Myeloproliferative disorders (MPDs) are predominantly diseases of older age, with a rapid increase in incidence between the sixth and eighth decades of life. In this issue of Blood, Vickers addresses the reasons for this so-called log-log incidence curve.
Traditionally, explanations have been based on the time taken to acquire multiple oncogenic events, a model which applies well to many solid tumors. However, both chronic myeloid leukemia, with the BCR-ABL fusion gene, and the MPDs, with the JAK2 V617F mutation, are disorders with a log-log age-incidence curve, but a single genetic event that explains many of the pathobiological features of the disease. If these disorders are genuinely caused by one abnormality, why do they take so long to manifest?
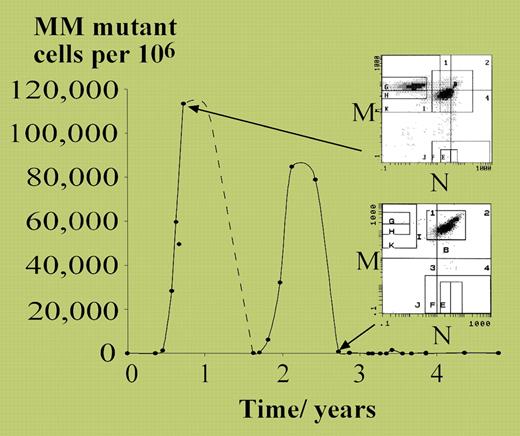
Vickers started with the detailed characterization of a patient with polycythemia vera (PV) who transiently acquired loss of heterozygosity at the MN blood group in a substantial proportion of red cells (see figure). The patient developed 2 separate expansions of MM-homozygous erythrocytes, each peaking at approximately 10% of all red cells, and each presumably representing differentiated progeny of a single progenitor cell. From these observations, it was possible to estimate that only approximately 18 neoplastic stem cells per year were contributing to this patient's polycythemic erythropoiesis. This would accord well with models of stem-cell hierarchy for normal hematopoiesis, in which there appears to be a large pool of quiescent stem cells undergoing rare stochastic progression into more proliferative progenitors.1
MM mutant cells measured over 5 years in a patient with polycythemia rubra vera. See the complete figure in the article beginning onpage 1675.
MM mutant cells measured over 5 years in a patient with polycythemia rubra vera. See the complete figure in the article beginning onpage 1675.
Apart from the insights into PV stem-cell biology these calculations yield, they also provide a basis for understanding the age-incidence curves of MPDs. Under the assumption that JAK2 V617F is necessary and sufficient for the development of PV, Vickers explored how several parameters affecting stem-cell behavior might produce a log-log age-incidence curve. Two major insights were obtained. First, the incidence curve is best approximated by models in which the accumulation of polycythemic stem cells (through symmetrical division) is relatively slow (0.2-0.4 divisions/cell/year), and the loss of stem cells through differentiation or apoptosis is a relatively high fraction (0.8-0.95) of the rate of symmetrical division. Models of chronic myelogenous leukemia (CML) incidence give similar conclusions.2 A slow net accumulation of polycythemic stem cells would explain clinical observations that patients with MPDs often have elevated blood counts for some years before formal diagnosis. The second interesting insight, which follows from the first, is that 60%-99% of all clones initiated by a V617F mutation are predicted to extinguish without causing disease, presumably due to the tenuous existence of small clones of cells with only weakly activating mutations, a process that has been modeled elsewhere.3
There has been extensive discussion about the existence of “pre-JAK2” genetic lesions in V617F-positive disease4,5 and also about a “pre-BCR-ABL” phase of CML. On both issues, the jury remains out. The article by Vickers does not prove that a single mutation causes MPDs, but it does provide a potential explanation for how a single somatic mutation could produce a disease predominantly affecting the elderly.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal