion varied from 59 to 183 pg/mL in primary AML blasts. FLG 29.1 and HL60 stably transfected with the herg1 cDNA (hERG1-HL60) gave the highest secretion of VEGF. In the former, secretion was strongly inhibited by Way. On the whole, a significant correlation emerged between herg1 expression and the amount of secreted VEGF in both primary AML blasts and AML cell lines (Figure 5).
Formation of an FLT-1/hERG1/β1 complex in primary AML blasts and normal hematopoietic precursors
We tested whether a FLT-1/hERG1/β1 complex was formed in primary AML blasts of different FAB as well, by using the approach illustrated in Figure 2A. Here again, the hERG1B protein co-immunoprecipitated with FLT-1 (Figure 6A, top panel) and the complex included the β1 integrin (Figure 6A, middle panel). The bottom panel of Figure 6A shows the results of reprobing the membrane with the same antibody used to obtain immunoprecipitation. Thus, the results we have obtained with AML blasts match those obtained in AML cell lines.
Physical association between FLT-1 receptor, hERG1, and β1 integrin subunit in primary AML blasts: effect on cell migration. (A) Co-immunoprecipitation of FLT-1 and hERG1 in primary AML blasts. Cell lysates from 5 AML cases of different FAB (respectively, M4, M2, M6, M5, and M2; 3 of the cases correspond to samples reported in Table 1: 1 is A45, 3 is A64, and 5 is A53), cultured in the presence of serum were immunoprecipitated with anti FLT-1 antibody; blots were probed with anti pan hERG1 antibody (top), anti-β1 antibody (middle), and anti FLT-1 antibody (bottom). (B) Co-immunoprecipitation of FLT-1 and hERG1 in primary peripheral CD34+ cells. Cell lysates were obtained from CD34+ (lane 1) and CD34+ treated for 12 hours in vitro with IL-3, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor31 (lane 2); blot was probed with anti-pan hERG1 antibody (top) and anti-FLT-1 antibody (bottom). (C) Effect of hERG1 expression on primary AML blast migration. The migration assay was performed as reported in the legend to Figure 4 on leukemic blasts isolated from PB of 6 AML samples. Cells were treated as reported in the legend to Figure 4B. Left, hERG1+ samples (A34, A42, A7). Right, hERG1− samples (A54, A10, A13). Values are reported as number of migrated cells/mL. The average number of migrated cells of hERG1+ samples (570 567 ± 83 749) was significantly higher (Student t test, P = .033*) than in hERG1− samples (217 000 ± 72 266). The average of migrated cells of flt1-negative cells was: hERG1+ samples (182 500 ± 11 000), hERG1− samples (120 000 ± 7400). (D) Effect of Way (1 μM) on migration of primary leukemia cells expressing or not expressing hERG1 channels. The migration assay was performed as described in the legend to Figure 4 on 3 hERG1+ leukemia samples (2 of which correspond to samples in panel A, on the left) and on one hERG1− sample (A60; on the right). Cells were treated (▒) or not (□) with Way 123 398 (1 μM). Values are reported as percentage of the control and each determination represents the average of 3 individual chambers (bars ± SEM). *Statistically significant differences between samples indicated by the horizontal bars. * = P < .05, Student t test. Error bars represent standard deviation.
Physical association between FLT-1 receptor, hERG1, and β1 integrin subunit in primary AML blasts: effect on cell migration. (A) Co-immunoprecipitation of FLT-1 and hERG1 in primary AML blasts. Cell lysates from 5 AML cases of different FAB (respectively, M4, M2, M6, M5, and M2; 3 of the cases correspond to samples reported in Table 1: 1 is A45, 3 is A64, and 5 is A53), cultured in the presence of serum were immunoprecipitated with anti FLT-1 antibody; blots were probed with anti pan hERG1 antibody (top), anti-β1 antibody (middle), and anti FLT-1 antibody (bottom). (B) Co-immunoprecipitation of FLT-1 and hERG1 in primary peripheral CD34+ cells. Cell lysates were obtained from CD34+ (lane 1) and CD34+ treated for 12 hours in vitro with IL-3, granulocyte colony-stimulating factor, and granulocyte-macrophage colony-stimulating factor31 (lane 2); blot was probed with anti-pan hERG1 antibody (top) and anti-FLT-1 antibody (bottom). (C) Effect of hERG1 expression on primary AML blast migration. The migration assay was performed as reported in the legend to Figure 4 on leukemic blasts isolated from PB of 6 AML samples. Cells were treated as reported in the legend to Figure 4B. Left, hERG1+ samples (A34, A42, A7). Right, hERG1− samples (A54, A10, A13). Values are reported as number of migrated cells/mL. The average number of migrated cells of hERG1+ samples (570 567 ± 83 749) was significantly higher (Student t test, P = .033*) than in hERG1− samples (217 000 ± 72 266). The average of migrated cells of flt1-negative cells was: hERG1+ samples (182 500 ± 11 000), hERG1− samples (120 000 ± 7400). (D) Effect of Way (1 μM) on migration of primary leukemia cells expressing or not expressing hERG1 channels. The migration assay was performed as described in the legend to Figure 4 on 3 hERG1+ leukemia samples (2 of which correspond to samples in panel A, on the left) and on one hERG1− sample (A60; on the right). Cells were treated (▒) or not (□) with Way 123 398 (1 μM). Values are reported as percentage of the control and each determination represents the average of 3 individual chambers (bars ± SEM). *Statistically significant differences between samples indicated by the horizontal bars. * = P < .05, Student t test. Error bars represent standard deviation.
We had previously shown that when CD34+ collected from PB of normal subjects are stimulated with IL-3, granulocyte colony-stimulating factor and granulocyte-macrophage colony-stimulating factor, herg1 is upregulated and cells enter into the cell cycle.25 Based on this information, we performed co-immunoprecipitation experiments on resting and stimulated normal peripheral CD34+ cells. A strong hERG1 band was observed only in the immunoprecipitate obtained from stimulated CD34+ cells (Figure 6B, top panel), and mostly contained the hERG1B isoform. We conclude that an FLT-1/hERG1/β1 complex occurs in human hematopoietic immature cells, whether they are activated normal CD34+ or AML blasts.
Role of hERG1 channels in leukemia cell migration in vitro
As a final step, we investigated whether herg1 expression, in addition to flt-1, might stimulate leukemia blast migration, in vitro. We studied 6 AML samples. All expressed the flt-1 receptor, and 3 of them expressed herg1 as well. Migration was studied in the same conditions applied to AML cell lines (Figure 4A). Cell migration was significantly higher in herg1-positive (hERG1+) compared with herg1-negative (hERG1−) samples (Figure 6C). Interestingly, flt-1-negative leukemias were almost unable to migrate through FN, irrespective of herg1 expression (Figure 6C). Pretreatment with 1 μM Way significantly reduced cell migration of hERG1+, but not of hERG1−, samples. (Figure 6D). Altogether, our results indicate that in vitro, the cell migration mediated by FLT-1 depends on hERG1 channel activity.
Overexpression of herg1 confers a higher invasiveness to leukemia cells in vivo
To test whether the FLT-1/hERG1/β1 complex regulates leukemia cell migration in vivo, we used a repopulation assay in NOD-SCID mice. We applied 2 different approaches: (1) inoculation of herg1− and herg1+ leukemia blasts from primary AML; and (2) inoculation of either HL60 or hERG1-HL60 cells that overexpress the herg1 gene (Table 3). The degree of BM engraftment and PB invasion was determined by measuring the amount of human CD45-positive (hCD45+) cells with flow cytometry (Figure 7). In addition, we determined (1) the level of angiogenesis, the state of normal hemopoiesis, and the presence of leukemia blasts in the BM; and (2) the presence and extent of leukemia blast invasion into extramedullary organs (Figure 8 and Figure 7). The BM engraftment was approximately the same irrespective of herg1 expression in the blasts from primary leukemias, as well as in HL60 and hERG1-HL60 (Figure 7). However, the efficiency of bloodstream invasion was significantly higher in herg1+ compared with herg1− blasts from primary AML, as well as in hERG1-HL60 compared with HL60 cells (Figure 7). This result was paralleled by a higher presence of immature cells in the PB of mice inoculated with either herg1+ AML blasts or hERG1-HL60 cells (Figure 7 insets). BM angiogenesis, in particular the total vascular area, was significantly higher in mice inoculated with either herg1+ blasts or hERG1-HL60 cells (Figure 8A). An increased density of undifferentiated leukemia cells and a concomitant decrease of endogenous hemopoiesis were detected in the BM of mice injected with either herg1+ blasts or hERG1-HL60 cells (Figure 8B). The hERG1-positive blasts were often found around new vessels in the BM of herg1+/hERG1-HL60-injected mice (Figure 8B inset). Finally, only mice injected with herg1+/hERG1-HL60 blasts displayed a substantial hepatic (Figure 8C) and splenic (Figure 8D) invasion. A quantitative estimation of these results is also given in Figure 7.
Characteristics of NOD-SCID mice inoculated with herg1− and herg1+ leukemia blasts, and with HL60 and hERG1-HL60 cells.
Characteristics of NOD-SCID mice inoculated with herg1− and herg1+ leukemia blasts, and with HL60 and hERG1-HL60 cells.
In vivo phenotypes of herg1-positive and herg1-negative leukemia blasts. Repopulation assay in NOD-SCID mice, inoculated with (1) herg1-negative (herg1−) and herg1-positive (herg1+) leukemia blasts from primary AML; two herg1− cases (cases A10 and A13 in Table 1) and two herg1+ cases (cases A34 and A42 in Table 1) were injected; (2) HL60 and of HL60 cells transfected with herg1a cDNA (hERG1-HL60). In these cells, herg1a mRNA expression was checked by RQ-PCR (Table 3). Data are representative of at least 3 mice per group. (A) Immunohistochemistry (IHC) with anti-mCD34 antibody on BM of mice inoculated with herg− (left) and herg+ (right) leukemia blasts from primary AML (magnification 250×). The arrows indicate CD34-positive vessels. (B) IHC with anti-hMHCI antibody on BM of mice inoculated with HL60 (left) and hERG1-HL60 (right) (magnification 200×). The arrows indicate niches of leukemia blasts. Inset, IHC with anti-hERG1 antibody on BM of mice injected with hERG1-HL60 (magnification 400×). The arrow indicates hERG1-positive leukemia cells. (C) IHC with anti-hMHCI on the liver of mice inoculated with herg− (left) and herg+ (right) leukemia blasts (magnification 400×). The arrow indicates niches of leukemia blasts. (D) IHC with anti-hMHCI on spleen of mice inoculated with HL60 (left) and hERG1-HL60 (right) (magnification 200×). The arrow indicates niches of leukemia blasts. Images were acquired on a Leica DM 4000B microscope with a Leica DFC 320 photocamera (Leica Microsystems) (PL Fluotar 40×/0.70, PL Fluotar 100×/1030 OIL objective). To determine BM angiogenesis, BM sections were stained with anti-CD34 antibodies. Vascular morphometric parameters were quantified following the procedure used by Korkolopoulou et al,30 with Leica DC Viewer software.
In vivo phenotypes of herg1-positive and herg1-negative leukemia blasts. Repopulation assay in NOD-SCID mice, inoculated with (1) herg1-negative (herg1−) and herg1-positive (herg1+) leukemia blasts from primary AML; two herg1− cases (cases A10 and A13 in Table 1) and two herg1+ cases (cases A34 and A42 in Table 1) were injected; (2) HL60 and of HL60 cells transfected with herg1a cDNA (hERG1-HL60). In these cells, herg1a mRNA expression was checked by RQ-PCR (Table 3). Data are representative of at least 3 mice per group. (A) Immunohistochemistry (IHC) with anti-mCD34 antibody on BM of mice inoculated with herg− (left) and herg+ (right) leukemia blasts from primary AML (magnification 250×). The arrows indicate CD34-positive vessels. (B) IHC with anti-hMHCI antibody on BM of mice inoculated with HL60 (left) and hERG1-HL60 (right) (magnification 200×). The arrows indicate niches of leukemia blasts. Inset, IHC with anti-hERG1 antibody on BM of mice injected with hERG1-HL60 (magnification 400×). The arrow indicates hERG1-positive leukemia cells. (C) IHC with anti-hMHCI on the liver of mice inoculated with herg− (left) and herg+ (right) leukemia blasts (magnification 400×). The arrow indicates niches of leukemia blasts. (D) IHC with anti-hMHCI on spleen of mice inoculated with HL60 (left) and hERG1-HL60 (right) (magnification 200×). The arrow indicates niches of leukemia blasts. Images were acquired on a Leica DM 4000B microscope with a Leica DFC 320 photocamera (Leica Microsystems) (PL Fluotar 40×/0.70, PL Fluotar 100×/1030 OIL objective). To determine BM angiogenesis, BM sections were stained with anti-CD34 antibodies. Vascular morphometric parameters were quantified following the procedure used by Korkolopoulou et al,30 with Leica DC Viewer software.
On the whole, the phenotype conferred to AML cells in vitro by herg1 overexpression (enhanced migration through FN after VEGF165 addition, caused by the assembly of a FLT-1/hERG1/β1 complex) was also observed in vivo and appears to contribute to the exit of leukemia blasts into the bloodstream with subsequent invasion of extramedullary sites.
Correlation between herg1 expression and clinical parameters and outcome
To follow up these results, we analyzed the correlation between herg1 expression and clinical features and outcome of 42 of the AML patients enrolled in the present study that were undergoing standard chemotherapy. Patients were identified as either herg1+ (n = 26) or herg1− (n = 16). Although our cohort was not numerous, we noted that (1) complete remission was obtained in 61% of herg1+ versus 82% of herg1− patients (P = .222); (2) the percentage of relapses was 79% in herg1+ versus 21% in herg1− patients (P = .035); and (3) the median time to relapse was 11.9 months in herg1+ versus 25.3 months in herg1− patients (P = .014; Table 4). Finally, the overall survival in the same cohort of patients was analyzed. The median overall survival of herg1+ AML patients was 12 months, compared with 23 months for herg1− patients (Figure 9). The difference is statistically significant.
Clinical outcome in DNR/Ara-C–treated patients
| . | Total . | herg1+ . | herg1− . | P . |
|---|---|---|---|---|
| CR | 22/32 (69%) | 13/21 (61%) | 9/11(82%) | .222 |
| Relapse | 14/22 (64%) | 11/14 (79%) | 3/14 (21%) | .035 |
| Time to relapse | 14.8 ± 1.3 | 11.9 ± 2.8 | 25.3 ± 6.1 | .014 |
| . | Total . | herg1+ . | herg1− . | P . |
|---|---|---|---|---|
| CR | 22/32 (69%) | 13/21 (61%) | 9/11(82%) | .222 |
| Relapse | 14/22 (64%) | 11/14 (79%) | 3/14 (21%) | .035 |
| Time to relapse | 14.8 ± 1.3 | 11.9 ± 2.8 | 25.3 ± 6.1 | .014 |
Time to relapse is expressed in months.
Ara-C indicates aracytin; CR, complete remission; DNR, daunorubicin.
Survival time and herg1 expression in AML patients. Survival from diagnosis was analyzed in 42 patients affected by AML and treated with daunorubicin-aracytine standard regimen. Data are presented in relation to the presence or absence of herg1 (number of herg1+ patients was 26; number of herg1− patients was 16). Survival curves have been computed using the Kaplan-Meier method and statistical comparisons between curves were based on log-rank tests (P = .026).
Survival time and herg1 expression in AML patients. Survival from diagnosis was analyzed in 42 patients affected by AML and treated with daunorubicin-aracytine standard regimen. Data are presented in relation to the presence or absence of herg1 (number of herg1+ patients was 26; number of herg1− patients was 16). Survival curves have been computed using the Kaplan-Meier method and statistical comparisons between curves were based on log-rank tests (P = .026).
Discussion
In this article, we provide evidence that FLT-1 activation induced a sustained cell migration in AML cells, and that the effect depended on the formation of a macromolecular signaling complex containing FLT-1, hERG1B K+ channels and the β1 subunit of integrin receptors. What is more, proper activity of hERG1 channels was crucial for the switching on of FLT-1 signaling activity, as well as for AML cell migration. In particular, we have shown that FLT-1 and hERG1B were constitutively associated in leukemia cells and their assembly was potentiated by VEGF/PlGF. Activated integrin receptors were also recruited in the complex, thus stimulating tyrosine phosphorylation of FLT-1 and activation of both MAPK and PI3K/Akt signaling pathways. In turn, FLT-1 p-Tyr and downstream signaling appeared to be regulated by hERG1 channel activity, which was probably sustained by integrins themselves.19 Therefore, besides ligand binding, the proper signaling activity of FLT-1 was determined by a cooperation between integrins and hERG1 channels.
The physiologic stimulation of membrane complex formation, through cooperation of VEGF/PlGF and integrin activation, triggered sustained cell migration in leukemic cell lines. This process appeared to be mediated by the PI3K/Akt pathway in a similar manner to what occurs in multiple myeloma cells17 and was also impaired by hERG1 channel inhibitors (Figure 4). We can conclude that both the engagement of β1 integrin and hERG1 channel activity leads to stimulation of the PI3K/Akt signaling pathway, and is necessary for the migratory phenotype.
To explain how hERG1 is linked to cell migration, we recently proposed a model that assumes that the activation of cell signaling downstream of integrins depends on K+ channel activity. Whether the effect of hERG1 activation depends on direct interaction between the ion channel and the proteins of the intracellular signaling apparatus or is mediated by changes in membrane potential is still unknown.19
A physical link between FLT-1, hERG1B, and β1 was also found to occur in cells from primary AML, in which we observed not only co-expression of herg1 and flt-1 but also co-immunoprecipitation of the 3 membrane proteins forming the complex. The same applies to human CD34+ hematopoietic precursors after appropriate stimulation with growth factors and cytokines. We had previously shown that such stimulation induces an upregulation of herg1 gene and entry into the cell cycle.25 Hence, we hypothesize that the FLT-1/hERG1/β1 complex assembly is stimulated in activated, growth-induced, immature hematopoietic cells, either normal CD34+ or AML cells. Moreover, the presence of both FLT-1 receptors and functional hERG1 channels conferred a pro-migratory phenotype to AML blasts.
In addition, we showed that AML cells (both primary blasts and cell lines) secreted VEGF and that the amount of secreted VEGF was correlated with herg1 expression. These results agree with our recent observation that hERG1 channel activity modulated VEGF secretion in glioblastoma cells.28 Data obtained in FLG 29.1 cells confirmed that the specific hERG1 inhibitor almost halved VEGF secretion (Table 3). These results suggest that a hERG1-dependent autocrine loop between VEGF and FLT-1 occurs in leukemia cells.
From a pathologic standpoint, stimulation of leukemia cell migration sustains transendothelial migration and, hence, bloodstream invasion. The latter, accompanied by subsequent infiltration of extramedullary sites, is a fundamental step in progression of the disease.39 In particular, Recher et al45 showed that integrin-dependent signaling, and the consequent activation of focal adhesion kinase with ensuing induction of cell motility, are negative prognostic factors in AML. Our experiments in leukemia-engrafted immunodeficient mice showed that herg1 overexpression (both in primary AML blasts and in cells with stable herg1 expression) regulated the leukemia blast exit into the bloodstream and the subsequent invasion into extramedullary sites. In other words, functional hERG1 channel expression onto the plasma membrane conferred a selective advantage to leukemia blasts, making them more efficient in leaving the BM microenvironment and entering the blood stream. A similar effect was demonstrated by Fragoso et al11 for FLT-1 in acute lymphoblastic leukemia cells. FLT-1 neutralization with monoclonal antibodies affects leukemia localization in the BM, increases leukemia apoptosis, impairs the exit of leukemia cells, and prolongs the survival of injected mice.
We hypothesize that hERG1 channels regulate different aspects of AML pathophysiology (Figure 10), such as (1) cell survival and proliferation in the BM,25 possibly through the MAPK pathway; (2) cell motility and transendothelial migration, possibly through the PI3K/Akt pathway; and 3) VEGF secretion inside and outside the BM. Taken together, these effects could engender an increased malignancy of herg1+ blasts in vivo. In fact, the VEGF secreted by herg1+ AML cells could produce increased angiogenesis in the BM and extramedullary organs (Figures 7, 8). In addition, herg1+ AML blasts could have increased motility and hence undergo a sustained transendothelial migration into extramedullary sites. This scenario is supported by data obtained in NOD-SCID mice, and is also reinforced by the clinical data presented in this article. In fact, although clinical data reported in the article are far from conclusive, we showed that herg1+ patients had a higher probability of relapse, and a shorter time to relapse, and their overall survival was significantly shorter compared with that of herg1− patients.
Proposed model for the role of hERG1 channels and the FLT-1/hERG1/β1 complex in regulating (i) leukemia cell proliferation and migration within the BM, and (ii) AML cell exit into PB stream and subsequent transendothelial migration into extramedullary organs.
Proposed model for the role of hERG1 channels and the FLT-1/hERG1/β1 complex in regulating (i) leukemia cell proliferation and migration within the BM, and (ii) AML cell exit into PB stream and subsequent transendothelial migration into extramedullary organs.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr J. Mitcheson (University of Leicester, Leicester, United Kingdom) for critically reading the manuscript and kindly revising the English. The authors also thank Dr A. Galli for use of the ABI PRISM 7700 Sequence Detection System facility, Mr A. Rosso for technical support, Mr E. Torre for assistance in preparing samples for histologic analysis, Dr F. Rossi for irradiating the mice, and Dr P. Dello Sbarba for kindly providing PD 098059.
This work was supported by Associazione Italiana per la Ricerca sul Cancro, Associazione Genitori contro le Leucemie e Tumori Infantili Noi per Voi, and Ente Cassa di Risparmio di Firenze, PRIN 2006 (A.A.); Associazione Italiana Contro le Leucemie (AIL Firenze and AIL Pistoia) (S.P.); Associazione Italiana per la Ricerca sul Cancro (M.F.B.); and FAR 2004-2006 (A.B.).
Authorship
Contribution: S.P. designed and performed research. M.F.B., P.A.B., B.B., V.B., R.B., and L.P. performed research. A.B. wrote the paper. V.B. analyzed data. A.A. designed research and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Annarosa Arcangeli, MD, PhD, Department of Experimental Pathology and Oncology, University of Florence, Viale G.B. Morgagni, 50, 50134 Firenze, Italy; e-mail: annarosa.arcangeli@unifi.it.

![Figure 1. hERG1 and VEGF receptors expression and activity in human acute myeloid leukemia cell lines. (A) RT-PCR analysis of herg1 (top panel, 575-bp band), flt1 (second panel, 550-bp band), kdr (third panel, 660-bp band) and gapdh (bottom panel, 138-bp band) transcripts in AML cell lines. Lane ST, molecular weight standard (100 bp; New England Biolabs); lane C+, SH-SY5Y cell line for herg1, HUVEC cell line for flt1 and kdr. (B) hERG1 protein(s) expression on AML cell lines. Cell lysates from AML cell lines, cultured in the presence of serum, were blotted and probed with the anti-pan hERG1 antibody. The top bands, weighing 135 to 150 kDa, refer to the hERG1A isoform; the bottom bands, weighing 75 to 100 kDa, refer to the hERG1B isoform. Reprobing of the membrane with antitubulin antibody is reported in the bottom panel. (C) FLT-1 protein expression in AML cell lines. Cell lysates from cells grown in the presence of serum were blotted and probed with the anti FLT-1 antibody. The arrow indicates the 180 kDa band corresponding to FLT-1. Reprobing of the membrane with antitubulin antibody is reported in the bottom panel. (D) Effect of VEGF165 addition on FLT-1 tyrosine phosphorylation. Proteins extracted from AML cell lines, treated with BSA (250μg/mL) or VEGF165 (100 ng/mL) for 30 minutes, were immunoprecipitated using anti FLT-1 antibody and the blot revealed using anti p-Tyr antibody. Reprobing the membrane with anti FLT-1 antibody is reported in the bottom panel. Preliminary experiments showed that VEGF165 triggered FLT-1 p-Tyr within 15 minutes, reaching a maximum after 30 minutes[b]. IP indicates immunoprecipitation.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2006-02-003772/5/m_zh80140704860001.jpeg?Expires=1769109268&Signature=LNF4KvTSa03d1OzjstTDwLoM5J1LnGFBUpKoT7uIQJjyZiGlXSrNDAJ7jz~5b~N51YTWTsPsgH-WP4CE1S4G29ULHSEUgGCjahfIalMuoMG2ltur3bZoXTr2oFrIM54KT3OzTpSyfOJIGwWwuGtFH8eD5Ni-0VClnYGRgAkn2kkJy2DxKZOg29oUxOj467CTzQOW1oUpudLIvODNbmSJOQX5VM5OtL2SrO8kCx3jY5VU5AbAIMSacq~WBKLd1neckDmG6CZPB9hCI5eWFPW4WgrbXPyR5y~LzyQLc7VBYnAEg1qWB4SDthamJn~RPy65r6KEz-pJ-eXK55ARhG6umw__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 2. Physical association between FLT-1 receptor, hERG1, and β1 integrin subunit in AML cell lines. (A) Co-immunoprecipitation of FLT-1 and hERG1B in AML cell lines. Cell lysates from AML cell lines cultured in the presence of serum were immunoprecipitated with anti FLT-1 antibody; blots were probed with anti-pan hERG1 antibody (top), anti-hERG1B antibody (upper middle), anti-β1 antibody (lower middle), and anti-FLT-1 antibody (bottom). The pan hERG1 antibody recognizes both hERG1A and hERG1B isoforms.27 Note that with the anti-hERG1B antibody only the higher molecular weight bands are visible, whereas the lower bands can be detected only after long exposure of the membrane (as reported in Lee et al33). Bands relative to the hERG1B protein are indicated by the arrows in the top and middle panels. (B) Co-immunoprecipitation of FLT-1, hERG1B, and β1 integrin after addition of VEGF, PlGF, and β1 activating antibody in FLG 29.1 cells. Cells were treated for 30 minutes with BSA (250μg/mL), VEGF165 (100 ng/mL), PlGF (50 ng/mL), the β1 activating antibody TS2/16 (20 μg/mL) or both VEGF165 and TS2/16. Proteins were extracted and immunoprecipitated using anti FLT-1 antibody; blot was sequentially revealed using anti-hERG1B antibody (top), anti-pan hERG1 antibody (upper middle), anti-β1 antibody (lower middle), or with anti FLT-1 antibody (bottom). Inset (left), Densitometric analysis of the amount of hERG1B protein co-immunoprecipitated with FLT-1 after stimulation. The analysis was performed as described in “Materials and methods”; data are the means (± standard error of the mean [SEM]) of 3 separate experiments. *Statistically significant differences between samples are indicated by the horizontal bars. VEGF-treated cells vs BSA-treated cells, Student t test, P = .03; PlGF-treated cells vs BSA-treated cells, Student t test, P = .026; TS2/16-treated cells vs BSA-treated cells, Student t test not significant (NS); VEGF plus TS2/16-treated cells vs BSA-treated cells, Student t test, P = .03; VEGF plus TS2/16-treated cells vs VEGF/PlGF-treated cells, Student t test, NS. Inset (right), densitometric analysis of the amount of β1 integrin co-immunoprecipitated with FLT-1 after stimulation. VEGF plus TS2/16-treated cells vs TS2/16–treated cells, Student t test, P = .03. (C) Co-immunoprecipitation of FLT-1, hERG1B and β1 integrin in AML cell lines after addition of VEGF and β1 activating antibody. AML cell lines were stimulated, and proteins were extracted and immunoprecipitated as noted. Blots were probed with anti pan hERG1 antibody (top), anti-β1A antibody (middle), and anti-FLT-1 antibody (bottom). Inset, Densitometric analysis of the amount of hERG1B protein co-immunoprecipitated with FLT-1 after stimulation with BSA or VEGF plus TS2/16. The analysis was performed as described in Document S1; data are the means (± SEM) of 3 separate experiments. *Statistically significant differences between samples comprised in the horizontal bars, P < .05, Student t test.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/4/10.1182_blood-2006-02-003772/5/m_zh80140704860002.jpeg?Expires=1769109268&Signature=TEvvkPXyFT3RMcSuxt-HOESczcqGHOiqE4fVixpZhtEHhbfAKULqPxamXk-fU2w9XhFnO5VlAd-mCw0SlkDmSVdWLpyIefr9ywaBGW5-ACIaRAfxwj-UjmCyUPUTufC6BUg7HBaJUdyuAaiE5mdRoC92rf2Ccn~3EDCqvnUXWu0cU5tHJD48QnfsJw6F2GCV01mAqMC9VrMthiE-XNKq1LtiCFdH5nKCuOCZ9IvaGKcnFpGAH7wcQSZu~dzyGXib1Lg0tJh5gx0SB3q6b-PhQwxsU6v3H8SGyK17w16oKtVyANt0CdD81P~KgXg329l~roghE14nlwGscgmoRwnalg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
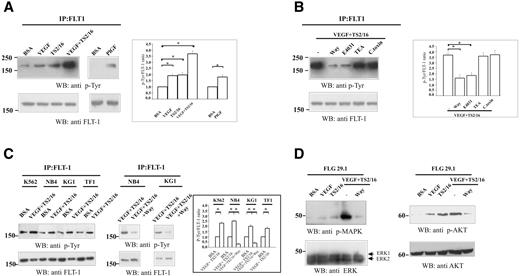
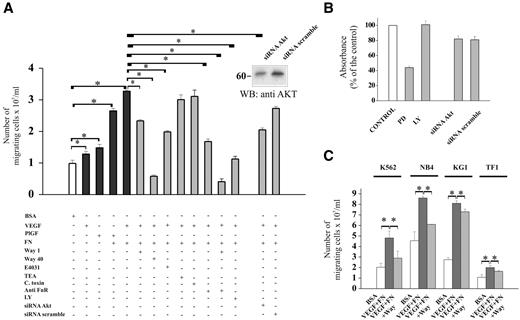
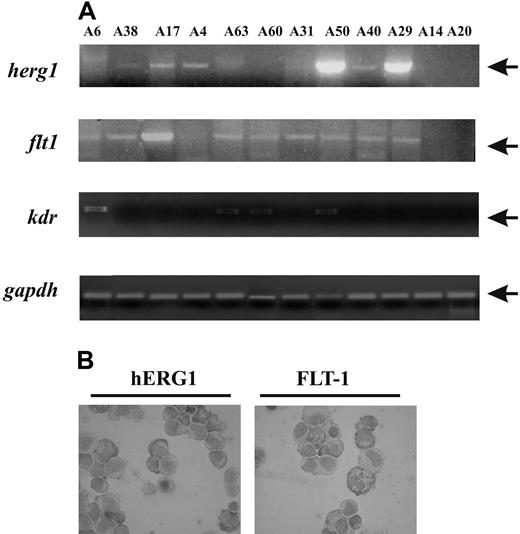
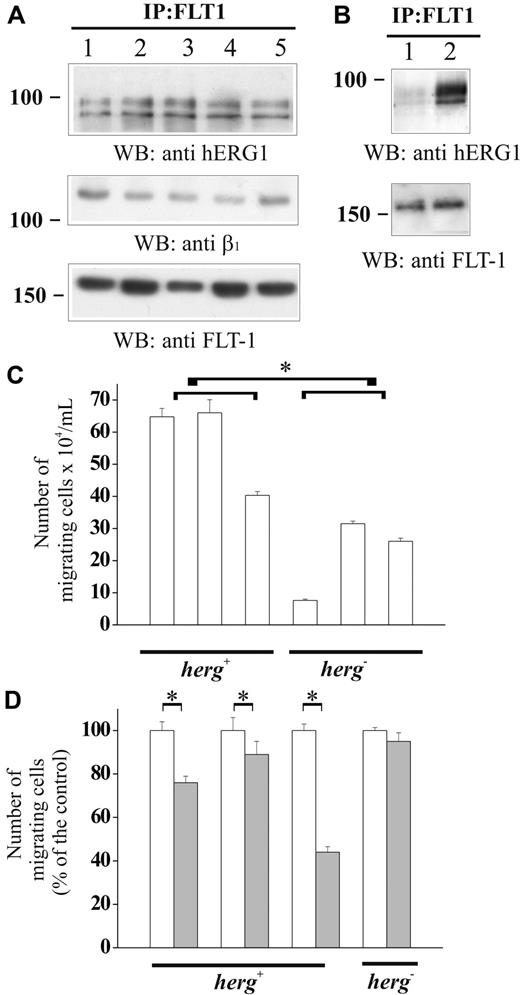
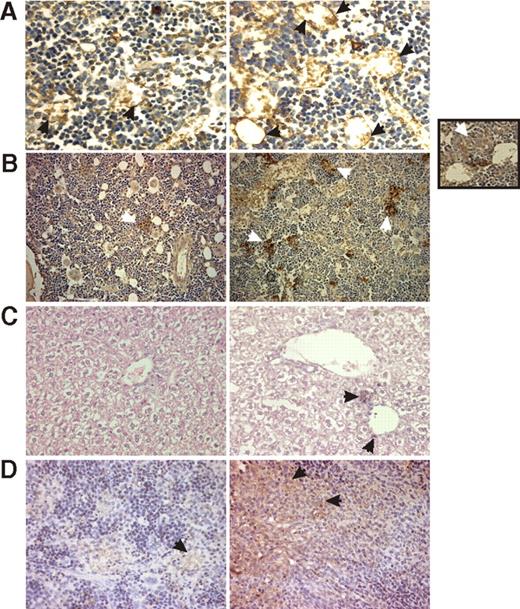
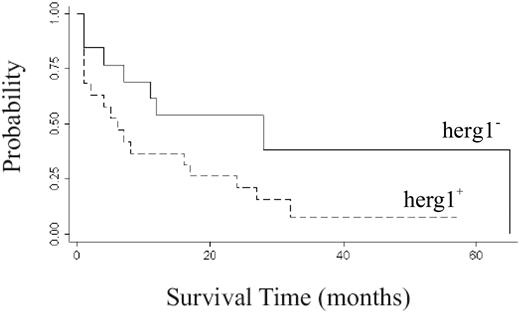
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal