Abstract
Patients with antiphospholipid syndrome are characterized by the association of thrombosis or pregnancy morbidity and the presence of antiphospholipid autoantibodies. Particularly, anti-β2-glycoprotein (β2 GPI) autoantibodies correlate with thrombosis, suggesting an antibody-induced gain of prothrombotic function and/or an antibody-induced loss of antithrombotic function of β2 GPI. In the search for potential antithrombotic properties of β2 GPI, we found that β2 GPI inhibits von Willebrand factor (VWF)–induced platelet aggregation. In addition, platelet adhesion to a VWF-coated surface was decreased by 50% in the presence of β2 GPI (P < .03). β2 GPI binds to the A1 domain of VWF but preferably when the A1 domain is in its active glycoprotein Ibα-binding conformation. Anti-β2 GPI antibodies isolated from a subset of antiphospholipid syndrome patients neutralized the β2 GPI-VWF interactions and thus the inhibitory activity of β2 GPI. In comparison to healthy individuals, the amounts of active VWF in circulation were increased 1.5-fold (P < .001) in patients positive for lupus anticoagulant (LAC) due to anti-β2 GPI antibodies. Thus, β2 GPI is a biologically relevant inhibitor of VWF function by interfering with VWF-dependent platelet adhesion. Anti-β2 GPI autoantibodies neutralize this inhibitory function and are associated with increased levels of active VWF. This mode of action could contribute to the thrombosis and consumptive thrombocytopenia observed in patients with anti-β2 GPI antibodies.
Introduction
Antiphospholipid syndrome is defined by the presence of antiphospholipid antibodies in plasma of patients with thrombotic complications or pregnancy morbidity.1-3 In 1990, it was found that these antiphospholipid antibodies actually recognize phospholipid-binding plasma proteins instead of phospholipids themselves.4,5 In time, a large number of different plasma proteins have been described that are recognized by certain subpopulations of so-called antiphospholipid antibodies. However, there is now a wealth of evidence that particularly autoantibodies directed against β2-glycoprotein I (GPI) expressing lupus anticoagulant (LAC) activity are the antibodies that are related with clinical manifestations observed in antiphospholipid syndrome.6-8
β2 GPI circulates in plasma as a 48-kDa glycoprotein at a relatively high concentration (3 μM). The sequence of β2 GPI is highly conserved among species, suggesting that β2 GPI is of biologic relevance.9 However, the few known individuals with β2 GPI deficiency10 as well as β2 GPI–deficient mice lack obvious hemostatic abnormalities.11 This is surprising in view of various in vitro studies that have suggested that β2 GPI can act as inhibitor of the intrinsic pathway of coagulation and fibrinolysis.12 Furthermore, β2 GPI has previously been reported to cause partial inhibition of ADP-induced platelet aggregation.13 In search for a physiologic role of β2 GPI, we have re-evaluated the effect of β2 GPI on platelet adhesion and aggregation. Both of these events involve a complex process in which several components play a role, including von Willebrand factor (VWF).
VWF is a plasma protein that circulates with a concentration of about 40 nM. VWF is a multimeric protein involved in platelet adherence to the injured vessel wall at high shear rates and platelet-platelet interactions and it is a carrier protein for factor VIII. Adhesion of platelets at high shear rates is a multistep process in which VWF bridges the subendothelial collagen matrix to the GpIb-IX-V receptor complex on platelets.14 VWF and platelets are unable to interact spontaneously. To induce an interaction, a conversion of the VWF A1 domain into a glycoprotein Ibα (GpIbα)–binding conformation is required.15 Activation of the A1 domain can be induced by binding of VWF to a surface or by incubation with modulators such as ristocetin.16,17 In von Willebrand disease (VWD) type 2B, VWF circulates in a GpIbα-binding conformation, due to a gain-of-function mutation in the A1 domain of VWF.
In the present paper, we show that β2 GPI preferentially binds to the GpIbα-binding conformation of the A1 domain of VWF. This property of β2 GPI makes it a biologically relevant inhibitor of platelet-VWF interaction. Autoantibodies against β2 GPI neutralize the inhibitory effect of β2 GPI, which could contribute to the increased thrombotic risk observed in the antiphospholipid syndrome.
Patients, materials, and methods
Proteins
Plasma β2 GPI was purified as described,18 and analysis via gel filtration revealed a single peak at 48 kDa. Plasma-derived VWF was purified from VWF/factor VIII concentrate (Haemate-P; Behringwerke AG, Marburg, Germany) as described.19 VWF was biotinylated with EZ-link sulfo-NS-LC biotin according to the manufacturer's instructions (Pierce Biotechnology, Etten-Leur, the Netherlands). Recombinant full-length β2 GPI was a generous gift from Dr M. Iverson (La Jolla Pharmaceutical Company, San Diego, CA).20 Expression and purification of VWF/A1 (encompassing residues 1261-1468), VWF/A1-R1306Q, wild-type (wt)-VWF, VWF/delta-A1, VWF/delta-A2, and VWF/delta-A3 was performed as described.15,21-23 Sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) showed that all proteins were purified to homogeneity. The multimeric structure of wt-VWF, VWF/delta-A1, VWF/delta-A2, and VWF/delta-A3 was confirmed using 0.1% SDS, 1% agarose gel electrophoresis as described.24
Antibodies
Anti-GpIbα antibody 6D1 was obtained from Dr B. S. Coller (New York, NY). Anti-VWF antibody monoclonal antibody (MoAb) 9 was kindly provided by Dr C. V. Denis (Le Kremlin-Bicêtre, France).25 Llama anti-VWF antibody ALX0081 was described previously26 and inhibits binding of VWF to GpIb. Anti-β2 GPI antibodies 27G7 and 1F12 were kindly provided by Dr J. Arnout (Leuven, Belgium) and antibody 2B2 by Dr A. Tincani (Brescia, Italy). Monoclonal anti-β2 GPI antibodies 3B7 and 4F3 were raised in our laboratory by immunizing mice with plasma-purified β2 GPI.27 HRP-conjugated antibodies against VWF and HRP-conjugated streptavidin were obtained from Dakocytomation (Glostrup, Denmark).
Patients
Thirty patients with a diagnosis of systemic lupus erythematosus (SLE; n = 25), lupuslike disease (LLD; n = 3), or primary antiphospholipid syndrome (PAPS; n = 2) were selected from a group of 198 patients that was described previously28 : 10 patients without LAC activity, 10 patients with LAC activity due to anti–β2 GPI antibodies, and 10 patients with LAC activity not due to anti-β2 GPI antibodies. Patients with SLE meet at least 4 criteria of the American College of Rheumatology for classification of SLE, and patients with LLD meet 1 to 3 of these criteria. PAPS patients have antiphospholipid antibodies and a history of thrombosis in the absence of other signs for a systemic autoimmune disease. Patient characteristics are described in Table 1. The Institutional Review Board of the University Medical Center Utrecht approved this study, and informed consent was obtained from all patients in accordance with the Declaration of Helsinki. Blood samples were taken at an arbitrary visit of patients to the outpatient department and were collected by venipuncture into 3.8% trisodium citrate (0.13 M) as anticoagulant. To obtain platelet-poor plasma (PPP), samples were centrifuged twice at 2000g for 10 minutes and stored at −80°C. All patient samples were measured with 2 LAC assays, an activated partial thromboplastin time (PTT-LA; Diagnostica Stago, Asnières, France), and a dilute Russell viper venom time (dRVVT; Dade-Behring, Marburg, Germany) as described.29 Plasma of 16 healthy individuals was kindly provided by Dr J. C. Meijers (Amsterdam, the Netherlands). For normal pooled plasma (NPP), platelet-depleted plasma of 40 healthy volunteers was pooled and stored in aliquots at −80°C.
Patient characteristics
| . | LAC positive due to anti–β2 GPI antibodies, n = 10* . | LAC positive not due to anti-β2 antibodies, n = 10 . | LAC-negative SLE patients, n = 10 . |
|---|---|---|---|
| SLE | 5 | 10 | 10 |
| LLD | 3 | 0 | 0 |
| PAPS | 2 | 0 | 0 |
| ACL IgG | 9 | 7 | 3 |
| ACL IgM | 4 | 3 | 2 |
| Anti–β2GPI IgG | 10 | 0 | 0 |
| Anti–β2GPI IgM | 3 | 1 | 0 |
| Antiprothrombin IgG | 8 | 5 | 4 |
| Antiprothrombin IgM | 5 | 4 | 0 |
| Thrombosis | 10 | 1 | 3 |
| . | LAC positive due to anti–β2 GPI antibodies, n = 10* . | LAC positive not due to anti-β2 antibodies, n = 10 . | LAC-negative SLE patients, n = 10 . |
|---|---|---|---|
| SLE | 5 | 10 | 10 |
| LLD | 3 | 0 | 0 |
| PAPS | 2 | 0 | 0 |
| ACL IgG | 9 | 7 | 3 |
| ACL IgM | 4 | 3 | 2 |
| Anti–β2GPI IgG | 10 | 0 | 0 |
| Anti–β2GPI IgM | 3 | 1 | 0 |
| Antiprothrombin IgG | 8 | 5 | 4 |
| Antiprothrombin IgM | 5 | 4 | 0 |
| Thrombosis | 10 | 1 | 3 |
ACL indicates anticardiolipin antibodies.
Determined as described in Galli et al.4
Purified IgGs and affinity-purified antibodies from patient plasma
To obtain affinity-purified patient antibodies, total IgG was first purified from patient plasma (positive for either anti-β2 GPI or antiprothrombin antibodies) using protein G-Sepharose as instructed. Purified IgG was then applied to β2 GPI– or prothrombin-coupled Sepharose as described.29 Purity of affinity-purified IgG was checked using SDS-PAGE. No differences were observed between antibodies isolated from patients with SLE, PAPS, or LLD.
Laboratory assays
VWF antigen levels were quantified as described.24 Active VWF in plasma was determined using the AU/VWFa-11–based immunosorbent assay as described.26 The ratio of the slope for different plasma samples over the slope for NPP was calculated and represents the relative amount of active VWF. Antibody AU/VWFa-11 does not interfere with the interaction between VWF and β2 GPI. Plasma samples of patients with acquired thrombotic thrombocytopenic purpura (TTP) were used as positive control.26 VWF propeptide concentrations were measured by an enzyme-linked immunosorbent assay (ELISA).30 The ratio of VWF-propeptide/mature VWF-antigen concentration was calculated using molar concentrations of both proteins. ADAMTS13 activity was determined using the fluorescence resonance energy transfer assay.31 Mean ADAMTS13 activity of 40 healthy donors was 101% (± 40%; mean ± 2SD).
Platelet aggregation with ADP, collagen, and ristocetin-activated VWF
Freshly drawn blood from healthy volunteers was collected into 3.8% trisodium citrate (0.13 M). Donors did not take any medication during 10 days prior to blood collection. Platelet-rich plasma (PRP) was prepared by centrifugation at 150g for 10 minutes (room temperature). Washed platelets were isolated as described.32 Aggregation studies were performed with an optical aggregometer (Chrono-log Corporation, Havertown, PA). PRP was stimulated with various concentrations of ADP (0-5.0 μM), collagen (0-2.0 μg/mL), or ristocetin (0.8-1.3 mg/mL) in the absence or presence of additional β2 GPI (2.2 μM), an anti-β2 GPI antibody with LA activity (27G7), or an anti-β2 GPI antibody without LA activity (1F12). Final concentration of the antibodies was 2.2 μM. Washed platelets were stimulated with ADP (50 μM) and epinephrine (50 μM), collagen (2 μg/mL), or ristocetin-activated VWF (10 μg/mL VWF and 0.3 mg/mL ristocetin) in the presence or absence of 2.2 μM β2 GPI. Anti-GpIbα antibody 6D1, anti-VWF llama antibody ALX0081, and anti-VWF-RGD MoAb 9 were used at a final concentration of 20 μg/mL. Patient antibodies (100 μg/mL) were preincubated for 5 minutes at 37°C with washed platelets and 2.2 μM β2 GPI before stimulation with 2 μg/mL collagen.
Platelet adhesion to VWF
Perfusions over VWF were carried out with reconstituted blood. To obtain reconstituted blood, washed platelets in a 4% human albumin solution were mixed with red cells to a platelet count of 200 × 109 platelets/L and a hematocrit of 0.4 (40%).33 Glass coverslips coated with VWF (37 nM) were incubated with plasma-derived β2 GPI (1.1 or 2.2 μM) for 1 hour at 22°C and blocked with 4% human albumin/PBS. Perfusions with or without the addition of 1 μM β2 GPI were performed at a shear rate of 300 s−1. After perfusion, slides were washed, fixed, and stained30 and platelet adhesion was evaluated using computer-assisted analysis with OPTIMAS 6.0 software (Dutch Vision Systems, Breda, The Netherlands). All perfusions were performed 3 times.
Binding of β2 GPI and VWF
Recombinant β2 GPI (10 μg/mL in Tris-buffered solution [TBS]; 100 mM NaCl, 50 mM Tris, pH 7.4) was immobilized onto a hydrophilic microtiter plate (Maxisorb, NUNC, Denmark) for 1 hour at 37°C. Wells were blocked with 2% nonfat dried milk in TBS/0.1% Tween-20 for 1 hour at 37°C, washed with the same buffer, and incubated with serial dilutions of biotinylated wt-VWF (0-10 μg/mL), biotinylated wt-VWF preincubated with ristocetin (1 mg/mL, 5 minutes at room temperature), VWF/A1, or VWF/A1-R1306Q (0-10 μg/mL). Plates were washed 3 times, and bound VWF was detected using HRP-conjugated streptavidin. Binding of deletion mutation was determined in the absence of ristocetin. A single concentration of wt-VWF, VWF/delta-A1, VWF/delta-A2, or VWF/delta-A3 (20 μg/mL) was allowed to bind to immobilized β2 GPI for 1 hour at 37°C. Bound VWF was detected using HRP-conjugated polyclonal anti-VWF antibodies. HRP activity was measured with o-phenylenediamine as a substrate (Merck, Darmstadt, Germany) and absorbance was measured at 490 nm. Surface plasmon resonance binding assays were performed employing a Biacore3000 system (Biacore AB, Uppsala, Sweden). β2 GPI was immobilized on a CM5 sensor chip.
Competition with VWF, ristocetin, and ristocetin-activated VWF
Specificity of the interaction between β2 GPI and VWF/A1-R1306Q was assessed in an immunosorbent assay, in which β2 GPI (10 μg/mL) was immobilized in microtiter wells (Costar, Cambridge, MA). Wells were blocked with 2% nonfat dried milk in TBS/0.1% Tween-20 for 1 hour at 37°C and incubated with biotinylated VWF/A1-R1306Q (1.1 μg/mL) in the presence or absence of wt-VWF, ristocetin, or wt-VWF preincubated with 1 mg/mL ristocetin (5 minutes at room temperature). After washing, wells were incubated with HRP-conjugated streptavidin (Dakocytomation) and binding was detected by measuring HRP activity.
Competition with anti–β2 GPI antibodies
Wells coated with recombinant β2 GPI were incubated for 1 hour at 37°C with affinity-purified anti-β2 GPI or antiprothrombin antibodies (3 or 20 μg/mL) for 1 hour at 37°C. Subsequently, VWF/A1-R1306Q was allowed to bind β2 GPI in the absence or presence of these antibodies. After washing, wells were incubated with HRP-conjugated streptavidin (Dakocytomation) and binding was detected by measuring HRP activity.
Binding of VWF to immobilized β2 GPI in the presence of patient plasma
β2 GPI (10 μg/mL) was immobilized in microtiter wells (Costar) and wells were blocked with 2% nonfat dried milk in TBS/0.1% Tween-20 for 1 hour at 37°C. After washing, wells were incubated for 2 hours at 37°C with plasma of healthy individuals (n = 29), plasma of patients tested negative for LAC activity (n = 10), patients tested positive for LAC activity not due to anti-β2 GPI antibodies (n = 10), or patients tested positive for LAC activity due to anti-β2 GPI antibodies (n = 10). Wells were subsequently washed, and VWF/A1-R1306Q was allowed to bind to immobilized β2 GPI. Bound VWF was detected using HRP-conjugated streptavidin.
Statistical analysis
Analyses of data were performed using the GraphPad Prism program (GraphPad Prism version 4.0 for Windows, GraphPad Software, San Diego, CA). All data were expressed as mean ± SD. The t test for paired observations was used to compare data obtained in aggregation and perfusion experiments. Statistical analyses of patient-related data were performed with SPSS software package (version 12.0.1, Chicago, Illinois). Differences between patient groups were tested using the Kruskal-Wallis test, followed by the Mann-Whitney U test for calculation of differences between separate groups. Differences were considered statistically significant when P values were less than .05.
Results
β2 GPI selectively inhibits ristocetin-induced platelet aggregation in PRP
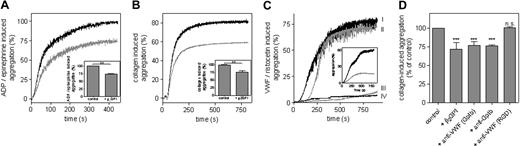
The effect of β2 GPI on platelet aggregation was first addressed in PRP. Aggregation was induced with various concentrations of ADP (0-5.0 μM), collagen (0-2.0 μg/mL), or ristocetin (0.8-1.3 mg/mL). Total aggregation measured after stimulation with the highest concentration agonist was set at 100% for each measurement, and relative aggregation at other agonist concentrations was calculated. Platelet aggregation induced by ADP, collagen, and ristocetin was dose dependent (Figure 1). ADP- and collagen-induced aggregations remained unaffected after addition of extra β2 GPI (2.2 μM) to PRP (Figure 1A,B). In contrast, platelet agglutination induced by ristocetin could only be achieved at higher ristocetin concentrations after addition of β2 GPI (Figure 1C). Plasma contains 3.0 μM β2 GPI. To neutralize endogenous plasma β2 GPI, anti–β2 GPI antibodies were added to PRP. First, addition of anti–β2 GPI antibody 1F12 (which lacks LAC activity) did not affect platelet aggregation by any of the agonists. Addition of monoclonal anti-β2 GPI antibody 27G7 (an antibody with LAC activity) did not affect ADP- or collagen-induced platelet aggregation (Figure 1A,B), whereas ristocetin-induced agglutination was observed at lower ristocetin concentrations (Figure 1C). This indicates that β2 GPI in plasma modulates ristocetin-induced platelet agglutination.
β2 GPI selectively inhibits ristocetin-induced aggregation in PRP. Aggregation of PRP was induced with various concentrations of ADP (0-5.0 μM; A), collagen (0-2.0 μg/mL; B), or ristocetin (0.8-1.3 mg/mL; C) in the absence (■) or presence (●) of β2 GPI (2.2 μM), monoclonal anti-β2 GPI antibody 1F12 without LA activity (□; 2.2 μM), or monoclonal anti-β2 GPI antibody 27G7 with LA activity (2.2 μM; ○). A typical example of each aggregation is shown. All aggregation experiments were performed 3 times using blood of different donors.
β2 GPI selectively inhibits ristocetin-induced aggregation in PRP. Aggregation of PRP was induced with various concentrations of ADP (0-5.0 μM; A), collagen (0-2.0 μg/mL; B), or ristocetin (0.8-1.3 mg/mL; C) in the absence (■) or presence (●) of β2 GPI (2.2 μM), monoclonal anti-β2 GPI antibody 1F12 without LA activity (□; 2.2 μM), or monoclonal anti-β2 GPI antibody 27G7 with LA activity (2.2 μM; ○). A typical example of each aggregation is shown. All aggregation experiments were performed 3 times using blood of different donors.
Aggregation of washed platelets induced by various stimuli is inhibited by β2 GPI
Subsequently, we examined the effect of β2 GPI on aggregation of washed platelets. Since ADP is a weak platelet activator in the absence of plasma fibrinogen, platelets were stimulated with ADP (50 μM) in combination with epinephrine (50 μM) to induce sufficient aggregation. Furthermore, collagen (2 μg/mL) or plasma-derived VWF (10 μg/mL) preincubated with ristocetin (0.3 mg/mL) were used as platelet activators. Addition of 2.2 μM β2 GPI resulted in significantly reduced platelet aggregation, regardless of the agonist (Figure 2). In ADP- or collagen-induced platelet aggregation, addition of β2 GPI selectively reduced the total extent of platelet aggregation, whereas the slope of the aggregation curve remained unaffected. This effect could be mimicked by the addition of an antibody against GpIbα (6D1) or VWF (ALX0081), both inhibiting the interaction between GpIbα and VWF (Figure 2D). Platelet aggregation remained unaffected by the addition of MoAb 9, an antibody that blocks the αIIbβ3-binding site on VWF (Figure 2D). These results suggest that β2 GPI inhibits ADP- and collagen-stimulated aggregation of washed platelets in a VWF-GPIb-dependent manner.
Aggregation of washed platelets induced by various stimuli is inhibited by β2 GPI. Aggregation of washed platelets was induced with ADP (50 μM)/epinephrine (50 μM; A) or collagen (2 μg/mL; B) in the absence (  ) or presence (
) or presence (  ) of β2 GPI (2.2 μM). Relative levels of total aggregation (mean ± SD; n = 3; **P < .001) in the absence or presence of β2 GPI (2.2 μM) are depicted in the insets of panels A and B. (C) Aggregation of washed platelets was induced with ristocetin-activated VWF (10 μg/mL VWF and 0.3 mg/mL ristocetin) in the absence (line I) or presence (line IV) of β2 GPI (2.2 μM). β2 GPI (2.2 μM) was also added in the presence of LAC-negative anti–β2 GPI antibody 1F12 (2.2 μM; line III) or LAC-positive anti–β2 GPI antibody 27G7 (2.2 μM; line II). A typical example of the aggregation curves is shown. All aggregation experiments were performed 3 times, using blood of different donors. (C inset) Ristocetin-independent platelet aggregation induced by VWF/R1306Q (10 μg/mL) in the absence (
) of β2 GPI (2.2 μM). Relative levels of total aggregation (mean ± SD; n = 3; **P < .001) in the absence or presence of β2 GPI (2.2 μM) are depicted in the insets of panels A and B. (C) Aggregation of washed platelets was induced with ristocetin-activated VWF (10 μg/mL VWF and 0.3 mg/mL ristocetin) in the absence (line I) or presence (line IV) of β2 GPI (2.2 μM). β2 GPI (2.2 μM) was also added in the presence of LAC-negative anti–β2 GPI antibody 1F12 (2.2 μM; line III) or LAC-positive anti–β2 GPI antibody 27G7 (2.2 μM; line II). A typical example of the aggregation curves is shown. All aggregation experiments were performed 3 times, using blood of different donors. (C inset) Ristocetin-independent platelet aggregation induced by VWF/R1306Q (10 μg/mL) in the absence ( ) or presence (
) or presence ( ) of β2 GPI (2.2 μM). (D) Total aggregation of washed platelets induced with 2 μg/mL collagen was set at 100%. Relative aggregation (%) in the presence of β2 GPI (2.2 μM), anti-VWF antibody ALX0081 recognizing the GpIbα-binding site (20 μg/mL), anti-GpIbα antibody 6D1 (20 μg/mL), or anti-VWF antibody MoAb 9 recognizing the αIIbβ3-binding site (20 μg/mL) was calculated. Data represent the mean (± SD) of 5 experiments. ***P < .001.
) of β2 GPI (2.2 μM). (D) Total aggregation of washed platelets induced with 2 μg/mL collagen was set at 100%. Relative aggregation (%) in the presence of β2 GPI (2.2 μM), anti-VWF antibody ALX0081 recognizing the GpIbα-binding site (20 μg/mL), anti-GpIbα antibody 6D1 (20 μg/mL), or anti-VWF antibody MoAb 9 recognizing the αIIbβ3-binding site (20 μg/mL) was calculated. Data represent the mean (± SD) of 5 experiments. ***P < .001.
Aggregation of washed platelets induced by various stimuli is inhibited by β2 GPI. Aggregation of washed platelets was induced with ADP (50 μM)/epinephrine (50 μM; A) or collagen (2 μg/mL; B) in the absence (  ) or presence (
) or presence (  ) of β2 GPI (2.2 μM). Relative levels of total aggregation (mean ± SD; n = 3; **P < .001) in the absence or presence of β2 GPI (2.2 μM) are depicted in the insets of panels A and B. (C) Aggregation of washed platelets was induced with ristocetin-activated VWF (10 μg/mL VWF and 0.3 mg/mL ristocetin) in the absence (line I) or presence (line IV) of β2 GPI (2.2 μM). β2 GPI (2.2 μM) was also added in the presence of LAC-negative anti–β2 GPI antibody 1F12 (2.2 μM; line III) or LAC-positive anti–β2 GPI antibody 27G7 (2.2 μM; line II). A typical example of the aggregation curves is shown. All aggregation experiments were performed 3 times, using blood of different donors. (C inset) Ristocetin-independent platelet aggregation induced by VWF/R1306Q (10 μg/mL) in the absence (
) of β2 GPI (2.2 μM). Relative levels of total aggregation (mean ± SD; n = 3; **P < .001) in the absence or presence of β2 GPI (2.2 μM) are depicted in the insets of panels A and B. (C) Aggregation of washed platelets was induced with ristocetin-activated VWF (10 μg/mL VWF and 0.3 mg/mL ristocetin) in the absence (line I) or presence (line IV) of β2 GPI (2.2 μM). β2 GPI (2.2 μM) was also added in the presence of LAC-negative anti–β2 GPI antibody 1F12 (2.2 μM; line III) or LAC-positive anti–β2 GPI antibody 27G7 (2.2 μM; line II). A typical example of the aggregation curves is shown. All aggregation experiments were performed 3 times, using blood of different donors. (C inset) Ristocetin-independent platelet aggregation induced by VWF/R1306Q (10 μg/mL) in the absence ( ) or presence (
) or presence ( ) of β2 GPI (2.2 μM). (D) Total aggregation of washed platelets induced with 2 μg/mL collagen was set at 100%. Relative aggregation (%) in the presence of β2 GPI (2.2 μM), anti-VWF antibody ALX0081 recognizing the GpIbα-binding site (20 μg/mL), anti-GpIbα antibody 6D1 (20 μg/mL), or anti-VWF antibody MoAb 9 recognizing the αIIbβ3-binding site (20 μg/mL) was calculated. Data represent the mean (± SD) of 5 experiments. ***P < .001.
) of β2 GPI (2.2 μM). (D) Total aggregation of washed platelets induced with 2 μg/mL collagen was set at 100%. Relative aggregation (%) in the presence of β2 GPI (2.2 μM), anti-VWF antibody ALX0081 recognizing the GpIbα-binding site (20 μg/mL), anti-GpIbα antibody 6D1 (20 μg/mL), or anti-VWF antibody MoAb 9 recognizing the αIIbβ3-binding site (20 μg/mL) was calculated. Data represent the mean (± SD) of 5 experiments. ***P < .001.
Anti–β2 GPI antibodies abrogate an inhibitory effect of β2 GPI on platelet aggregation
We then investigated if the inhibitory effect of β2 GPI could be neutralized by anti–β2 GPI antibodies. To this end, monoclonal anti–β2 GPI antibodies 27G7 and 1F12 were compared, antibodies with and without LAC activity, respectively. Stimulation of washed platelets with ristocetin-activated VWF induced a total platelet aggregation of 78% (Figure 2C). Total aggregation was reduced to 10% in the presence of β2 GPI (2.2 μM), corresponding with 87% inhibition. Also, platelet aggregation induced by type 2B VWF was inhibited by β2 GPI (Figure 2C inset). β2 GPI-mediated inhibition remained unaltered in the presence of 2.2 μM 1F12. In contrast, addition of 2.2 μM 27G7 completely abolished the inhibitory effect of β2 GPI on platelet aggregation (Figure 2C). This suggests that anti-β2 GPI antibodies that display LAC activity neutralize the inhibitory function of β2 GPI on ristocetin-induced platelet agglutination.
β2 GPI interacts directly with VWF
To test the possibility that β2 GPI interacts directly with VWF, we developed an immunosorbent assay in which β2 GPI was immobilized onto microtiter wells. Biotinylated VWF (0-10 μg/mL) was found to bind poorly to immobilized β2 GPI, as only at concentrations of 5 μg/mL or higher could some signal be detected (Figure 3A). In contrast, binding was markedly enhanced when VWF was preincubated with ristocetin, and half-maximal binding was calculated to be 2.6 μg/mL (Figure 3A). Apparently, VWF needs to be in its active conformation to interact with β2 GPI. Alternatively, ristocetin may induce nonphysiologic VWF aggregates that stick nonspecifically to β2 GPI. To distinguish between these possibilities, we tested binding of VWF/R1306Q, a VWD type 2b mutant that is constitutively in its active conformation. As depicted in Figure 3A, VWF/R1306Q was as efficient as ristocetin-activated VWF in binding to immobilized β2 GPI. Binding of VWF to immobilized β2 GPI was further assessed in a quantitative manner via surface plasmen resonance (SPR) analysis. This confirmed that active VWF is indeed far more efficient than resting VWF in binding to β2 GPI (apparent Kd = 37 (± 8 nM) and 3.3 (± 0.2 μM), respectively.
β2 GPI interacts with the GpIb-binding conformation of the A1 domain of VWF. (A) β2 GPI (10 μg/mL) was immobilized onto microtiter wells (1 h at 37°C) and incubated with different concentrations of biotinylated VWF/R1306Q (▴; 0-10 μg/mL) or biotinylated wt-VWF (0-10 μg/mL) in the absence (○) or presence (●) of ristocetin (1 mg/mL). Bound VWF was detected with HRP-conjugated streptavidin. (B) Wells coated with β2 GPI were incubated with 20 μg/mL wt-VWF, VWF/delta-A1, VWF/delta-A2, or VWF/delta-A3. Bound VWF was detected using HRP-conjugated anti-VWF. **P = .003. (C) Immobilized β2 GPI was incubated with different concentrations of biotinylated VWF/A1 (0-10 μg/mL; ○) or VWF/A1-R1306Q (0-10 μg/mL; ●). Bound VWF was monitored with streptavidin-HRP. (D) β2 GPI-coated wells were incubated with biotinylated VWF/A1-R1306Q (1.1 μg/mL) in the presence of different concentrations of plasma-derived VWF (0-100 μg/mL) without (○) or with (●) ristocetin (1 mg/mL). Bound VWF/A1-R1306Q was monitored using HRP-conjugated streptavidin. All data represent the mean (± SD) of 3 experiments.
β2 GPI interacts with the GpIb-binding conformation of the A1 domain of VWF. (A) β2 GPI (10 μg/mL) was immobilized onto microtiter wells (1 h at 37°C) and incubated with different concentrations of biotinylated VWF/R1306Q (▴; 0-10 μg/mL) or biotinylated wt-VWF (0-10 μg/mL) in the absence (○) or presence (●) of ristocetin (1 mg/mL). Bound VWF was detected with HRP-conjugated streptavidin. (B) Wells coated with β2 GPI were incubated with 20 μg/mL wt-VWF, VWF/delta-A1, VWF/delta-A2, or VWF/delta-A3. Bound VWF was detected using HRP-conjugated anti-VWF. **P = .003. (C) Immobilized β2 GPI was incubated with different concentrations of biotinylated VWF/A1 (0-10 μg/mL; ○) or VWF/A1-R1306Q (0-10 μg/mL; ●). Bound VWF was monitored with streptavidin-HRP. (D) β2 GPI-coated wells were incubated with biotinylated VWF/A1-R1306Q (1.1 μg/mL) in the presence of different concentrations of plasma-derived VWF (0-100 μg/mL) without (○) or with (●) ristocetin (1 mg/mL). Bound VWF/A1-R1306Q was monitored using HRP-conjugated streptavidin. All data represent the mean (± SD) of 3 experiments.
The VWF A1 domain comprises a β2 GPI interactive site
To identify the region within VWF that mediates the interaction with β2 GPI, a number of VWF deletion mutants were analyzed for their capacity to bind β2 GPI. To avoid potential ristocetin-dependent complications, sufficient high concentrations of wt-VWF and the mutants (20 μg/mL) were used without added ristocetin. Binding of VWF/delta-A2 and VWF/delta-A3 was similar compared with binding of wt-VWF (Figure 3B). However, deletion of the A1 domain of VWF resulted in significant decreased binding to immobilized β2 GPI (P = .003, Figure 3B), indicating that the VWF A1 domain dominates the interaction with β2 GPI. This was further examined in binding experiments using recombinant VWF A1 domain, either wt or with the VWD type 2b R1306Q mutation. Again, experiments were performed in the absence of ristocetin. Binding of VWF/A1 to β2 GPI was inefficient at concentrations up to 10 μg/mL (Figure 3C). In contrast, VWF/A1-R1306Q displayed efficient dose-dependent and saturable binding to β2 GPI (half-maximum binding at 1.1 μg/mL; Figure 3C). Specificity of the interaction was challenged in competition assays, in which binding of biotinylated VWF/A1-R1306Q (1.1 μg/mL) was studied in the presence of different concentrations of VWF with or without 1 mg/mL ristocetin. Binding of VWF/A1-R1306Q to immobilized β2 GPI remained unaffected in the presence of up to 100 μg/mL VWF (Figure 3D) and in the presence of up to a 100-fold molar excess of ristocetin (data not shown). However, ristocetin-activated VWF increasingly inhibited binding to β2 GPI up to 87% at a concentration of 100 μg/mL VWF.
β2 GPI inhibits platelet adhesion to VWF under flow conditions
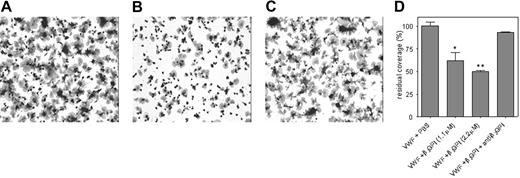
Because VWF-platelet interactions occur under conditions of flow in vivo, the inhibitory effect of β2 GPI was also explored in a perfusion system. Reconstituted blood was perfused over VWF-coated glass coverslips at a shear of 300 s−1. Platelet coverage on VWF in the absence of β2 GPI was set at 100% (Figure 4A,D). Preincubation of the VWF-coated surface with different concentrations of β2 GPI (1.1 μM or 2.2 μM) was associated with a significant and dose-dependent decrease of platelet coverage compared with the control (27.9% ± 2.5% and 50% ± 3.3%, respectively; P < .03, n = 3; Figure 4B,D). The inhibitory effect of β2 GPI was abolished upon the addition of anti-β2 GPI antibody 27G7 with LAC activity (Figure 4C,D). A coverslip coated with only β2 GPI did not support platelet adhesion (not shown).
Platelet adhesion to VWF is inhibited by β2 GPI. Glass coverslips were coated with VWF (10 μg/mL) and subsequently incubated with PBS (A), β2 GPI (2.2 μM; B), or β2 GPI and a monoclonal anti–β2 GPI antibody with LA activity (both 2.2 μM; C). Reconstituted blood supplemented with PBS, β2 GPI, or β2 GPI/anti–β2 GPI antibody was then perfused over these coverslips at a shear rate of 300 s−1. After perfusion, adhered platelets were fixed in PBS/0.5% glutaraldehyde, dehydrated in methanol, and stained with May-Grünwald and Giemsa. Adhered platelets were visualized using light microscopy (Leitz Diaplan; Leica, Rijswijk, the Netherlands) equipped with a CCD camera (JAI-CV-235C, Copenhagen, Denmark) and computer-assisted analysis (AMS 40-10 Saffron, Walden, United Kingdom). Original magnification was 400 × (40×/1.00 NA objective lens). Dark regions represent platelet aggregates. (D) Platelet adhesion was evaluated using computer-assisted analysis and expressed as the percentage of surface covered with platelets. To compare experiments, platelet coverage on VWF in the presence of PBS was set to be 100% and relative coverage was calculated for the other conditions. Data represent the mean (± SD) of 3 experiments. *P < .03, **P < .003.
Platelet adhesion to VWF is inhibited by β2 GPI. Glass coverslips were coated with VWF (10 μg/mL) and subsequently incubated with PBS (A), β2 GPI (2.2 μM; B), or β2 GPI and a monoclonal anti–β2 GPI antibody with LA activity (both 2.2 μM; C). Reconstituted blood supplemented with PBS, β2 GPI, or β2 GPI/anti–β2 GPI antibody was then perfused over these coverslips at a shear rate of 300 s−1. After perfusion, adhered platelets were fixed in PBS/0.5% glutaraldehyde, dehydrated in methanol, and stained with May-Grünwald and Giemsa. Adhered platelets were visualized using light microscopy (Leitz Diaplan; Leica, Rijswijk, the Netherlands) equipped with a CCD camera (JAI-CV-235C, Copenhagen, Denmark) and computer-assisted analysis (AMS 40-10 Saffron, Walden, United Kingdom). Original magnification was 400 × (40×/1.00 NA objective lens). Dark regions represent platelet aggregates. (D) Platelet adhesion was evaluated using computer-assisted analysis and expressed as the percentage of surface covered with platelets. To compare experiments, platelet coverage on VWF in the presence of PBS was set to be 100% and relative coverage was calculated for the other conditions. Data represent the mean (± SD) of 3 experiments. *P < .03, **P < .003.
Anti–β2 GPI antibodies abrogate an inhibitory effect of β2 GPI on platelet aggregation
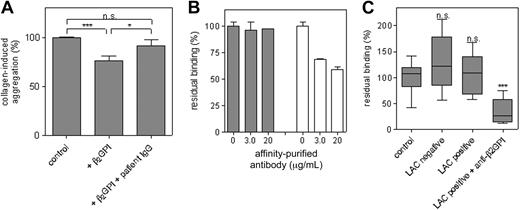
To further understand the physiologic implications of our findings, the effect of patient-derived anti–β2 GPI antibodies on platelet aggregation was investigated. For this, affinity-purified anti-β2 GPI antibodies were purified from plasma of 3 different patients with β2 GPI-dependent LACs. Washed platelets were stimulated with collagen (2 μg/mL) in the presence or absence of β2 GPI. Platelet aggregation was reduced by 24% ± 5% in the presence of β2 GPI (P < .001; Figure 5A). This reduction disappeared upon the addition of patient-derived antibodies, resulting in similar total platelet aggregation compared with the control situation, in which only collagen was added to the platelets. The effect of each patient-derived antibody preparation was studied using isolated platelets of 5 different blood donors, a typical example of which is shown in Figure 5A.
Patient anti–β2 GPI antibodies interfere with the interaction between VWF and β2 GPI. (A) Total aggregation of washed platelets induced with 2 μg/mL collagen was set at 100%. Relative aggregation in the presence of β2 GPI (2.2 μM) and in the presence of β2 GPI and patient IgG of 3 different patients (100 μg/mL) was calculated. All aggregation experiments were performed with platelets of 5 different blood donors. Data represent the mean (± SD) of the effect of antibodies isolated from 1 patient and are representative for the data found with the IgGs from the other 2 patients. *P = .02, ***P < .001. (B) β2 GPI–coated wells were incubated with biotinylated VWF/A1-R1306Q (1.1 μg/mL) in the presence of different concentrations of affinity-purified antiprothrombin antibodies (▩) or anti–β2 GPI antibodies (□). Bound VWF/A1-R1306Q was detected using streptavidin-HRP. Experiments were performed with antibodies purified from plasma of 3 different patients, and a typical example is presented. Data show the mean (± SD) of 3 experiments. (C) β2 GPI–coated wells were incubated with plasma of healthy individuals (n = 16), plasma of patients negative for LAC activity (n = 10), plasma of patients positive for LAC activity not due to anti–β2 GPI antibodies (n = 10), or plasma of patients positive for LAC activity due to anti–β2 GPI antibodies (n = 10) for 2 hours at 37°C. After washing, wells were incubated with biotinylated VWF/A1-R1306Q (1.1 μg/mL) for 30 minutes at 37°C. Bound VWF was detected with streptavidin-HRP. Residual binding determined in 3 independent experiments is presented in a box-and-whiskers plot. ***P < .001.
Patient anti–β2 GPI antibodies interfere with the interaction between VWF and β2 GPI. (A) Total aggregation of washed platelets induced with 2 μg/mL collagen was set at 100%. Relative aggregation in the presence of β2 GPI (2.2 μM) and in the presence of β2 GPI and patient IgG of 3 different patients (100 μg/mL) was calculated. All aggregation experiments were performed with platelets of 5 different blood donors. Data represent the mean (± SD) of the effect of antibodies isolated from 1 patient and are representative for the data found with the IgGs from the other 2 patients. *P = .02, ***P < .001. (B) β2 GPI–coated wells were incubated with biotinylated VWF/A1-R1306Q (1.1 μg/mL) in the presence of different concentrations of affinity-purified antiprothrombin antibodies (▩) or anti–β2 GPI antibodies (□). Bound VWF/A1-R1306Q was detected using streptavidin-HRP. Experiments were performed with antibodies purified from plasma of 3 different patients, and a typical example is presented. Data show the mean (± SD) of 3 experiments. (C) β2 GPI–coated wells were incubated with plasma of healthy individuals (n = 16), plasma of patients negative for LAC activity (n = 10), plasma of patients positive for LAC activity not due to anti–β2 GPI antibodies (n = 10), or plasma of patients positive for LAC activity due to anti–β2 GPI antibodies (n = 10) for 2 hours at 37°C. After washing, wells were incubated with biotinylated VWF/A1-R1306Q (1.1 μg/mL) for 30 minutes at 37°C. Bound VWF was detected with streptavidin-HRP. Residual binding determined in 3 independent experiments is presented in a box-and-whiskers plot. ***P < .001.
Patient anti–β2 GPI antibodies inhibit binding of β2 GPI to VWF
Patient-derived anti–β2 GPI antibodies were also examined for their effect on binding of VWF to β2 GPI. To this end, biotinylated VWF/A1-R1306Q was allowed to bind to immobilized β2 GPI in the presence of affinity-purified anti–β2 GPI antibodies. Affinity-purified antiprothrombin antibodies isolated from plasma of 3 different patients with LAC-positive SLE were used as control. Binding of VWF/A1-R1306Q remained unaffected in the presence of antiprothrombin antibodies. Addition of anti–β2 GPI antibodies significantly reduced binding dose-dependently to a maximum of 41% ± 4% in the presence of 20 μg/mL antibody. Due to restricted amounts of these affinity-purified antibodies, we were unable to study the effect of higher antibody concentrations. A typical example of the effect of patient antibodies on the interaction between VWF/A1-R1306Q and β2 GPI is shown in Figure 5B.
Similar experiments were performed using plasma of patients instead of purified antibodies. Immobilized β2 GPI was incubated with plasmas of healthy individuals (n = 16), plasmas tested positive for anti–β2 GPI antibodies and LAC activity (n = 10), plasmas with LAC activity not due to anti–β2 GPI antibodies (n = 10), and plasmas from patients with SLE who tested negative for LAC activity (n = 10). After incubation, wells were washed and incubated with biotinylated VWF/A1-R1306Q. Binding of VWF/A1-R1306Q after incubation with plasma of healthy individuals was set at 100% (Figure 5C), and relative binding of VWF/A1-R1306Q after incubation with patient plasma was calculated. After incubation with plasma tested negative for LAC activity or plasma positive for LAC activity not due to anti–β2 GPI antibodies, binding of VWF/A1-R1306Q remained unaffected (127% ± 51%, n = 10 and 106% ± 39%, n = 10, respectively) compared with normal plasma. However, binding was strongly decreased after incubation of immobilized β2 GPI with plasma of patients positive for anti-β2 GPI–dependent LAC activity (34% ± 24%, n = 10, relative to normal plasma; P < .001). This indicates that the interaction between β2 GPI and VWF is influenced by the presence of anti–β2 GPI antibodies in plasma.
Increased amounts of active VWF in patients with LAC activity caused by anti–β2 GPI antibodies
The results of the previous experiments suggest that β2 GPI interacts with the GpIbα-binding conformation of VWF and that patient antibodies neutralize this interaction. To support these observations, we quantified active VWF levels in plasma of patients with the antiphospholipid syndrome. First, plasma samples were diluted to obtain equal VWF antigen concentrations. Subsequently, serial dilutions were applied to AU/VWFa-11–coated wells, and bound active VWF was monitored using HRP-conjugated polyclonal anti-VWF antibodies. The slope of the initial linear part of the binding curve was calculated for each individual sample (Figure 6A). The ratio of the slope for different plasma samples over the slope for NPP was calculated and represents the relative amount of active VWF (Figure 6B). Plasma samples of patients with acquired TTP were included as positive control. In plasma of patients who tested positive for LAC activity not due to anti–β2 GPI antibodies (n = 10), the amounts of active VWF were similar to the amounts found in normal plasma (0.9 ± 0.2, Figure 6B). In contrast, in patients who tested positive for LAC activity due to anti–β2 GPI antibodies (n = 10), an increased amount of circulating active VWF was measured (1.4 ± 0.2; P < .0001; Figure 6B). Rearrangement of the patients into a group with (n = 11) and a group without (n = 9) thrombosis showed that the amount of active VWF was significantly increased in the group with thrombosis (P = .002; Figure 6C).
Increased amounts of active VWF in patients with anti–β2 GPI antibodies. (A) Plasma of a healthy individual (■), a patient positive for lupus anticoagulant not due to anti–β2 GPI antibodies (□), and a patient positive for lupus anticoagulant due to anti–β2 GPI antibodies (n = 10, ●) were diluted to obtain similar amounts of VWF antigen. Indicated concentrations of VWF were added onto the AU/VWFa-11–coated wells. The initial linear part of the binding curve is shown. Plasma sample of a patient with acquired TTP is included as positive control (○). (B) The ratio of the slopes of the initial linear parts for the different plasma samples (n = 29 for controls and n = 10 for each patient group) over the slope for NPP was calculated and represents the relative amount of circulating active VWF. Relative amounts of active VWF are presented in a box-and-whiskers plot. (C) Patients were divided into those with (n = 11) or without (n = 9) thrombosis, and relative amounts of active VWF in these groups are presented in a box-and-whiskers plot.
Increased amounts of active VWF in patients with anti–β2 GPI antibodies. (A) Plasma of a healthy individual (■), a patient positive for lupus anticoagulant not due to anti–β2 GPI antibodies (□), and a patient positive for lupus anticoagulant due to anti–β2 GPI antibodies (n = 10, ●) were diluted to obtain similar amounts of VWF antigen. Indicated concentrations of VWF were added onto the AU/VWFa-11–coated wells. The initial linear part of the binding curve is shown. Plasma sample of a patient with acquired TTP is included as positive control (○). (B) The ratio of the slopes of the initial linear parts for the different plasma samples (n = 29 for controls and n = 10 for each patient group) over the slope for NPP was calculated and represents the relative amount of circulating active VWF. Relative amounts of active VWF are presented in a box-and-whiskers plot. (C) Patients were divided into those with (n = 11) or without (n = 9) thrombosis, and relative amounts of active VWF in these groups are presented in a box-and-whiskers plot.
ADAMTS13 activity and endothelial-cell activation
In order to find a possible explanation for the increased amounts of active VWF in the circulation, a number of other parameters were measured. The ratio between VWF propeptide and mature VWF antigen is indicative for acute endothelial-cell activation.30 The levels of VWF antigen were equally increased in all patient groups and propeptide levels were concomitantly increased. The propeptide-mature VWF ratio therefore remained within normal range in all groups (Table 2). Furthermore, we measured ADAMTS13 activity, since this enzyme determines length and reactivity of VWF multimers. Mean ADAMTS13 activity was within the normal range in all patient groups (Table 2).
Propeptide and VWF antigen levels and activity of ADAMTS13
| . | LAC positive due to anti–β2GPI antibodies . | LAC positive due to antiprothrombin antibodies . | LAC-negative SLE patients . |
|---|---|---|---|
| VWF antigen, μg/mL | 23.4 ± 9.4 | 18.3 ± 4.5 | 22.1 ± 9.1 |
| VWF propeptide, μg/mL | 0.17 ± 0.10 | 0.13 ± 0.05 | 0.16 ± 0.14 |
| Propeptide/mature VWF, mol/mol | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.08 ± 0.02 |
| ADAMTS13 activity, % | 81.7 ± 28.5 | 71.7 ± 31.1 | 66.5 ± 20.8 |
| . | LAC positive due to anti–β2GPI antibodies . | LAC positive due to antiprothrombin antibodies . | LAC-negative SLE patients . |
|---|---|---|---|
| VWF antigen, μg/mL | 23.4 ± 9.4 | 18.3 ± 4.5 | 22.1 ± 9.1 |
| VWF propeptide, μg/mL | 0.17 ± 0.10 | 0.13 ± 0.05 | 0.16 ± 0.14 |
| Propeptide/mature VWF, mol/mol | 0.09 ± 0.03 | 0.09 ± 0.03 | 0.08 ± 0.02 |
| ADAMTS13 activity, % | 81.7 ± 28.5 | 71.7 ± 31.1 | 66.5 ± 20.8 |
All data represent mean (± SD), n = 10. Normal ranges are as follows: VWF antigen, 6.1-14.2 μg/mL; VWF propeptide, 0.6-1.6 μg/mL; propeptide/mature VWF ratio, 0.04-0.21; and ADAMTS13 activity, 61%-142 %.
Discussion
It is almost an axiom in hemostasis that every active (co)factor or enzyme has its own inhibitor(s) to prevent exaggerated hemostasis and unwanted thrombus formation. The first step in thrombus formation at higher shear stresses is binding of VWF to an injured vessel wall. This step uncovers a binding site for platelet GpIb within the VWF molecule. So far, direct inhibitors of the VWF-GpIb interaction have not been identified. Here, we report that β2 GPI interacts with VWF, albeit that this interaction is of low affinity (Figure 3). In contrast, β2 GPI binds with high affinity to VWF in its GpIbα-binding conformation (ie, active VWF; Figure 3). When in complex with β2 GPI, VWF is unable to induce platelet agglutination (Figure 2) or to act as an adhesive surface for platelets at physiologic shear rates (Figure 4), suggesting that β2 GPI prevents VWF from binding to GpIb. Indeed, the binding site for β2 GPI is located within the VWF A1 domain (Figure 3), which also comprises the GpIbα interactive site.15 Apparently, β2 GPI has the capacity to interfere with VWF-platelet interactions.
Part of the VWF molecules that are secreted from endothelial cells can be qualified as being ultralarge.34 These ultralarge (UL) VWF multimers are peculiar in that they are in a GpIb-binding conformation and therefore bind platelets spontaneously.35 To avoid the release of these active multimers in the circulation, UL multimers are subject to proteolysis by ADAMTS13.36 ADAMTS13-mediated proteolysis not only reduces the size of multimers but probably also induces conformational changes, the combination of which converts active VWF into an inactive derivative. As such, ADAMTS13 has been considered to be an important regulator of VWF function. In view of the inhibitory actions of β2 GPI toward the VWF-platelet interaction, it is tempting to speculate that β2 GPI is a novel player in the regulation of VWF function.
Direct binding studies indicate that the VWF A1 domain dominates the interaction with β2 GPI and binding to VWF lacking the A1 domain (VWF/delta-A1) was strongly reduced, whereas isolated recombinant A1 domain was able to interact with β2 GPI (Figure 3). Binding of full-length VWF to β2 GPI was considerably enhanced in the presence of ristocetin (Figure 3). Of note, ristocetin may potentially induce nonspecific binding. However, this seems highly unlikely with respect to the VWF-β2 GPI interaction that we describe in the current manuscript. First, binding is also observed in the absence of ristocetin, albeit with lower efficiency (Figure 3B). Second, mutant VWF/R1306Q is as efficient as ristocetin-activated VWF without the need for any ristocetin (Figure 3A). VWF/R1306Q comprises a VWD type 2b mutation that forces the protein in an active GpIb-binding conformation, allowing spontaneous interactions with platelets. Indeed, VWF/R1306Q induced spontaneous platelet aggregation, an event that was inhibited in the presence of β2 GPI (Figure 2 inset). This not only confirms the inhibitory capacity of β2 GPI but also shows that β2 GPI-active VWF interactions occur in solution. Thus, β2 GPI has the potential to neutralize active VWF in the circulation. Obviously, this inhibitory capacity has its limitations because thrombus formation does occur upon vascular injury in healthy persons with normal β2 GPI levels. Further studies are therefore needed to elucidate the precise mechanism by which β2 GPI contributes to the regulation of active VWF in vivo. It is important to mention in this regard that little if any active VWF circulates in plasma under normal conditions,26 making it unlikely that β2 GPI-VWF complexes circulate in plasma, since VWF is in its resting conformation.
β2 GPI is a major target for autoantibodies in antiphospholipid syndrome.2,3 Inhibition of VWF-platelet interactions by β2 GPI could be neutralized by β2 GPI antibodies, either monoclonal or patient derived (Figures 1,2, 4,5). Importantly, only antibodies displaying LAC activity were able to counteract β2 GPI-mediated inhibition. Antibodies that display LAC activity are known to correlate strongly with thrombotic complications.6 The underlying mechanism in this regard is poorly understood. One possible mechanism involves the induction of novel prothrombotic properties, for instance via activation of endothelial cells or leukocytes.2,3 On the other hand, anti–β2 GPI autoantibodies may interfere with intrinsic antithrombotic functions of this protein,10,11 and probably both mechanisms may occur simultaneously. The data obtained in the present study point to anti–β2 GPI antibodies with LAC activity to interfere with a previously unrecognized antithrombotic function. This may contribute to the increased thrombotic risk associated with the presence of this subset of anti-β2 GPI antibodies.
Having identified β2 GPI as an inhibitor of active VWF, a function that is neutralized in the presence of LAC-positive anti-β2 GPI antibodies, the next step was to analyze how these antibodies affect the presence of active VWF in the circulation. Using a well-defined group of antiphospholipid syndrome patients, we established that levels of active VWF were selectively increased in patients with anti-β2 GPI–dependent LAC activity (Figure 6B). The extent by which these levels were increased was similar to that found in patients with an acquired ADAMTS13 deficiency (acquired TTP).26 Since these levels predispose to thrombosis in TTP patients, it seems conceivable that similar levels of active VWF in antiphospholipid syndrome patients are associated with an increased thrombotic risk as well. Of course, additional studies using larger patients cohorts are needed to enforce this possibility. However, it is encouraging that in the small patient group included in the patient study, levels of active VWF were significantly increased in those patients with a history of thrombosis compared with those without thrombotic complications (Figure 6C).
The absence of β2 GPI is not a known risk factor for thrombosis, albeit that only a few families with β2 GPI deficiency have been described. Indeed, β2 GPI deficiency is extremely rare in the white population, whereas a few Japanese families have been described who harbored a frame-shift mutation resulting in the absence of detectable levels of β2 GPI.10 Although the siblings described did not suffer from thrombotic manifestations, a significant number of their ancestors died of stroke. While not having sufficient information to draw firm conclusions, it is possible that absence of β2 GPI is only a risk factor for thrombosis in conjugation with other challenges such as accelerated atherosclerosis at older age. In this respect, it is understandable that mice lacking β2 GPI do not spontaneously develop thrombosis.11
One intriguing issue is that we and others have recently reported that anti–β2 GPI autoantibodies induce binding of β2 GPI to GpIb, the platelet receptor for VWF.37,38 Apparently, when β2 GPI autoantibodies are present, the binding site for VWF on the β2 GPI molecule is lost, whereas an interactive site for GpIb is acquired. As such, β2 GPI is converted from an antagonist of platelet adhesion into an element that supports platelet adhesion.
We have previously shown that the amount of active VWF correlates strongly with a decreased platelet number in VWD type 2B.26 Thrombocytopenia is often observed in patients with antiphospholipid syndrome. It is noteworthy that in older studies on additional risk factors for thrombosis in antiphospholipid syndrome, besides antiphospholipid antibodies, only thrombocytopenia was found to be an independent risk factor for thromboembolic complications.39
In conclusion, we have revealed that active VWF comprises a binding site for β2 GPI. This interaction seems to prevent binding of VWF to platelets, thereby inhibiting thrombus formation. Antibodies recognizing β2 GPI compete for the interaction between active VWF and β2 GPI, resulting in loss of the inhibitory protective function of β2 GPI. The increased amount of circulating, unprotected, active VWF is able to interact spontaneously with platelets, which may contribute to the onset of thrombosis and consumptive thrombocytopenia in patients with a β2 GPI–dependent LAC.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Drs M. T. Pennings and R. T. Urbanus for providing us with purified plasma β2 GPI. M. Compier, L. Rens, and M. IJsseldijk are gratefully acknowledged for excellent technical assistance.
This study was supported by grants from the Netherlands Organization for Health Research and Development (ZON-MW 902-26-290) and from the Landsteiner Foundation of Blood Transfusion Research (LSBR grant no. 0114). The financial support of the Noaber foundation and the Hoge Dennen foundation is gratefully acknowledged.
Authorship
Contribution: J.J.J.H. performed experiments, analyzed data, and wrote and approved the manuscript; P.J.L. designed the research, performed experiments, analyzed data, and wrote and approved the manuscript; B.d.L. performed experiments, analyzed data, and approved the manuscript; R.H.W.M.D. provided valuable reagents for the study and approved the manuscript; R.F. designed research, analyzed data, and approved the manuscript; and P.G.d.G. designed research, analyzed data, and wrote and approved the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
J.J.J.H., P.J.L., and B.d.L. contributed equally to this study.
Correspondence: Philip G. de Groot, Laboratory for Thrombosis and Haemostasis, Department of Clinical Chemistry and Haematology, G03.550, University Medical Center Utrecht, PO Box 85500, 3584 CX Utrecht, the Netherlands, e-mail: Ph.G.deGroot@umcutrecht.nl.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal