Abstract
Naive B cells are ineffective antigen-presenting cells and are considered unable to activate naive T cells. However, antigen-specific contact of these cells leads to stable cell pairs that remain associated over hours in vivo. The physiologic role of such pairs has not been evaluated. We show here that antigen-specific conjugates between naive B cells and naive T cells display a mature immunologic synapse in the contact zone that is absent in T-cell–dendritic-cell (DC) pairs. B cells induce substantial proliferation but, contrary to DCs, no loss of L-selectin in T cells. Surprisingly, while DC-triggered T cells develop into normal effector cells, B-cell stimulation over 72 hours induces regulatory T cells inhibiting priming of fresh T cells in a contact-dependent manner in vitro. In vivo, the regulatory T cells home to lymph nodes where they potently suppress immune responses such as in cutaneous hypersensitivity and ectopic allogeneic heart transplant rejection. Our finding might help to explain old observations on tolerance induction by B cells, identify the mature immunologic synapse as a central functional module of this process, and suggest the use of naive B-cell–primed regulatory T cells, “bTregs,” as a useful approach for therapeutic intervention in adverse adaptive immune responses.
Introduction
Naive B cells are poor antigen-presenting cells (APC) for naive T cells. In many systems, they have been dispensable for CD4+ T-cell priming.1,2 Yet antigen presentation by naive B cells is not an immunologic null event. Animals can be rendered tolerant toward antigens presented by naive B cells.3 Indeed, evidence suggests that B cells can take part in antigen presentation via major histocompatibility complex (MHC) II molecules4-7 and might even be required to reach full T-cell effector potential.8
We have previously shown that naive B cells, despite low efficiency on a per cell basis, when loaded with specific peptide antigen can induce antigen-specific proliferation in naive T cells.9 However, the biophysics of the underlying T-B contact was very different from bona fide T-cell activation by dendritic cells (DCs).10 While T-cell contacts to DCs were dynamic and sequential, contacts to B cells were mostly very stable in vitro and in vivo.9,11 The functional significance of this discrepancy is not clear.
Information transfer between T cell and APC during cell-cell interaction is characterized by the formation of a supramolecular assembly of signaling and adhesion molecules termed immunologic synapse (IS).12 Its exact function is still debated yet it is generally accepted as a T-cell activating structure.13,14,14 A mature IS presents with a distinguished central part of the supramolecular activation cluster (c-SMAC) enriched in signaling molecules like T-cell receptor (TCR) and MHC, and a peripheral part (p-SMAC) enriched in adhesion molecules.15 The formation of a mature IS takes between 30 to 60 minutes.12 Encounters between DCs and T cells in early and late phases of immune responses, however, last only for several minutes.16,17 This is too short to form a mature IS. In contrast, antigen-specific contacts to naive B cells invariably last several hours.9 It is unclear whether a mature synapse can form under these circumstances.
Based on these arguments, we reasoned that the molecular organization of the IS between T cells and naive B cells might be different from the one found in T-DC pairs. We also wanted to test the potential consequences for the resulting activated CD4+ T cells. To further evaluate the outcome of antigen-specific CD4+ T-cell stimulation by naive B cells, we investigated the molecular structure of the underlying IS and the phenotype of CD4+ T cells after in vitro contact with specific-antigen loaded naive B cells or DCs.
We present evidence that stimulation of naive T cells by naive B cells results in formation of a mature IS which is absent in DC–T-cell pairs. In addition, while DC stimulation generates classical effector T cells, naive B-cell–activated T cells show regulatory capacity in vitro and in vivo.
Materials and methods
Mice
DO11.10 mice carrying a transgenic TCR that recognizes a peptide of chicken ovalbumin (AA 323-339), pOVA, in context with I-Ad,18 and OT-II mice carrying a TCR that recognizes the identical peptide in context with I-Ab19 were used as T-cell sources. C57BL/6 (H-2b) and Balb/c (H-2d) mice as sources of B cells and bone marrow DCs (bmDCs, DCs) were purchased from Harlan Germany. IL-10 KO mice were a kind gift from Werner Müller, Helmholtz Centre for Infection Research (HZI), Braunschweig, Germany. Animals were housed under specific pathogen-free conditions and treated according to institutional guidelines. All animal experiments were approved by the animal protection committee of the regional board of Braunschweig, Germany.
Cell preparation
Naive CD4+ T cells from spleens of DO11.10 or OT-II mice were enriched by negative isolation via immunomagnetic depletion (Miltenyi, Bergisch-Gladbach, Germany) to purities of more than 90% and 80%, respectively (75%-85% TCR transgenic). Separation of naive splenic B cells from Balb/c or C57BL/6 mice resulted in purities between 90% to 95% B220/MHCII+/+cells. Pre-activated B cells were generated by coculture of naive B cells with T cells in a 1:1 to 1:2 (T:B) ratio. After 72 hours, pre-activated B cells were isolated as described above. BmDCs (DCs) were generated in 8-day cultures as described20 and activated with 20ng/mL LPS (E.coli 0111, B4; Sigma, Deisenhofen, Germany) during the final 2 days of culture. Cell lines secreting murine granulocyte-macrophage colony-stimulating factor or IL-4 were kindly provided by Thomas Blankenstein, Max Delbrück Center for Molecular Medicine (MDC), Berlin. pOVA (10 μg/mL; Peptide Core facility, HZI, Braunschweig, Germany) was added to the cultures of B cells, and DCs overnight and for the final 4 hours, respectively, before use.
In vitro T-cell activation assays
Primary T-cell activation.
Naive T cells (105) were cocultured in 96-well round bottom plates (Nunc, Roskilde, Denmark) with 104 mature, antigen-loaded DCs or 105 antigen-loaded B cells unless noted otherwise. The liquid medium used was RPMI-based and fetal calf serum (FCS) supplemented (Gibco, Los Angeles, CA). For some experiments, 3-D collagen gels were used.10 Gels were digested by type VII collagenase (30 U/100mL gel for 30 minutes at 37°C; Sigma). For estimation of T-cell proliferation, naive T cells were stained with 5,6-carboxyfluorescein diacetate, succinimidyl ester (CFSE, 0.5 μM; Molecular Probes, Leiden, Netherlands). To obtain B-cell– and DC-primed T cells, cocultures underwent immunomagnetic depletion of non-CD4+ cells after 72 hours. Naive T cells were CFSE-labeled and cocultured with DCs as described in “Cell preparation.” Varying numbers of B-cell– (TofB) or DC (TofDC)–primed T cells were added to test their inhibitory effects on naive T cells, typically at a ratio of 1:1 (T primed: T naive). Readouts for proliferation or activation markers were taken at 72 hours by flow cytometry.
Transwell experiments
Transwell experiments were done in 24-well plates (Nunc) using 106 naive, CFSE-labeled CD4+ T cells plus 105 antigen-loaded mature DCs. In addition, 106 TofDCs, TofBs, or naive T cells were either directly added or placed in transwell chambers (Millicell, 0.4 μm; Millipore, Billerica, MA) in the same well. Readouts for proliferation were taken at 72 hours by flow cytometry.
Adoptive transfer and flow cytometric determination of in vivo distribution of T cells
Primed T cells were dually stained with CFSE and with Cell Tracker Orange (CTO), 5-(and-6)-(((4-chloromethyl)benzoyl)amino) tetramethylrhodamine-mixed isomers (CMTMR; Molecular Probes), or carbocyanine (DiD, Vybrant DiD; Molecular Probes). T cells, 5 × 106 per type, were intravenously injected into tail veins. At indicated timepoints, mice were anesthesized with Isoflurane (Deltaselect, Pfullingen, Germany), bled by retroorbital venous puncture and killed to obtain spleen, Peyer Patches, and mesenteric, inguinal, popliteal, axillar, and cervical lymph nodes. Flow cytometric analysis of transferred cells was done based on the dual dye labeling.
Flow cytometry of surface activation marker and intracellular cytokines
The anti-CD11c was from Caltag (Burlingame, CA); antibodies against other surface markers came from BD Pharmingen (San Jose, CA); CCL19-huFc-Protein and anti-huFc were from eBioscience (San Diego, CA); anti-IL-2, anti-IL-4, IL-10, and IFNγ were from Invitrogen (Karlsruhe, Germany); the Fix&Perm Kits with Golgiplug from BD Pharmingen; anti-Foxp3 (FJK 16s) and staining kits were from eBioscience. Flow cytometry was performed on a BD Pharmingen FacsCalibur. Mean fluorescence intensity (MFI) was shown against the secondary antibody control.
ELISA
OptEIA kits (BD Pharmingen) were used for determining IL-2, IL-10, and IFNγ.
Immunohistology/confocal microscopy
Fixed cell pairs were generated by mixing DO11.10 T cells with pOVA loaded naive Balb/c B cells in a ratio of 1:1, with pre-activated B cells 1:5, and with mature DCs 1:5 (T cell: APC), in 96-well round bottom plates in FCS-supplemented RPMI-based media for 2 hours. Cells were transferred onto Poly-L-Lysine (Sigma)–coated cover slips, fixed with warm 4% paraformaldehyde and permeabilized with 0.1% Triton X-100 (Sigma) in PBS. Blocking with 5% horse serum in 1% bovine serum albumin (BSA, Sigma). Staining in 1% BSA using 2 U/mL Alexa-488-Phalloidin (Molecular Probes), 5 μg/mL biotinylated anti-DO11.10 TCR (KJ1.26, Caltag), and 5 μg/mL Streptavidin-Cy3 (Dianova, Hamburg, Germany). Samples were mounted in Mowiol (Calbiochem, Darmstadt, Germany) with 2.5 mg/mL N-Propylgallate (Sigma) as antifading reagent. Analysis was done with an Olympus FluoView1000 confocal microscope (Olympus, Hamburg, Germany) performing 3-D-reconstructions of z-stacks (mean width 0.2 μm/slice), scanning with 40 μs/Pixel using Kalman mode. Images were taken with a PLAPO 100×/TIRFM-SP, NA 1.45 oil immersion lens at room temperature. Reconstructions were made with the Olympus FluoView Software FV10-ASW, version 1.6 as well as with LSM Image Browser version 4.0 (Zeiss, Jena, Germany).
2-photon-microscopy of explanted lymph nodes
Naive T cells, TofBs, and B cells were labeled with 1 μM CFSE, CTO, or 7-amino-4-chloromethylcoumarin (CMAC, also called CTB, Cell Tracker Blue, Molecular Probes). Inversion of colors had no effect on results. TofBs (107) were injected intravenously into the tail veins of Balb/c mice 48 hours before imaging; 2 × 107 naive T cells and 3 × 107 naive B cells were applied 24 hours before imaging. Inguinal lymph nodes were removed and immediately subjected to imaging in pH-stabilized culture media constantly warmed to 37°C. Three-D 2-photon microscopy was performed with a MaiTai laser (Spectra-Physics, Darmstadt, Germany) running at 800 nm, a multibeam scanhead (LaVision Biotech, Bielefeld, Germany) and an Olympus BX51WI stage equipped with a XLUMPL 20×/0.95 NA water dipping lens. Image detection was made with a cooled CCD-camera (Imager Intense; LaVision, Goettingen, Germany). For estimation of TofB distribution, RGB z-stacks of 200 × 200 μm images were recorded in 48 steps of 3μm. A total of 932 cells were quantified in 14 extended focus projections within B follicles (> 90% B cells), in T-cell zones (> 80% T cells), and in mixed T-B areas, respectively.
Contact hypersensitivity
Mice were sensitized by painting 100 μL 2,4-dinitrofluorobenzene (DNFB) solution (Sigma; 0.5% in acetone/olive oil 4:1) on the shaved backs on day 0 as described.21 On day 5, the left ear was challenged by applying 12 μL 0.3% DNFB, and the right ear was treated with acetone/olive oil alone. Ear swelling was measured in a blinded fashion with a spring-loaded micrometer (Mitutoyo, Hamburg, Germany) 48 hours after challenge (day 7). Contact hypersensitivity was determined as amount of swelling of the hapten-challenged ear compared with thickness of vehicle-treated ear and was expressed in μm (mean ± standard deviation [SD]). Mice that were ear-challenged without previous sensitization served as negative controls. The immunoregulatory effect of adoptively transferred primed T cells was tested by intravenously transferring 0.5 to 5 × 106 MACS-separated DC–primed or B-cell–primed T cells at day 1 (for inhibition of priming) or at day 4 (for inhibition of challenge), or at d 10 (for inhibition of priming at later stage after transfer). In the latter case, mice received an (un)specific antigen boost at day 1. Control mice received media only. Each group consisted of 8 mice.
Allogeneic heart transplantation
Heterotopic vascularized heart transplantation was carried out according to the method of Corry et al.22 Male C57BL/6 (H-2b) and female BALB/c (H-2d) were used as donors and recipients, respectively. Briefly, animals were anesthetized with isoflurane. The donor pulmonary artery was anastomosed to the recipient inferior vena cava and the donor ascending aorta was anastomosed to the recipient abdominal aorta. 2 experimental groups of 6 animals each were evaluated: Recipients of the control group were treated with saline and recipients of the TofB group received 5 × 106 cells via tail vein injection 24 hours prior to transplantation. No other immunosuppressive treatment was administered. Graft function was assessed by daily palpation. Rejection was defined as the lack of palpable cardiac contraction.
Statistical analysis
Survival analysis was performed using a Kaplan-Meier estimator as part of the statistical package in GraphPad Prism 4 (GraphPad Software, San Diego, CA). For all other statistics, Student t test was applied. Significance levels used are indicated in “Results.”
Results
Naive B cells signal to naive T cells via a mature immunologic synapse
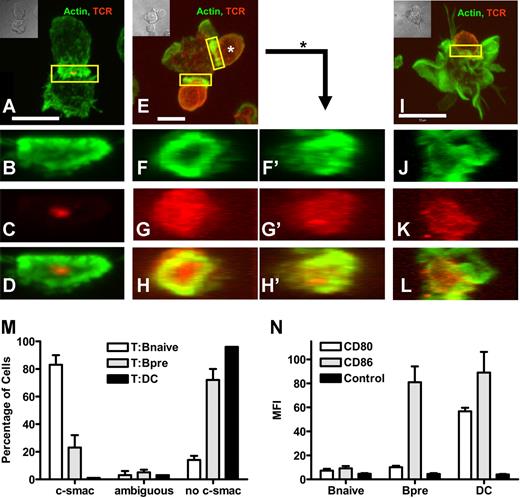
To study the molecular makeup of the interface between naive T cells and different APC, we established pairs of chicken ovalbumin-specific T cells18 interacting with either naive splenic B cells or mature bmDCs, each loaded with specific peptide. Since the initial description of the mature immunologic synapse,15 a large number of molecules were found to be characteristically located at c- and p-SMAC, respectively. The prototypic marker for c-SMAC is the TCR complex.15 A more recent study shows that f-actin is highly enriched in p-SMAC.23 Thus we chose to study the spatial distribution of f-actin and the peptide-specific TCR. T cells (Figure 1A,E,I) and naive B cells (Figure 1A) recruited the majority of their cortical actin cytoskeleton toward the contact zone, while DCs (Figure 1I) showed a very prominent actin scaffold that was not enhanced at the T-cell contact. While with T cells contacting naive B cells (Figure 1A) almost the entire TCR was located at the B-cell contact zone, surprisingly, the TCR in T cells contacting DCs remained scattered over the entire T-cell surface (Figure 1I). A three-dimensional reconstruction confirmed that in the contact plane to DCs no c-SMAC enrichment of TCR or p-SMAC enrichment of actin was detectable (Figure 1J,L). However, the contact zone to B cells presented as a structure with highly concentrated actin in the p-SMAC and the majority of TCR signal in the c-SMAC (Figure 1B-D). This pattern was consistently found in almost 90% of T naive B pairs, while more than 90% of the DC contacts did not show a mature IS (Figure 1M). To assess the impact of the activation state of the B cell, we also analyzed pairs of preactivated B cells and T cells (Figure 1E-H′). Preactivated B cells showed an intermediate phenotype of IS formation with heterogenous IS structures. In some cases this plasticity of IS phenotype could even be found on a single preactivated B cell contacting multiple naive T cells (Figure 1F-H and F'-H'). Quantitatively, the majority (80%) of preactivated B cells did not show a c-SMAC-(mature)–type of IS, while 20% did (Figure 1M). As parameter for the activation level we measured expression of the primary costimulatory molecules CD80 and CD86 and found an increase in CD86 in preactivated B-cells and an increase of CD80 and CD86 in DCs compared with naive B cells (Figure 1N). IS formation in B-T pairs was antigen-specific as no IS formation was seen when B cells were left unloaded (data not shown). Taken together, naive B cells loaded with specific antigenic peptide engage naive T cells under formation of a mature IS.
Naive B cells naive T cells form a mature immunologic synapse. Mature bmDC or naive or preactivated splenic B cells were loaded with pOVA. Cells were mixed with naive DO11.10 T cells and fixed after 120 minutes of interaction. Immunohistologic staining was performed for actin (green) and the clonotypic TCR (red). Individual pairs of T cells and naive B cells (A-D), T cells and preactivated B cells (E-H') or T cells and DC (I-L), respectively, were analyzed by 2-color confocal microscopy making Z-stacks over the entire range of a cell pair. T cells in contact with B cells have the entire TCR signal at the contact plane (A), while T cells attached to DC show TCR staining scattered over the body (I). T cells in contact with preactivated B cells show both accumulation of TCR and distribution over the whole T-cell surface (E). A three-dimensional reconstruction showing the en face view of the contact plane reveals no preferential accumulation of TCR in the contact with DC, see Actin-stain (J), TCR-stain (K), and merged image (L). In contrast, in T-cell–naive B-cell pairs the TCR signal is focused in the center of contact (B-D). Interactions with preactivated B cells show partially the formation of a mature IS (F-H'). Shown is a T-B interface resembling a mature IS, yet with substantially scattered TCR (T cell at 6 o'clock, F-H); and an interface between the same preactivated B cells interacting with a second T cell, showing almost no IS segregation (T cell at 2 o'clock, F'-H'). The scale bar defines 5 μm (A,E) and 10 μm (I), respectively. (M) Quantitative analysis of 300 random individual cell pairs for the distribution of TCR in the contact plane. (N) Mean Fluorescence Intensity (MFI) analysis of the expression of costimulatory molecules on APC. While naive splenic B cells show only a sparse expression of CD80 and CD86, CD86 is clearly up-regulated upon B-cell activation. DCs exhibit a high amount of CD80 as well as CD86 on their surface. (M,N) Bars represent mean plus or minus SD.
Naive B cells naive T cells form a mature immunologic synapse. Mature bmDC or naive or preactivated splenic B cells were loaded with pOVA. Cells were mixed with naive DO11.10 T cells and fixed after 120 minutes of interaction. Immunohistologic staining was performed for actin (green) and the clonotypic TCR (red). Individual pairs of T cells and naive B cells (A-D), T cells and preactivated B cells (E-H') or T cells and DC (I-L), respectively, were analyzed by 2-color confocal microscopy making Z-stacks over the entire range of a cell pair. T cells in contact with B cells have the entire TCR signal at the contact plane (A), while T cells attached to DC show TCR staining scattered over the body (I). T cells in contact with preactivated B cells show both accumulation of TCR and distribution over the whole T-cell surface (E). A three-dimensional reconstruction showing the en face view of the contact plane reveals no preferential accumulation of TCR in the contact with DC, see Actin-stain (J), TCR-stain (K), and merged image (L). In contrast, in T-cell–naive B-cell pairs the TCR signal is focused in the center of contact (B-D). Interactions with preactivated B cells show partially the formation of a mature IS (F-H'). Shown is a T-B interface resembling a mature IS, yet with substantially scattered TCR (T cell at 6 o'clock, F-H); and an interface between the same preactivated B cells interacting with a second T cell, showing almost no IS segregation (T cell at 2 o'clock, F'-H'). The scale bar defines 5 μm (A,E) and 10 μm (I), respectively. (M) Quantitative analysis of 300 random individual cell pairs for the distribution of TCR in the contact plane. (N) Mean Fluorescence Intensity (MFI) analysis of the expression of costimulatory molecules on APC. While naive splenic B cells show only a sparse expression of CD80 and CD86, CD86 is clearly up-regulated upon B-cell activation. DCs exhibit a high amount of CD80 as well as CD86 on their surface. (M,N) Bars represent mean plus or minus SD.
CD4+ T-cell stimulation by specific antigen-loaded naive B cells results in activated T cells with an unusual phenotype
We now asked whether the phenotype of activated CD4+ T cells based on such diverse IS structures would differ. We therefore focused on naive B cells and mature DCs as these APC had shown the most extreme forms of IS structure (Figure 1). We first looked at the induction of T-cell proliferation and changes in characteristic activation markers. After 72 hours of in vitro stimulation with pOVA-loaded naive C57BL/6 B cells, TCR-transgenic OT-II T cells showed clear induction of proliferation (Figure 1A). When appropriate numbers of APC were used (B:T = 1:1; DC:T = 1:10), equivalent proliferation levels were reached. In addition, efficient and, within the type of APC, equivalent up-regulation of CD69 and CD44 (not shown) as well as CD25 (Figure 2A top row) was induced. Interestingly, under identical conditions, only DCs, but not B cells, induced down-regulation of CD62L in T cells driven into proliferation (Figure 2A bottom row). CD62L is a key molecule mediating T-cell entry into lymph nodes; its down-regulation is a hallmark for effective CD4+ T-cell activation.24 This effect was seen for all peptide concentrations tested, while DCs showed a similar behavior only at very low antigen concentrations in vitro (Figure 2B). Loading APCs with intact protein instead of peptide led to similar results (data not shown). As it was possible that the observed discrepancy between effective induction of proliferation and ineffective down-regulation of CD62L was an artifact of the two-dimensional liquid culture system, we looked at T-cell priming in a three-dimensional collagen matrix featuring a network of type I collagen fibres, the major component of the extracellular matrix. Cell behavior measured in this three-dimensional matrix closely resemble values observed in vivo.9-11,25-27 Again, under conditions where equivalent and effective T-cell activation (based on CD69 levels) could be observed, DCs effectively down-regulated CD62L while B cells did not (Figure 2C). Next we wondered whether the induced phenotype of B cells was dominant over simultaneous DC stimulation. Thus, we looked at T-cell activation after contact with a mixture of B cells and DCs. As seen in Figure 2D, in the presence of 10% (compared with B cells) DCs, CD62L was down-regulated, indicating the dominance of T-cell activation by DCs over the one by B cells when both cell types were used as APC. This supremacy could be detected even at DC numbers as low as 1% (data not shown). The dominating role of DCs as APC in vivo has been demonstrated extensively.28 In conclusion, exclusive stimulation of T cells by naive B cells results in activated T cells that retain high levels of CD62L.
Antigen-specific T-cell activation by B cells leads to generation of T cells with aberrant phenotype. Naive splenic CD4+ T cells were cocultured in vitro with mature DC or B cells, both loaded with specific antigenic peptide. After 72 hours, proliferation and expression of surface markers were determined by fluorescence-assisted cell sorting (FACS). (A) Specific antigenic peptide loaded B cells efficiently induce proliferation and up-regulation of CD25 in CD4+ T cells. In contrast to DC, however, B cells fail to down-regulate CD62L. (B) B cells remain unable to down-regulate CD62L even at high antigen doses. (C) The failure to downmodulate CD62L levels by B cells is not restricted to a two-dimensional environment as it is also seen in a three-dimensional environment using a collagen matrix. (D) CD62L is down-regulated in cultures in which DC were added (10% of corresponding B-cell number). The data shown are representative of 3 to 5 independent experiments.
Antigen-specific T-cell activation by B cells leads to generation of T cells with aberrant phenotype. Naive splenic CD4+ T cells were cocultured in vitro with mature DC or B cells, both loaded with specific antigenic peptide. After 72 hours, proliferation and expression of surface markers were determined by fluorescence-assisted cell sorting (FACS). (A) Specific antigenic peptide loaded B cells efficiently induce proliferation and up-regulation of CD25 in CD4+ T cells. In contrast to DC, however, B cells fail to down-regulate CD62L. (B) B cells remain unable to down-regulate CD62L even at high antigen doses. (C) The failure to downmodulate CD62L levels by B cells is not restricted to a two-dimensional environment as it is also seen in a three-dimensional environment using a collagen matrix. (D) CD62L is down-regulated in cultures in which DC were added (10% of corresponding B-cell number). The data shown are representative of 3 to 5 independent experiments.
In vivo, B-cell–stimulated T cells, TofBs, preferentially migrate to lymph nodes
Our result that naive B-cell–activated T cells, TofBs, did not down-regulate CD62L made us investigate possible implications of this finding in vivo. As CD62L mediates the entry of T cells into lymph nodes,29 we reasoned that TofBs would predominantly migrate there. Indeed, when adoptively transferring intravenously equal numbers (5 × 106) of dually stained TofBs or DC-primed T cells (TofDCs), respectively, TofBs clearly outnumbered transferred TofDCs in peripheral lymph nodes. In contrast, both populations were found at equal proportions in blood and spleen, where CD62L is not involved in the entry of cells30 (Figure 3A-C). We also measured expression of CCR7, another important homing receptor for entry into lymph nodes. Using a monoclonal antibody as well as a CCL19-Fc fusion protein we found that TofBs retain CCR7 at levels similar to those in naive T cells (data not shown). However, as TofDCs showed comparable CCR7 expression, this molecule is unlikely to account for the differences in homing we observed.
In vivo B-cell–primed T cells (TofB) preferentially migrate to lymph nodes. T cells primed by peptide-loaded DC and B cells were differentially labeled with live cell dyes. Equal numbers of TofDCs and TofBs were adoptively transferred intravenously, and distribution of the cells was determined 24 hours after transfer by organ removal and FACS analysis. (A) T cells for adoptive transfer were labeled with CFSE; TofDCs were additionally labeled with CTO and TofBs with DiD. (B) Upon organ analysis after transfer, equal numbers of TofDCs and TofBs as transferred can be found in the spleen, whereas in lymph nodes TofBs clearly dominate. (C) Distribution of the transferred T-cell populations in lymphatic organs 24 hours after transfer. When analysis was performed at day 7 after transfer, a similar pattern was observed (data not shown). The data shown are representative of at least 3 independent experiments. Within the lymph nodes, TofBs migrate to T-cell areas and are excluded from B-cell follicles. A simplified drawing illustrates lymph node architecture showing B-cell areas (blue), T-cell dominated areas [green], and the imaged area shown above (red rectangle) at the transition of the 2 zones. (D) An image stitched together of 6 individual extended focus projections of each 200 × 200 × 144 μm shows the transition from a B-cell follicle dominated by B cells (blue) to a mixed T-B zone containing mostly naive T cells (green). Note that TofBs (red) are found only in the T-cell area. Scale bar: 100 μm. (E) A quantification of TofB distribution analyzing 14 image stacks from within and outside B-cell follicles. TofBs clearly show T-cell homing behavior and localize within T-cell–dominated areas but are excluded from B-cell follicles. Bars represent mean plus or minus SD.
In vivo B-cell–primed T cells (TofB) preferentially migrate to lymph nodes. T cells primed by peptide-loaded DC and B cells were differentially labeled with live cell dyes. Equal numbers of TofDCs and TofBs were adoptively transferred intravenously, and distribution of the cells was determined 24 hours after transfer by organ removal and FACS analysis. (A) T cells for adoptive transfer were labeled with CFSE; TofDCs were additionally labeled with CTO and TofBs with DiD. (B) Upon organ analysis after transfer, equal numbers of TofDCs and TofBs as transferred can be found in the spleen, whereas in lymph nodes TofBs clearly dominate. (C) Distribution of the transferred T-cell populations in lymphatic organs 24 hours after transfer. When analysis was performed at day 7 after transfer, a similar pattern was observed (data not shown). The data shown are representative of at least 3 independent experiments. Within the lymph nodes, TofBs migrate to T-cell areas and are excluded from B-cell follicles. A simplified drawing illustrates lymph node architecture showing B-cell areas (blue), T-cell dominated areas [green], and the imaged area shown above (red rectangle) at the transition of the 2 zones. (D) An image stitched together of 6 individual extended focus projections of each 200 × 200 × 144 μm shows the transition from a B-cell follicle dominated by B cells (blue) to a mixed T-B zone containing mostly naive T cells (green). Note that TofBs (red) are found only in the T-cell area. Scale bar: 100 μm. (E) A quantification of TofB distribution analyzing 14 image stacks from within and outside B-cell follicles. TofBs clearly show T-cell homing behavior and localize within T-cell–dominated areas but are excluded from B-cell follicles. Bars represent mean plus or minus SD.
To assess more precisely the localization of TofBs within the lymph node we used two-photon microscopy imaging studies. We found that adoptively transferred TofBs resided primarily in T-cell zones and were basically excluded from B-cell follicles (Figure 3D,E). Thus, B-cell–primed T cells migrate preferentially to the T-cell zones of peripheral lymph nodes where cell entry from the circulation is known to be mediated by CD62L.
TofBs (but not TofDCs) regulate priming of naive T cells
The propensity of TofBs to home into lymph nodes might affect their involvement in immune responses. Classical CD4+ T-cell activation results predominantly in short-lived effector cells that home to peripheral sites of infection/inflammation.31-33 In addition, a fraction of activated cells turns into effector memory (EM) and central memory (CM) cells.34 While EM are long-lived in peripheral organs, CM remain localized in central lymphatic tissue, most notably the spleen.34 TofBs share a CD4+ CD62L+ phenotype with CM, yet do not preferentially home to the spleen (Figure 3). We also tested for a recently identified key memory T-cell marker, CD127 (IL-7 receptor alpha chain), which is important for cells of the CD8+35,36 and to some degree also of the CD4+ lineage.37,38 TofBs showed very low levels of CD127 on days 3 and 7 of the priming process (data not shown). Collectively, there was no evidence that TofBs belong to the memory T-cell lineage. Interestingly, there are reports that absence of expression of human CD127 correlates with a regulatory function.39 Such regulatory T cells of natural occurrence (nTreg) are characterized by CD4, CD25 double positivity.40 In addition, the subgroup of CD62Lhigh nTregs was shown to have regulatory activity in autoimmune processes.41,42 Thus, we wanted to see whether the CD4+/CD25+/CD62Lhigh TofBs also show regulatory capacity.
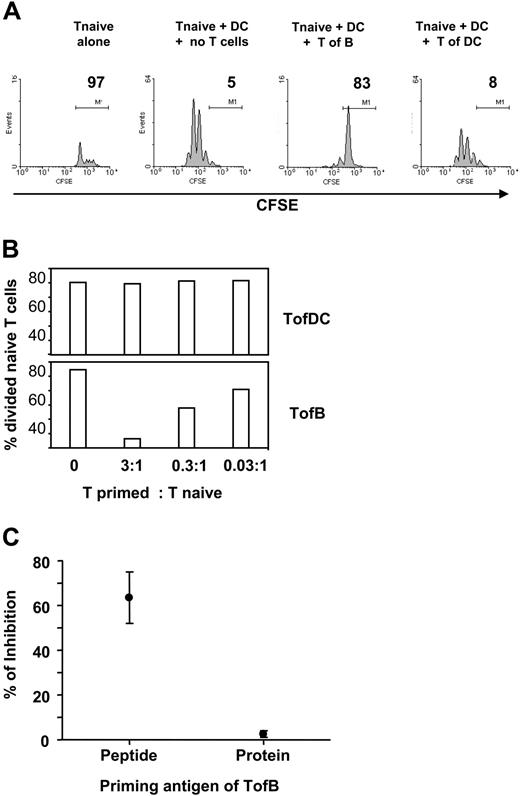
The key activity of Tregs is the (down)regulation of an immune response, the inhibition of T-cell proliferation.43 We therefore tested the effect of TofBs on fresh, naive T cells during priming by specific-antigen-loaded DCs in vitro. Indeed, TofBs inhibited almost completely the induced proliferation in naive T cells, whereas TofDCs did not (Figure 4A). The effect could be titrated and depended on the ratio of Treg versus T naive (Figure 4B). The inhibitory effect was seen upon priming by peptide but not by protein antigen (Figure 4C). The reason for the inability of protein to induce Tregs is most likely the well-known inherent incapability of B cells to take up and present complete protein. More than 100 times higher amounts of protein on a weight basis than peptide were necessary to induce T-cell proliferation in our system (data not shown). Thus, the great majority of T cells remain naive (based on low expression of CD69, CD25, CD44, and absence of CFSE-diluted proliferation, data not shown) after contact to B cells loaded with protein and, therefore, do not show regulatory capacity.
TofBs show regulatory capacity and suppress the proliferation of naive T-cells. Differentially primed T cells were added to in vitro cocultures of naive, CFSE-labeled CD4+ T cells and mature DCs loaded with antigenic pOVA. At 72 hours, proliferation of the naive T cells was determined by FACS. (A) Upon addition of TofBs but not of TofDCs, proliferation of naive T cells determined by CFSE-dilution is markedly inhibited. Equal numbers of cells (105) were used. No proliferation is observed when naive T cells are cultured in the absence of DCs. (B) The regulatory effect of TofBs can be titrated. When TofBs were added at a ratio of 3:1 to naive T cells, the effect was more pronounced. At a ratio of 0.03:1, minor effects can be seen. In contrast, TofDCs do not show inhibition even at a 3:1 ratio over naive T. (C) The inhibitory effect can only be observed after B-cell loading with specific peptide (pOVA) but not with complete protein (Ovalbumin). The data shown are representative of 2 (panel B) to 5 (panels A,C) independent experiments. Dots represent the mean plus or minus SD.
TofBs show regulatory capacity and suppress the proliferation of naive T-cells. Differentially primed T cells were added to in vitro cocultures of naive, CFSE-labeled CD4+ T cells and mature DCs loaded with antigenic pOVA. At 72 hours, proliferation of the naive T cells was determined by FACS. (A) Upon addition of TofBs but not of TofDCs, proliferation of naive T cells determined by CFSE-dilution is markedly inhibited. Equal numbers of cells (105) were used. No proliferation is observed when naive T cells are cultured in the absence of DCs. (B) The regulatory effect of TofBs can be titrated. When TofBs were added at a ratio of 3:1 to naive T cells, the effect was more pronounced. At a ratio of 0.03:1, minor effects can be seen. In contrast, TofDCs do not show inhibition even at a 3:1 ratio over naive T. (C) The inhibitory effect can only be observed after B-cell loading with specific peptide (pOVA) but not with complete protein (Ovalbumin). The data shown are representative of 2 (panel B) to 5 (panels A,C) independent experiments. Dots represent the mean plus or minus SD.
We also included pre-activated B cells in our studies and compared them with naive B cells and DCs in their capacity to induce regulatory T cells. We found that T cells generated by antigen-specific contact with pre-activated B cells (TofBpre) inhibit the proliferation of naive T cells in vitro to some degree, yet they do so clearly less than TofB(naive). In addition, TofBs retain considerably less CD62L than TofB(naive) (data not shown). Taken together and in accordance with our findings on IS formation (Figure 1), TofBpre show an intermediate phenotype with characteristics between those found in TofB(naive) and TofDCs.
Taken together, we provide evidence that naive B-cell–primed T cells, TofBs, can suppress the proliferation of naive T cells in vitro.
B cells substantially induce IL-2 and IFNγ in CD4+ T cells following antigen-specific contact. B cells are the source of IL-10 found in T-B cocultures but the generation of regulatory T cells is IL-10 independent
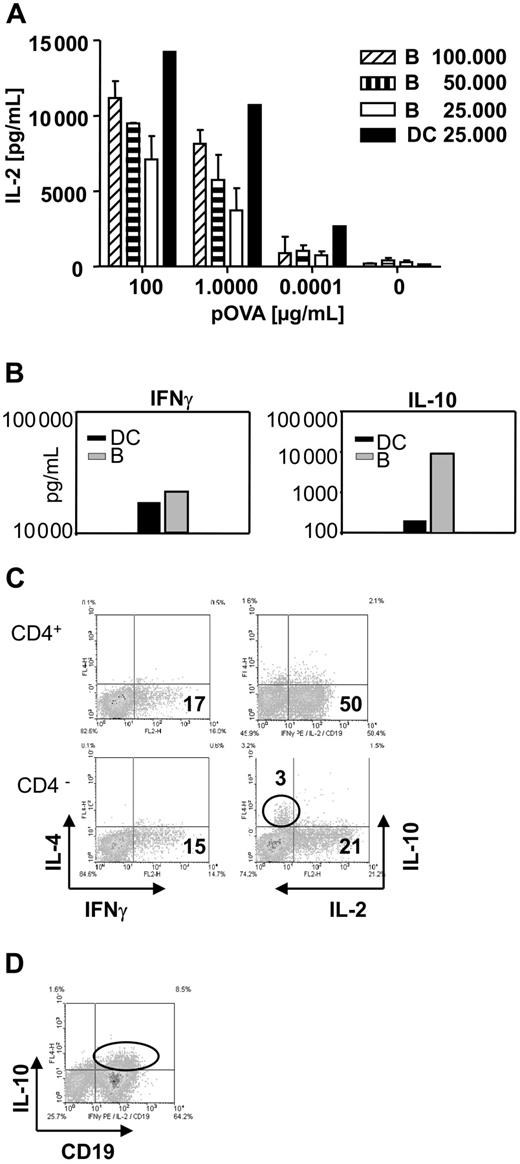
To explore the mechanism by which TofBs exert their regulatory effect, we characterized the cytokine profile in T-APC-cocultures. In T-DC– and T-B–cocultures, substantial and approximately equivalent amounts of IL-2 and IFNγ were present (Figure 5A,B). When identical numbers of B cells and DCs were used in their respective cocultures, approximately half the amount (1415 pg/mL vs 720 pg/mL) of IL-2 was induced after B-cell contact (Figure 5A). Interestingly, we found much higher levels of IL-10 in the supernatants of T-B–cocultures than in supernatants of T-DC–cocultures (Figure 5B). This appeared noteworthy at this point as one regulatory T-cell subset (TR cells) is known to act via secretion of IL-10.44,45 However, when tested at single cell level by intracellular cytokine staining, B cells were revealed as the source of IL-10 (Figure 5C,D). Previously, IL-10 produced by APC was found to be involved in induction of a tolerogenic T-cell phenotype.46-48 However, subsequent testing in our study with B cells from IL-10 KO mice revealed that IL-10 was dispensable for the generation of TofBs with regulatory phenotype (data not shown). Thus, B-cell priming leads to substantial induction of Th1 cytokines in T cells. B cells are the source of concomitantly found IL-10 but the mechanism of induction of T cells with regulatory phenotype is IL-10 independent.
Cytokine profile during the activation of T cells by B cells shows substantial amounts of Th1 cytokines but also high levels of B-cell–derived IL-10. Supernatants were taken from DC–T-cell and from B-cell–T-cell cocultures and analyzed for cytokines by ELISA. Cells were analyzed for intracellular cytokines by FACS at 48 hours of culture. (A) B cells induce substantial amounts of IL-2 in supernatants of B-cell–T-cell cocultures, but DCs are at least twice as powerful on a per-cell basis. Bars represent mean plus or minus SD. (B) While equivalent amounts of IFNγ can be observed in supernatants of DC–T-cell and B-cell–T-cell cocultures, a marked increase in the amount of IL-10 is found in cultures containing B cells. (C) Intracellular cytokine staining reveals that IL-10 is produced by the CD4 negative population. The level of induced IL-4 is low for all cells. (D) Costaining with the specific B-cell marker CD19 identifies B cells as the source of IL-10. The data shown are representative of 3 independent experiments.
Cytokine profile during the activation of T cells by B cells shows substantial amounts of Th1 cytokines but also high levels of B-cell–derived IL-10. Supernatants were taken from DC–T-cell and from B-cell–T-cell cocultures and analyzed for cytokines by ELISA. Cells were analyzed for intracellular cytokines by FACS at 48 hours of culture. (A) B cells induce substantial amounts of IL-2 in supernatants of B-cell–T-cell cocultures, but DCs are at least twice as powerful on a per-cell basis. Bars represent mean plus or minus SD. (B) While equivalent amounts of IFNγ can be observed in supernatants of DC–T-cell and B-cell–T-cell cocultures, a marked increase in the amount of IL-10 is found in cultures containing B cells. (C) Intracellular cytokine staining reveals that IL-10 is produced by the CD4 negative population. The level of induced IL-4 is low for all cells. (D) Costaining with the specific B-cell marker CD19 identifies B cells as the source of IL-10. The data shown are representative of 3 independent experiments.
The mechanism of regulation by TofBs requires close cell-cell proximity and is independent of Foxp3
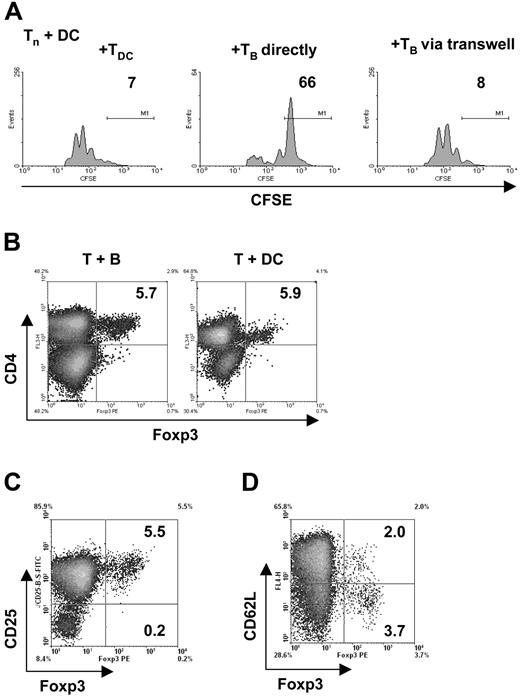
Naturally occurring, “classic,” CD4+ CD25+ Tregs were shown to mainly act independently of soluble mediators such as cytokines and to depend on very close cell-cell interactions as recently reviewed.49 It was possible that TofBs also required the proximity of interacting cells for proliferation inhibition. We tested this hypothesis by using a Transwell membrane assay. This assay tests whether for a given effect cells need to be in close proximity, either because direct cell-cell contact is necessary or because potentially required soluble messengers are released in limited amounts and work only at short distances. Indeed, regulation by TofBs requires naive T cells to be in close proximity (Figure 6A). A molecular marker for classical CD4+ CD25+ Tregs is the transcription factor Foxp3.50 If TofBs use identical mechanisms as these Tregs, a great part of TofBs in contrast to TofDCs should be Foxp3+. We found, however, that this was not the case. Only 5% of TofBs were Foxp3+, the same amount as within TofDCs (Figure 6B). This proportion was also unchanged from naive T cells (not shown). Foxp3+ cells resided exclusively in the CD4+ CD25+ population (Figure 6C) but no correlation was seen with expression of CD62L (Figure 6D). Most of the Foxp3+ Tregs were CD62L low. In conclusion, B-cell priming of T cells does not lead to an increased frequency of Foxp3+ Tregs. Therefore, we believe that TofBs act via pathways different from the ones of classical Tregs and do not require increased expression of Foxp3 yet need close cell-cell proximity.
The generation of T cells with regulatory capacity requires immediate vicinity of cells but is independent of Foxp3. (A) To in vitro cocultures of naive, CFSE-labeled CD4+ T cells and mature DC loaded with antigenic peptide (pOVA 323-339), TofDC or TofB were added directly and via a semipermeable transwell membrane. At 72 hours, proliferation of the naive T cells was determined by FACS. Close cell contact is needed for exertion of the regulatory effect of TofB, as separation from naive T cells plus DC via a transwell membrane abrogates the effect. (B-D) The level of the transcription factor Foxp3 was determined via intracellular staining and FACS after 72 hours of cocultures of naive splenic CD4+ T cells with peptide-loaded mature DC or B cells, respectively. (B) Preferential induction of Foxp3+ T cells could not be observed after B-cell contact; equally low numbers were found in Tof B and TofDC. (C) The Foxp3+ T cells are also CD25+. Depicted is the result for the CD4+ TofB population only. (D) The positivity of Foxp3 is independent of the CD62L level. Depicted is the result for the CD4+ TofB population only. The data shown are representative of at least 3 independent experiments.
The generation of T cells with regulatory capacity requires immediate vicinity of cells but is independent of Foxp3. (A) To in vitro cocultures of naive, CFSE-labeled CD4+ T cells and mature DC loaded with antigenic peptide (pOVA 323-339), TofDC or TofB were added directly and via a semipermeable transwell membrane. At 72 hours, proliferation of the naive T cells was determined by FACS. Close cell contact is needed for exertion of the regulatory effect of TofB, as separation from naive T cells plus DC via a transwell membrane abrogates the effect. (B-D) The level of the transcription factor Foxp3 was determined via intracellular staining and FACS after 72 hours of cocultures of naive splenic CD4+ T cells with peptide-loaded mature DC or B cells, respectively. (B) Preferential induction of Foxp3+ T cells could not be observed after B-cell contact; equally low numbers were found in Tof B and TofDC. (C) The Foxp3+ T cells are also CD25+. Depicted is the result for the CD4+ TofB population only. (D) The positivity of Foxp3 is independent of the CD62L level. Depicted is the result for the CD4+ TofB population only. The data shown are representative of at least 3 independent experiments.
TofBs inhibit priming of T cells during immune responses in vivo
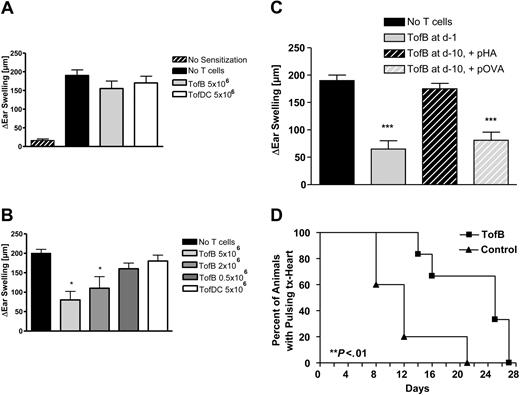
Having demonstrated immunoinhibitory effects in vitro we then tested the effects of TofBs in vivo. First, we used a hapten hypersensitivity assay, where TofB were added to recipients at different timepoints to dampen an immune response elicited by priming and challenge with DNFB. The resulting inflammatory response was significantly reduced when TofBs were added to recipients shortly before priming (Figure 7B). In contrast, when TofBs were added shortly before challenge, no effect was seen (Figure 7A). This discrepancy complies with our finding that TofBs migrate preferentially to lymph nodes where they are most effective during priming. Thus, we could show that TofBs mediate immunoregulation during the priming phase of immune responses in vivo.
TofBs inhibit priming of T cells during immune responses in vivo. (A-C) In a hapten hypersensitivity assay, Balb/C mice were sensitized against DNFB by epicutaneous application on the back. Ears were challenged with DNFB 5 days later and ear swelling was measured 48 hours after challenge. T cells primed by different APCs loaded with T-cell antigen–specific, hapten-unrelated, peptide (pOVA) were intravenously applied at different time points to modulate ear swelling. Ear swelling response is expressed as the difference (μm, mean ± SD) between the thickness of the challenged ear and the vehicle-treated ear. *, P less than.05; ***, P less than .005 versus positive control. (A) Adoptive transfer of primed TofDCs, and TofBs at 24 hours before challenge. Transferred cells were unable to modulate the ear swelling induced by the challenge. (B) In contrast, when T cells were transferred before priming (at d −1), a specific inhibitory effect of TofBs could be seen. The transfer of TofBs but not of TofDCs resulted in a decreased immune response post challenge. (C) TofBs are functioning at 10 days after transfer yet require specific antigen restimulation. The suppressive effect shown after transfer at d −1 (as in panel B) is lost when cells were transferred already at d −10 before priming. However, when hapten priming and specific antigen restimulation (pOVA) occurred together, TofBs were rescued to suppress. In contrast, the application of an unspecific control antigen (pHA; Hemagglutinin peptide) had no effect. (D) In ectopic allogeneic heart transplantation in the mouse model, TofBs effectively inhibit organ rejection. At d −1 before ectopic transplantation of C57BL/6 donor hearts, Balb/c recipients of the treatment group were injected with TofBs, while the control group received only a saline injection. No other immunosuppressive treatment was administered. Graft function was assessed by daily palpation. Rejection was defined as the lack of palpable cardiac contraction. **, P less than.01 vs. control group.
TofBs inhibit priming of T cells during immune responses in vivo. (A-C) In a hapten hypersensitivity assay, Balb/C mice were sensitized against DNFB by epicutaneous application on the back. Ears were challenged with DNFB 5 days later and ear swelling was measured 48 hours after challenge. T cells primed by different APCs loaded with T-cell antigen–specific, hapten-unrelated, peptide (pOVA) were intravenously applied at different time points to modulate ear swelling. Ear swelling response is expressed as the difference (μm, mean ± SD) between the thickness of the challenged ear and the vehicle-treated ear. *, P less than.05; ***, P less than .005 versus positive control. (A) Adoptive transfer of primed TofDCs, and TofBs at 24 hours before challenge. Transferred cells were unable to modulate the ear swelling induced by the challenge. (B) In contrast, when T cells were transferred before priming (at d −1), a specific inhibitory effect of TofBs could be seen. The transfer of TofBs but not of TofDCs resulted in a decreased immune response post challenge. (C) TofBs are functioning at 10 days after transfer yet require specific antigen restimulation. The suppressive effect shown after transfer at d −1 (as in panel B) is lost when cells were transferred already at d −10 before priming. However, when hapten priming and specific antigen restimulation (pOVA) occurred together, TofBs were rescued to suppress. In contrast, the application of an unspecific control antigen (pHA; Hemagglutinin peptide) had no effect. (D) In ectopic allogeneic heart transplantation in the mouse model, TofBs effectively inhibit organ rejection. At d −1 before ectopic transplantation of C57BL/6 donor hearts, Balb/c recipients of the treatment group were injected with TofBs, while the control group received only a saline injection. No other immunosuppressive treatment was administered. Graft function was assessed by daily palpation. Rejection was defined as the lack of palpable cardiac contraction. **, P less than.01 vs. control group.
We then tested whether TofBs maintain their function over a longer time span and whether function at later timepoints requires specific antigen restimulation. To this end we extended the hapten hypersensitivity assay, injecting TofBs 10 days before sensitization. Without specific restimulation, TofBs were not able to suppress priming at day 0. However, the cells were still present and functional as application of specific antigen at day – 1 re-activated suppressor function (Figure 7C). Thus, TofBs are present at least 10 days after intravenous transfer but only regulate upon specific antigenic restimulation.
Finally, we wanted to test TofBs in a model of ectopic allogeneic heart transplantation. Based on our observation that TofBs preferentially home to lymph nodes, we expected them to work most efficiently when applied before initiation of immune response, before an immune challenge occurs. Indeed, TofBs were able to successfully suppress rejection and prolong functioning of transplanted allogeneic hearts significantly (Figure 7D). Thus, TofBs are able to suppress solid organ transplant rejection in vivo.
Discussion
The outcome of interactions between resting B lymphocytes and naive T cells has always spurred a controversial discussion.51 Initially, contacts were believed to be nonproductive in vivo.1 Later, evidence accumulated that B cells can induce tolerance.3,52 Under some circumstances, B cells were even found to be effective T-cell activators.53 It is known that naive B cells show a lesser number of MHC-II and coactivation molecules on their surface than DCs,54 and Figure 1N. In that sense, B cells may be similar to immature DCs, which can also induce T-cell tolerance.55-57 However, to our knowledge this is the first report that tolerization after B-cell contact can be mediated, at least in part, by the de novo generation of T cells with regulatory phenotype. Such pathways have hitherto been discussed for immature dendritic cells58,59 and recent reports have suggested a similar mechanism for human macrophages.60
What triggers the differential response of T cells toward these distinct APC? The result of T-cell priming is defined at the level of earliest interactions.61,62 Thus, individual IS structures might be decisive for this process. We demonstrate formation of a mature IS between a naive B cell and an antigen-specific T cell and its absence in T-DC pairs. This appears surprising at first glance as naive B cells are very ineffective antigen presenters and synapses are generally considered structures leading to effector T-cell generation.63 However, many studies demonstrating presence of mature IS actually used B cells as APC.15,64,65 In contrast, a thorough analysis of singular T-DC interactions revealed the predominance of multifocal structures without a typical p-SMAC–c-SMAC appearance.66 The function of theses structures is usually judged by the measurement of T-cell proliferation.12 Our data show that T-cell proliferation alone is not sufficient to characterize the phenotype of a T cell. Only analysis of proliferated T cells in secondary coculture assay allowed us to identify a regulatory role of these cells.
The function of the synapse, especially of the c-SMAC, is intensely debated.67 As it was shown that phospho-tyrosine levels are low to absent in c-SMAC,68 the current concept considers c-SMAC as an area of TCR internalization and signal termination.14 Signaling in p-SMAC is constantly maintained in microclusters that are then transported to c-SMAC.69,70 Such microclusters might also be present in T-B synapses because B cells can induce long-lasting Ca2+signaling in T cells.9 As shown here, this could be the basis for deviation of T-cell effector function to a phenotype with regulatory properties. Thus, synapses formed between naive B cells and naive T cells may be the prototype of a novel type of IS, the regulatory synapse.
We show that inhibition of T-cell proliferation by TofBs is cell contact–dependent or at least requires close cell-cell interactions. In addition we show that most TofBs localize in T-cell zones of lymph nodes. This suggests interactions between TofBs and T cells or DCs in the T-cell zone being the mode of action in vivo. Contacts of Tregs with T-DC clusters impacting on recruitment of signaling molecules in involved naive T cells have been demonstrated before.71 However, the intrinsic molecular mechanism of how Tregs can mediate cell-contact inhibition is insufficiently defined. Many independent pathways seem to exist, none of which can alone account for all aspects of regulation. Signaling molecules like CTLA-4 and Cbl-b as well as immunosuppressive cytokines such as IL-10, TGF beta and more may have important roles (for recent reviews see Miyara and Sakaguchi72 and Graca et al73 ). TGF beta may be especially important during peripheral generation of Treg, most notably CD4+ CD25+ Foxp3+ T cells.74,75 The Forkhead box transcription factor Foxp3 is considered to be the most specific intracellular marker for naturally occurring (n)Tregs.76 Thus, it was unexpected that TofBs did not show enhanced Foxp3 expression. However, the group of Treg phenotypes is expanding.77 In fact, also Foxp3 independent Tregs have been described.45,78
The most obvious molecular T-cell marker differentially regulated by B cells and DCs, respectively, in this study, was CD62L, L-Selectin. This molecule is down-regulated on lymphocytes within 30 minutes after activation79 by cleavage near the cell surface by a member of the A Disintegrin and Metalloprotease (ADAM) family ADAM-17.80 However, other sheddases or additional factors may be required.81 Whether B cells activate sheddases differently than DCs is not clear. Spatial distribution of Tregs was shown to be of great importance for their role in immune responses.82-84 Likewise, manipulation of molecules involved in lymphocyte homing and trafficking greatly affects lymphocyte function.83 In that respect, it is important that our data provide a model correlating the level of CD62L and, thus, the entry into peripheral lymphatic organs with the regulatory function in vivo.
Our demonstration that antigen presentation by naive B cells induces Tregs might help to explain older findings on induction of specific tolerance in vivo against antigens presented by naive B cells.3,85 Our data might also shed light on results from a recent study where prolonged subcutaneous infusion of low peptide doses transformed mature T cells into CD4+ 25+ suppressors.86 In accordance with our data, this approach proved effective in inducing tolerance prospectively (priming phase), but it was open whether ongoing immune responses could be suppressed (challenge phase). Involvement of B-cell antigen presentation to generate Tregs in both systems seems to be an attractive model to explain their mode of action. Indeed, Matzinger has suggested that B cells, by virtue of their large quantities, may indeed participate in T-cell priming in a tolerizing manner.85 In light of our data, this might be a consequence of de novo generation of Tregs. Such processes may play a role in vivo as B-cell–deficient individuals tend to develop autoaggressive phenomena.87,88 Interestingly, T cells specifically activated in vivo show substantial proliferation yet remain CD62L high in up to 50% of the proliferating cells (unpublished observations). This could be interpreted as result of DC-triggering low antigen doses or as B-cell participation in T-cell priming. It is tempting to speculate that a fraction of these in vivo activated T cells is regulatory as well.
More importantly, even if physiologic relevance of naive B-cell triggered T-cell activation in vivo may be limited facing overwhelming competition by professional APC, the process described by us can have important clinical implications. As we show, B cells with their specific way of inducing T-cell activation may prove powerful for cell-based immunosuppression. Currently, DCs are the main focus of strategies against alloreactivity, transplant rejection, autoimmune diseases and allergy.89-94 However, generation of DCs for therapeutic applications requires sophisticated handling, is expensive, time-consuming, and difficult to standardize.92 In that respect, B cells may be a better option for obtaining defined APC. Indeed, genetically modified B cells have been used to tolerize animals against epitopes of pathophysiologic relevance.95 Our model provides a more direct approach without the need for transgenic manipulation. It can be envisioned that the use of B-cell primed regulatory T cells, TofBs or “bTregs,” may be a new way of significantly improving current cell-based therapies.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Dunja Bruder, Markus Gereke, and Wiebke Hansen for help with initial experiments. We thank Werner Müller and Andreas Lengeling for providing IL-10 deficient animals. Jiong Tian is gratefully acknowledged for passing microsurgical expertise to S.R. Peter H. Krammer, Burkhart Schraven, Waldemar Kolanus and Jan Buer are acknowledged for critical reading of the manuscript.
This study was supported by the Deutsche Forschungsgemeinschaft (SPP 1160 (GU 769/1–1 and GU 769/1–2) to M.G.), by the European Union (FP6, Nest, 043243, Mamocell) to M.G., by the Interdisciplinary Center of Clinical Research (IZKF) grant Lo2/017/07 to K.L., and by the fund Innovative Medical Research (IMF) of the University of Münster Medical School grant Lo11/06 03 to K.L
Authorship
P.R., B.D., S.R., F.G., S.B., and K.L. performed experiments and analyzed data; M.G. conceived the study; and P.R. and M.G. wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Matthias Gunzer, PhD, Helmholtz Centre for Infection Research, Junior Research Group Immunodynamics, Inhoffenstraße 7, D-38124 Braunschweig, Germany; e-mail: mgunzer@helmholtz-hzi.de.


![Figure 3. In vivo B-cell–primed T cells (TofB) preferentially migrate to lymph nodes. T cells primed by peptide-loaded DC and B cells were differentially labeled with live cell dyes. Equal numbers of TofDCs and TofBs were adoptively transferred intravenously, and distribution of the cells was determined 24 hours after transfer by organ removal and FACS analysis. (A) T cells for adoptive transfer were labeled with CFSE; TofDCs were additionally labeled with CTO and TofBs with DiD. (B) Upon organ analysis after transfer, equal numbers of TofDCs and TofBs as transferred can be found in the spleen, whereas in lymph nodes TofBs clearly dominate. (C) Distribution of the transferred T-cell populations in lymphatic organs 24 hours after transfer. When analysis was performed at day 7 after transfer, a similar pattern was observed (data not shown). The data shown are representative of at least 3 independent experiments. Within the lymph nodes, TofBs migrate to T-cell areas and are excluded from B-cell follicles. A simplified drawing illustrates lymph node architecture showing B-cell areas (blue), T-cell dominated areas [green], and the imaged area shown above (red rectangle) at the transition of the 2 zones. (D) An image stitched together of 6 individual extended focus projections of each 200 × 200 × 144 μm shows the transition from a B-cell follicle dominated by B cells (blue) to a mixed T-B zone containing mostly naive T cells (green). Note that TofBs (red) are found only in the T-cell area. Scale bar: 100 μm. (E) A quantification of TofB distribution analyzing 14 image stacks from within and outside B-cell follicles. TofBs clearly show T-cell homing behavior and localize within T-cell–dominated areas but are excluded from B-cell follicles. Bars represent mean plus or minus SD.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/5/10.1182_blood-2006-10-053793/6/m_zh80130704130003.jpeg?Expires=1765900644&Signature=K2nk~xO4XlZVd~yKgM0WYr9mLNstEtpwiA~dA5upA0lkDpHG6ZQkICpLDPxqAv4Nm~P1403ene-v5T76CwDAt2pATzRnvjw7OcgE0JVCJZDClAg0oU31guPzIaMrcPOlgFWvkoSrJOHQTzhbbSfmIXKFmN488sRVMw6HggpYO9u50k5KIV9ecXsZ0JVahSdcNxDIL2R85a6qn357CtEJ6p8qI82j1F45JzWTBotF1IbdF915IFdOpfsFr8n7EAnFlKI6I1IhkGjk7KL0zMfI3lVC7aLFLglZFphZil3F8bzl5lclane2ZZTGGclkRb~uIH2WMtFl2Xsn38nZaesMGg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal