Abstract
Activation of the innate immune system promotes polyclonal antibody secretion to eliminate invading pathogens. Inherent in this process is the potential to activate autoreactive B cells and induce autoimmunity. We showed previously that TLR-stimulated dendritic cells and macrophages regulate B cell tolerance to Smith antigen, in part through the secretion of interleukin-6 (IL-6). In this manuscript, we show that neutralization of IL-6 fails to abrogate macrophage-mediated repression and identify soluble CD40 ligand (CD40L) as a second repressive factor secreted by macrophages. CD40L selectively repressed Ig secretion by chronically antigen-experienced (anergic) immunoglobulin transgenic and nontransgenic B cells but not by transiently stimulated B cells. The importance of macrophages in maintaining B cell tolerance was apparent in lupus-prone MRL/lpr mice. Compared with C57BL/6 mice, macrophages from MRL/lpr mice were significantly less efficient at repressing immunoglobulin secretion coincident with diminished IL-6 and CD40 ligand production. These data indicate that macrophages regulate autoreactive B cells by secreting repressive factors that prohibit terminal differentiation of B cells. The regulation of autoreactive B cells by macrophages is diminished in lupus-prone mice suggesting a role in autoimmunity.
Introduction
Recognition of microorganisms by Toll-like receptors (TLRs) promotes inflammation and stimulates the innate immune system to produce antibody, responses that are beneficial in clearing infections. However, TLR ligation of autoreactive B cells can lead to transient or persistent autoimmunity.1 Studies of rheumatoid factor-specific B cells show that immune complexes containing TLR and B-cell receptor (BCR) ligands induce proliferation of autoreactive B cells.2-4 Likewise, anti–double-stranded DNA (dsDNA)–specific B cells proliferate in response to BCR-mediated internalization of chromatin.5 Because most nuclear self-antigens contain BCR and TLR ligands, these findings suggest that stimulation of autoreactive B cells through the BCR and/or TLR activates some autoreactive B cells
During T-dependent immune responses, CD40 stimulation induces B-cell proliferation, increases the expression of costimulatory molecules, and promotes germinal center formation leading to high affinity, class-switched antibodies. Continuous exposure to CD40 ligand (CD40L) promotes the formation of memory cells by blocking B lymphocyte–induced maturation protein-1 (Blimp-1) expression and arresting plasma cell differentiation.6 CD40/CD40L interactions also regulate autoreactive B cells that encounter activated CD4+ TH cells.7 Hen egg lysozyme (HEL)–specific B cells that have been continuously exposed to self-antigen up-regulate Fas in response to CD40L stimulation. Subsequent encounter with an activated HEL-specific T cell induces Fas-dependent B-cell apoptosis, thereby preventing autoimmunity. Thus, persistent exposure to self-antigen modulates Fas and CD40 to induce apoptosis or prevent terminal differentiation.
We recently identified a mechanism of tolerance that regulates autoreactive B cells during innate immune responses. In response to lipopolysaccharide (LPS), dendritic cells (DCs) and macrophages (MΦs) regulate HEL-, p-azophenylarsonate (Ars)- and low-affinity (2-12H/Vκ8), Smith antigen (Sm)-specific B cells through the secretion of interleukin-6 (IL-6).8 Regulation of immunoglobulin (Ig) secretion is selective in that chronically antigen-experienced (anergic) B cells are repressed, whereas transiently stimulated naive B cells are not. This indicates that tolerance within the B-cell compartment extends beyond antigen-induced receptor desensitization and that persistent BCR ligation affects other receptors. Herein, we report that in addition to IL-6, MΦs secrete soluble CD40L (sCD40L), which selectively represses Ig secretion by chronically antigen-experienced B cells. Regulation was also apparent in nontransgenic (non-Tg) B cells where anti-nucleosome responses were repressed by sCD40L. sCD40L-mediated repression did not reflect changes in proliferation; rather, it revealed a reduction in the number of B cells that differentiated into antibody secreting cells (ASCs). Finally, we show that MΦs derived from autoimmune-prone MRL/lpr mice failed to repress Sm-specific B cells coincident with diminished production of IL-6 and sCD40L, suggesting that MΦ-mediated tolerance may play a role in regulating autoimmunity. Collectively, the data show that the history of antigen binding determines whether LPS-induced Ig secretion is repressed or enhanced by IL-6 and sCD40L stimulation. These findings identify a second soluble mediator that facilitates the regulation of autoreactive B cells by MΦs and reveal how naive and autoreactive B cells are differentially regulated to ensure immunity in the absence of autoimmunity during innate immune responses.
Materials and methods
Mice
2-12H/Vκ89 (80% follicular [FO], 1% marginal zone [MZ]), 2-12H10 (70% FO, 12% MZ),17 and Ars/A111 (78% FO, 0.9% MZ)11 (K.A., L.J.W., unpublished observations, November 2002) Ig transgenic (IgTg) mice have been described. HEL-Ig (MD4; 70% FO, 7% MZ) and HEL-Ig × sHEL (MD4 × ML5; 72% FO, 0.3% MZ) IgTg mice,12,13 C57BL/6 (70% FO, 7% MZ), IL-6−/−, CD40L−/−, and MRL/lpr mice were from The Jackson Laboratory (Bar Harbor, ME). Animals were 8 to 16 weeks old and maintained in an accredited animal facility.
Cell lines
CD40L-transfected Chinese hamster ovary (CHO cells) (m40L-2) and control CHO-K1 cells (Dr D. Conrad, Virginia Commonwealth University, Richmond, VA) were prepared and maintained as described previously.14
Antibodies and other reagents
Neutralizing anti-CD40L, hamster IgG (isotype control for anti-CD40L), neutralizing anti-IL-6, and recombinant IL-6 (rIL-6) were from BD Biosciences (San Jose, CA). Recombinant sCD40L (rsCD40L) was from R&D Systems (Minneapolis, MN). The trimeric form of CD40L has the most biologic activity; however, the manufacturer reports no trimeric protein by Silver stain. 187.1 (anti-κ), HB100 (anti-IgMa), 33-60 (anti-IgM), B7.6 (anti-IgM), MR1 (anti-CD40L), and 54.1 (3-83 idiotype, an isotype control for anti-IL-6) were purified from hybridoma culture supernatant using Protein G Sepharose (GE Healthcare, Chalfont St Giles, United Kingdom or MEP HyperCel (BioSepra, Marlborough, MA).
B-cell purification and LPS stimulation
Splenic B cells were isolated from 2-12H/Vκ8, HEL-Ig, HEL-Ig × sHEL, Ars/A1, and C57BL6 mice by negative selection using the StemSep B-cell enrichment kit (StemCell Technologies, Vancouver, BC). B cells were 83%-93% pure. Purified B cell preparations were contaminated with T cells (< 3%), DCs/MΦs (< 6%), and an unidentified population (CD43+CD19−CD11b−CD11c−CD3−). Triplicate cultures of B cells (1 × 105, purity determined by flow cytometry) were stimulated with LPS (30 μg/mL; Sigma, St Louis, MO) for 4 days. MΦs, rsCD40L, rIL-6, MΦ-conditioned media (CM) (25% of final volume), or anti-CD40L were added at day 0.
Bone marrow–derived MΦ cultures
Single-cell suspensions of bone marrow were prepared from the femurs of C57BL/6 mice. Mononuclear cells were isolated using Lympholyte Separation Medium (CEDARLANE Laboratories, Burlington, ON) then cultured in macrophage-colony-stimulating factor (20 ng/mL; Peprotech, Rocky Hill, NJ) for 7 days. BMMΦ cultures were 98% CD11b+, I-A−, and B7.2−. CM was made from 1 × 104 bone marrow–derived MΦ (BMMΦ) cultured for an additional 4 days in the presence or absence of LPS (30 μg/mL).
Enzyme-linked immunosorbent assays
IgMa/κ (encoded by 2-12H/Vκ8 or Ars/A1) was measured as described previously.9 A standard curve was generated using mouse IgMa/κ (TEPC 183; Sigma). IgM (C57BL/6) was detected using anti-mouse IgM (clone 33-60) and biotin-labeled anti-mouse IgM (B7.6). Mouse anti-HEL IgMa in supernatants was measured as described previously.15 Mouse anti-HEL IgMa (clone E1; Dr T. Tedder, Duke University, Durham, NC)16 was used to generate a standard curve. Nucleosome-specific Ig was captured with histones (10 μg/well; Immunovision, Springdale, AR) and dsDNA (1 μg/well; Sigma) and detected with goat anti-mouse Ig-AP (Southern Biotechnologies, Birmingham, AL). Mouse anti-histone (clone PL2-6; Dr M. Monestier, Temple University, Philadelphia, PA) was used to generate a standard curve. Data were plotted as percent of control calculated relative to cultures of LPS-stimulated B cells. IL-6 was measured using anti-IL-6 and biotin-labeled anti-IL-6 according to the manufacturer's directions (BD Biosciences).
Cell sorting
FO (B220+CD23+CD21/35loCD138−) and MZ (B220+CD23−CD21/35+CD138−) B cells were sorted from 2-12H mice and were more than 90% pure when reanalyzed. Macrophages (CD11b+CD11c−) were sorted from 8-week-old C57BL/6, C57BL6.lpr, MRL, and MRL/lpr or 24-week-old MRL/lpr. All sorted cells were more than 97% pure. CM was made from 1 × 105 MΦs cultured for an additional 4 days in the presence of LPS (30 μg/mL). All sorts were done using a MoFlo high speed sorter (Dako North America, Inc, Carpinteria, CA).
Enzyme-linked immunosorbent spot
The number of ASCs was determined as described previously17 by culturing LPS-stimulated B cells (1 × 105) in the presence or absence of rsCD40L, rIL-6, DCs, or MΦs. On day 3, the cells were washed and transferred to plates coated with 1 U/well Smith antigen (Sm; Immunovision) for 8 hours. The ASC were detected using biotin-labeled anti-IgMa followed by streptavidin-horseradish peroxidase (BD Biosciences). The plates were analyzed using an ImmunoSpot Analyzer and ImmunoSpot software package (Cellular Technology, Cleveland, OH).
CFSE-based proliferation assay
B cells (1 × 106 cells/mL) were labeled with 5-carboxyfluorescein diacetate succinimidyl ester (CFSE; final concentration, 0.4 μmol/L; Invitrogen) for 10 minutes at 37°C. CFSE-labeled cells were LPS-stimulated in the presence or absence of rsCD40L (100 ng/mL) as described above. After 3 days, the cells were harvested and CFSE fluorescence intensity was analyzed using a FACSCalibur (BD Biosciences) with WinMDI software (The Scripps Research Institute, San Diego, CA). The proliferative index was calculated by dividing the total number of cells in all generations by the calculated number of original parent cells.
Intracellular IgM staining
LPS-stimulated B cells were cultured in the presence or absence of rsCD40L, as described above. Intracellular IgM was measured on day 3 by blocking surface IgM with unlabeled anti-IgM (20 μg/mL, clone B7.6) in phosphate-buffered saline/2% bovine serum albumin. The cells were washed and fixed with 1% paraformaldehyde (Electron Microscopy Sciences, Hatfield, PA). Fixed cells were permeabilized in 0.05% saponin (Sigma) followed by staining with Cy5.5-labeled anti-IgM. The cells were analyzed using a FACSCalibur.
Reverse transcription–real-time quantitative polymerase chain reaction
RNA was extracted from 5 × 105 B cells in TRIzol (Invitrogen) and reverse-transcribed with random hexamer primers and Moloney murine leukemia virus reverse transcriptase (Invitrogen). Reverse transcription–polymerase chain reaction (RT-PCR) reactions were performed using SYBR Green PCR Master Mix (Applied Biosystems, Foster City, CA) and 1 μmol/L primers in a 20-μl final volume. Relative expression of X-box-binding protein-1 (XBP-1) and Blimp-1 were determined using 18S rRNA as an internal control. XBP-1 primers: 5′-ACACGCTTGGGAATGGACAC-3′ and 5′-CCATGGGAAGATGTTCTGGG-3′; Blimp-1 primers: 5′-TGTTGGATCTTCTCTTGGAAAA-3′ and 5′-GTGTAAAGTAGACTGCCTTGA-3′.18 18s rRNA primers were 5′-TCAAGAACGAAAGTCGGAGGTT-3′ and 5′-GGACATCTAAGGGCATCACAG-3′.19 Data were analyzed using the 2ΔΔCT method: relative expression = 2ΔΔCT, where ΔΔCT = ((cycle threshold [CT] gene of interest) – (CT 18s rRNA) in experimental sample) – (CT gene of interest) – (CT 18s rRNA) time zero).20
Quantum dot staining of CD40L
C57BL/6 and MRL/lpr BMMΦs were plated on poly-d-Lysine–coated coverslips for 2 hours then LPS-stimulated (15 μg/mL; List Biological Laboratories Inc, Campbell, CA) for 3 hours. Cells were fixed with 1.5% paraformaldehyde, permeabilized with ice-cold 100% methanol, and stained with biotinylated anti-mouse CD40L (clone MR1) followed by secondary staining with Quantum dot (Qdot) 655 streptavidin (Invitrogen). Samples were mounted using 90% glycerol and observed at room temperature using the Olympus FV500 Confocal Laser Scanning Microscope. Images were captured using a 60×/1.4 numerical aperture oil plan apochromat objective lens and the Olympus Flowview with Tiempo acquisition software. Data analysis was done using ImageJ 1.37C software (http://rsb.info.nih.gov/ij/). For each field, the threshold was set based on background intensity. The percentage of CD40L-expressing cells and the fluorescence was quantitated from 500 cells (100 cells/experiment). Fluorescence of positively staining cells was quantitated by subtracting background staining from each treatment condition (secondary only) then calculating the fold increase in CD40L staining of stimulated cells over unstimulated cells.
Statistical analysis
One-sample t test was used when comparing antibody secretion in treated and untreated cultures; Exact Wilcoxon rank sum test was used to test for differences in antibody secretion between experimental groups. Statistical analyses were performed with SAS statistical software (ver. 9.1; SAS Institute Inc, Cary, NC).
Results
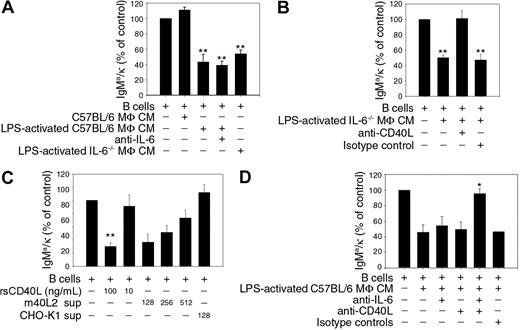
B cells expressing the 2-12H Ig transgene paired with a Vκ8 Ig light chain transgene bind the small nuclear ribonucleoprotein, Sm, with low affinity.21 Sm-specific B cells fail to secrete Ig upon LPS stimulation unless the splenic B-cell preparations lack DCs and MΦs.8,9 We previously described a novel mechanism of tolerance in which DCs and MΦs regulate autoreactive B cells during innate immune responses.8 IL-6, secreted by DCs and MΦs, represses Ig secretion by chronically antigen-experienced B cells but has no affect on Ig secretion by naive B cells.8 We were surprised, however, that repression of autoantibody secretion by CM prepared from MΦs was not alleviated by the addition of anti-IL-6, although MΦs secrete significant amounts of IL-6 (36.1 ± 6.8 ng/mL) (Figure 1A).8 To investigate whether other factors secreted by MΦs repressed autoantibody secretion, we prepared CM from LPS-stimulated MΦs from IL-6 deficient (IL-6−/−) mice. MΦ CM prepared from IL-6−/− mice repressed 47% of Ig secretion (Figure 1A), indicating that in addition to IL-6, MΦs secrete other factors that repress Ig secretion by autoreactive B cells.
sCD40L represses Ig secretion by Sm-specific B cells. Sm-specific (2-12H/Vκ8) B cells (1 × 105) were stimulated with LPS (30 μg/mL) and cocultured with CM from C57BL/6 BMMΦs, CM from LPS-activated C57BL/6 BMMΦs, anti-IL-6 (50 μg/mL) or CM from LPS-activated IL-6−/− BMMΦs (A), LPS-activated IL-6−/− BMMΦ CM, anti-CD40L (10 μg/mL) or isotype-matched control antibody (10 μg/mL) (B), the indicated concentration of rsCD40L or supernatant from CD40L transfected (m40L2) or untransfected cells (CHO-K1) (C), LPS-activated C57BL/6 BMMΦ CM, anti-IL6 (50 μg/mL), anti-CD40L (10 μg/mL) or isotype-matched control antibodies (10 μg/mL) (D). IgMa/κ levels were quantitated on day 4 by ELISA. Titrations of CD40L-transfected cell supernatants represent 8.6 pg/mL (1:128), 4.3 pg/mL (1:256), and 2.1 pg/mL (1:512) as determined by ELISA. LPS-stimulated B cells (100%) secreted 1.3-8.5 μg/mL. Statistical analysis was performed using one-sample t test by comparing treated cultures to untreated cultures (control) except in panel D where antibody/C57BL/6 BMMΦ CM-treated cultures were compared with cultures treated with C57BL/6 BMMΦ CM lacking antibody. Data represent triplicate samples in each of 2-4 independent experiments. Error bars represent plus or minus SEM. (*P ≤ .05; **P ≤ .001.)
sCD40L represses Ig secretion by Sm-specific B cells. Sm-specific (2-12H/Vκ8) B cells (1 × 105) were stimulated with LPS (30 μg/mL) and cocultured with CM from C57BL/6 BMMΦs, CM from LPS-activated C57BL/6 BMMΦs, anti-IL-6 (50 μg/mL) or CM from LPS-activated IL-6−/− BMMΦs (A), LPS-activated IL-6−/− BMMΦ CM, anti-CD40L (10 μg/mL) or isotype-matched control antibody (10 μg/mL) (B), the indicated concentration of rsCD40L or supernatant from CD40L transfected (m40L2) or untransfected cells (CHO-K1) (C), LPS-activated C57BL/6 BMMΦ CM, anti-IL6 (50 μg/mL), anti-CD40L (10 μg/mL) or isotype-matched control antibodies (10 μg/mL) (D). IgMa/κ levels were quantitated on day 4 by ELISA. Titrations of CD40L-transfected cell supernatants represent 8.6 pg/mL (1:128), 4.3 pg/mL (1:256), and 2.1 pg/mL (1:512) as determined by ELISA. LPS-stimulated B cells (100%) secreted 1.3-8.5 μg/mL. Statistical analysis was performed using one-sample t test by comparing treated cultures to untreated cultures (control) except in panel D where antibody/C57BL/6 BMMΦ CM-treated cultures were compared with cultures treated with C57BL/6 BMMΦ CM lacking antibody. Data represent triplicate samples in each of 2-4 independent experiments. Error bars represent plus or minus SEM. (*P ≤ .05; **P ≤ .001.)
To identify the other factor(s), we neutralized CM from IL-6−/− MΦs with a panel of antibodies. The addition of neutralizing anti-CD40L completely restored Ig secretion (Figure 1B). To confirm these findings, we added rsCD40L to cultures containing Sm-specific B cells. As shown in Figure 1C, rsCD40L (100 ng/mL) inhibited 74% of Ig secretion. This was a direct effect of sCD40L on B cells because sorted B cells (99% pure) were similarly repressed (data not shown). We were concerned that 100 ng/mL rsCD40L was not physiologically relevant and might be due to low bioactivity. To assess this, we treated purified Sm-specific B cells with supernatant from a CHO cell line expressing CD40L. As little as 9 pg/mL (1:128 dilution) repressed 63% of secretion. Repression was due to sCD40L because supernatant from nontransfected parental CHO-K1 cells failed to repress Ig secretion. The trimeric form of sCD40L contains the most biological activity, although monomers do have low levels of activity.22 Because the rsCD40L preparation is likely to contain a mixture of both oligomerized and nonoligomerized forms, we treated Sm-specific B cells with monomeric and oligomeric sCD40L to determine which form repressed Ig secretion. LPS-stimulated Ig secretion was similar in the presence or absence of monomeric sCD40L (rsMCD40L); however, when a crosslinking antibody was added to oligomerize the sCD40L, Ig secretion was repressed 63% (data not shown). This indicates that the oligomerized form of sCD40L represses autoantibody secretion. The amount of trimer in the rsCD40L preparation was below the limit of detection by silver staining, confirming the low bioactivity of the recombinant protein. To determine whether MΦs secrete repressive factors other than IL-6 and sCD40L, we neutralized LPS-activated MΦ CM with anti-IL-6 and anti-CD40L. Neutralization with either anti–IL-6 or anti-CD40L failed to restore Ig secretion (Figure 1D). However, neutralization with both antibodies restored 95% of secretion. Collectively, the data indicate that MΦs repress autoantibody secretion through the production of IL-6 and sCD40L. Although both factors are equally competent to regulate secretion, only one factor is required.
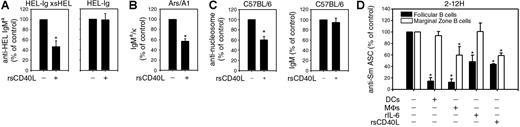
We previously reported that only chronically antigen-experienced B cells are regulated by IL-6, suggesting that persistent antigen exposure reprograms the IL-6 receptor to repress LPS-induced Ig secretion.8 To determine whether sCD40L exhibited the same specificity, we compared the effects of rsCD40L on naive and chronically antigen-experienced B cells. rsCD40L repressed 53% of Ig secretion by HEL-specific B cells that had been continuously exposed to sHEL (HEL-Ig × sHEL) (Figure 2A). In contrast, rsCD40L did not affect LPS-induced Ig secretion by naive HEL-specific B cells (HEL-Ig). To assess whether sCD40L regulated other chronically antigen-experienced B cells, we measured Ig secretion by Ars-specific B cells. Ars/A1 IgTg mice express a heavy and light chain pair that binds Ars; however, B cells from these mice cross-react with single-stranded DNA, conferring an anergic phenotype.11,23 Similar to the effects on HEL-specific B cells, rsCD40L repressed 43% of LPS-induced Ig secretion by purified Ars-specific B cells (Figure 2B). To determine whether autoreactive B cells in a population of nonautoreactive cells could be regulated by sCD40L, we measured the amount of nucleosome-specific Ig produced by C57BL/6 B cells. As shown in Figure 2C, LPS-induced anti-nucleosome secretion was repressed 40% by rsCD40L, whereas total IgM secretion in the same cultures was unaffected. These data indicate that during innate immune responses, B cells continuously exposed to self-antigen are repressed by sCD40L. Further, the repressive effects of MΦs are not restricted to Sm-specific B cells.
Soluble mediators selectively repress chronically Ag-experienced B cells and differentially regulate autoreactive FO and MZ B cells. B cells (1 × 105) from HEL-Ig × sHEL and HEL-Ig (A), Ars/A1 (B), or C57BL/6 mice (C) were stimulated with LPS (30 μg/mL) in the presence or absence of rsCD40L (100 ng/mL) for 4 days. Anti-HEL IgMa (HEL-Ig and HEL-Ig × sHEL), IgMa/κ (Ars/A1), IgM, and anti-nucleosome Ig (C57BL/6) were quantitated by ELISA. LPS-stimulated B cells (100%) secreted 9-15 μg/mL (HEL-Ig × sHEL), 16-47 μg/mL (HEL-Ig), 2-9 μg/mL (Ars/A1), 56-156 ng/mL anti-nucleosome Ig (C57BL/6), and 19-43 μg/mL IgMb (C57BL/6) (100%) secreted. (D) 1 × 105 Sm-specific (2-12H) FO and MZ B cells were sorted and stimulated with LPS (30 μg/mL) in the absence or presence of BMDCs (1 × 104), BMMΦs (1 × 104), rIL-6 (20 ng/mL), or rsCD40L (100 ng/mL). The number of ASCs was determined on day 3 using an Sm-specific enzyme-linked immunosorbent spot (ELISPOT). LPS-stimulated FO B cells (100%) yielded 2.8 × 104 to 1 × 105 spots/106 cells, whereas MZ B cells (100%) yielded 2.3 × 104-1.3 × 105 spots/106 cells. Statistical analysis was performed using 1-sample t test by comparing treated and untreated cultures. Data represent at least 3 experiments. Error bars represent plus or minus SEM. (*P ≤ .05.)
Soluble mediators selectively repress chronically Ag-experienced B cells and differentially regulate autoreactive FO and MZ B cells. B cells (1 × 105) from HEL-Ig × sHEL and HEL-Ig (A), Ars/A1 (B), or C57BL/6 mice (C) were stimulated with LPS (30 μg/mL) in the presence or absence of rsCD40L (100 ng/mL) for 4 days. Anti-HEL IgMa (HEL-Ig and HEL-Ig × sHEL), IgMa/κ (Ars/A1), IgM, and anti-nucleosome Ig (C57BL/6) were quantitated by ELISA. LPS-stimulated B cells (100%) secreted 9-15 μg/mL (HEL-Ig × sHEL), 16-47 μg/mL (HEL-Ig), 2-9 μg/mL (Ars/A1), 56-156 ng/mL anti-nucleosome Ig (C57BL/6), and 19-43 μg/mL IgMb (C57BL/6) (100%) secreted. (D) 1 × 105 Sm-specific (2-12H) FO and MZ B cells were sorted and stimulated with LPS (30 μg/mL) in the absence or presence of BMDCs (1 × 104), BMMΦs (1 × 104), rIL-6 (20 ng/mL), or rsCD40L (100 ng/mL). The number of ASCs was determined on day 3 using an Sm-specific enzyme-linked immunosorbent spot (ELISPOT). LPS-stimulated FO B cells (100%) yielded 2.8 × 104 to 1 × 105 spots/106 cells, whereas MZ B cells (100%) yielded 2.3 × 104-1.3 × 105 spots/106 cells. Statistical analysis was performed using 1-sample t test by comparing treated and untreated cultures. Data represent at least 3 experiments. Error bars represent plus or minus SEM. (*P ≤ .05.)
Our data show that multiple soluble factors secreted by DCs and MΦs regulate autoreactive B cells, suggesting a possible redundant function. Alternatively, DCs and MΦs might exhibit specialized functions with different soluble factors regulating unique B-cell subsets. To examine this, we isolated FO and MZ B cells from 2-12H mice and assessed the effects of DCs, MΦs, rsCD40L, and rIL-6 on their differentiation into ASCs. The 2-12H mice express the same heavy chain as the 2-12H/Vκ8 mice, but it pairs with endogenous light chains, resulting in antigen receptors of various affinities for Sm.10 We used these mice because the number of MZ B cells is higher compared with 2-12H/Vκ8 mice.9,17 As shown in Figure 2D, differentiation of FO B cells into ASC was significantly reduced by the addition of DCs, MΦs, rIL-6, and rsCD40L to the cultures. In contrast, MZ B cells were unaffected by DCs and rIL-6; however, addition of MΦs or rsCD40L reduced the number of ASCs by 41% and 42%, respectively. This indicates that MZ B cells are partially regulated by MΦ secretion of sCD40L, but not IL-6. Collectively, the data indicate that the use of multiple factors is redundant in the repression of FO B cells but sCD40L plays a unique role in repressing MZ B cells.
Because termination of cell division is required for plasma cell differentiation, we reasoned that impaired cell-cycle arrest may be the mechanism through which sCD40L repressed Ig secretion. To assess this, we labeled naive (C57BL/6) and autoreactive (2-12H/Vκ8) B cells with CFSE and calculated the proliferative index (PI); an indication of the average number of divisions per cell (Figure 3A). LPS-stimulated C57BL/6 B cells treated with rsCD40L (PI = 6.6 ± 0.26) showed enhanced proliferation compared with untreated cells (PI = 4.7 ± 0.18). Likewise, LPS-stimulated 2-12H/Vκ8 B cells treated with rsCD40L (PI = 5.1 ± 0.25) proliferated more than untreated cells (PI = 3.7 ± 0.39), revealing that rsCD40L has similar affects on the proliferation of naive and autoreactive B cells. This was consistent with comparable increases in the numbers of viable B cells in rsCD40L-treated C57BL/6 and 2-12H/Vκ8 cultures after 3 days (data not shown). Thus, the ability of sCD40L to repress Ig secretion was not due to prolonged proliferation.
Regulation of ASC differentiation by sCD40L is due to a block in Blimp-1, and XBP-1 transcription and not due to failure to exit the cell cycle. Proliferation of LPS-stimulated (30 μg/mL), CSFE-labeled C57BL/6 (A) and Sm-specific (2-12H/Vκ8) B cells (B), in the presence (—) or absence (▬) of rsCD40L (100 ng/mL), was determined on day 3 by FACs analysis. Data are representative of 5 experiments. C57BL/6 and Sm-specific (2-12H/Vκ8) B cells were stimulated with LPS (30 μg/mL) in the presence or absence of rsCD40L (100 ng/mL) for 3 days and the frequency of (C) intracellular IgMhi cells or (D) ASCs was determined by fluorescence-activated cell-sorting analysis or ELISPOT, respectively. LPS-stimulated C57BL/6 cultures (100%) had 5.6 × 104 to 7.8 × 104 ASCs/106 cells and 2-12H cultures had 7.6 × 104 to 3.8 × 105 ASCs/106 cells. Blimp-1 (E) and XBP-1 (F) mRNA levels were measured by real-time PCR in Sm-specific B cells after 3 days of LPS stimulation (30 μg/mL) in the presence or absence of rsCD40L (100 ng/mL). Statistical analysis was performed using 1-sample t test by comparing treated B-cell cultures with untreated, LPS-stimulated, B-cell cultures (control). Data represent at least 3 experiments (*P ≤ .05). Error bars represent plus or minus SEM.
Regulation of ASC differentiation by sCD40L is due to a block in Blimp-1, and XBP-1 transcription and not due to failure to exit the cell cycle. Proliferation of LPS-stimulated (30 μg/mL), CSFE-labeled C57BL/6 (A) and Sm-specific (2-12H/Vκ8) B cells (B), in the presence (—) or absence (▬) of rsCD40L (100 ng/mL), was determined on day 3 by FACs analysis. Data are representative of 5 experiments. C57BL/6 and Sm-specific (2-12H/Vκ8) B cells were stimulated with LPS (30 μg/mL) in the presence or absence of rsCD40L (100 ng/mL) for 3 days and the frequency of (C) intracellular IgMhi cells or (D) ASCs was determined by fluorescence-activated cell-sorting analysis or ELISPOT, respectively. LPS-stimulated C57BL/6 cultures (100%) had 5.6 × 104 to 7.8 × 104 ASCs/106 cells and 2-12H cultures had 7.6 × 104 to 3.8 × 105 ASCs/106 cells. Blimp-1 (E) and XBP-1 (F) mRNA levels were measured by real-time PCR in Sm-specific B cells after 3 days of LPS stimulation (30 μg/mL) in the presence or absence of rsCD40L (100 ng/mL). Statistical analysis was performed using 1-sample t test by comparing treated B-cell cultures with untreated, LPS-stimulated, B-cell cultures (control). Data represent at least 3 experiments (*P ≤ .05). Error bars represent plus or minus SEM.
Germinal center B cells stimulated through CD40 are blocked from terminally differentiating into ASCs.6,24,25 To determine whether sCD40L regulated autoreactive B cells by a mechanism similar to cell fate decisions in the germinal center, we quantitated intracellular IgM and enumerated plasma-cell formation. As shown in Figure 3C, 7% of Sm-specific B cells became intracellular IgMhi after 3 days of LPS-stimulation. Addition of rsCD40L reduced the number of intracellular IgMhi cells by 50%. Paralleling this decrease, the presence of rsCD40L inhibited the number of ASC by 49% (Figure 3D). In contrast, the number of intracellular IgMhi cells and the number of ASCs in LPS-stimulated C57BL/6 cultures was minimally affected by treatment with rsCD40L (Figure 3C,D). It is noteworthy that the number of intracellular IgMhi cells in nonautoreactive mice (C57BL/6) is significantly higher than autoreactive mice (2-12H/Vκ8). This might indicate that despite removal of the regulatory mechanisms conferred by DCs/MΦs, some cells maintain intrinsic regulatory mechanisms that repress Ig secretion. These data indicate that sCD40L prevents Ig secretion by inhibiting the differentiation of autoreactive B cells into plasma cells.
Terminal differentiation of B cells requires expression of the transcriptional activators, Blimp-1 and XBP-1. We reasoned that sCD40L might prevent autoantibody production by directly or indirectly regulating Blimp-1 and XBP-1. Real-time PCR analysis showed that Blimp-1 (Figure 3E) and XBP-1 (Figure 3F) mRNA was up-regulated by LPS stimulation of autoreactive B cells. However, treatment with rsCD40L reduced Blimp-1 and XBP-1 mRNA levels by 47% and 58%, respectively. Collectively, the data show that despite maintaining comparable proliferation, sCD40L blocks the formation of ASCs through the regulation of transcription factors required for terminal differentiation. This allows MΦs to regulate autoreactive MZ and FO B cells during innate immune responses.
The defects underlying autoimmune disease are poorly defined. Our data identify a unique mechanism of tolerance wherein MΦs repress Ig secretion through the secretion of IL-6 and sCD40L. To test the idea that MΦ-mediated tolerance is defective in lupus-prone mice, we compared the ability of C57BL/6 and MRL/lpr-derived MΦs to repress Ig secretion by Sm-specific B cells. MRL/lpr mice provide a good model to study the breakdown in B-cell tolerance because they develop an autoimmune disease similar to human SLE. As shown in Figure 4A, MΦs from C57BL/6 mice repressed 96% of Ig secretion when cocultured at a ratio of 20:1 (20 B cells to 1 MΦ), whereas MΦs from MRL/lpr mice repressed 82% of secretion. At a ratio of 100:1, MΦs from C57BL/6 mice repressed 77%, whereas MRL/lpr MΦs repressed 40% of Ig secretion. This indicates that MΦs derived from lupus-prone mice are defective in regulating autoantibody secretion by autoreactive B cells. To distinguish defects in the production of soluble mediators from the effects of cell-cell contact, we assessed the ability of CM from MRL/lpr MΦs to regulate Ig secretion. Similar to the results obtained from intact cells, CM from C57BL/6 MΦs repressed 77% of Ig secretion, whereas CM from MRL/lpr MΦs repressed 48% (Figure 4B). Because the defect in MΦ CM was comparable with the defect found when intact MΦs were cocultured with Sm-specific B cells, we reasoned that defects in the production of soluble mediators might be responsible. To address this, we quantitated IL-6 and CD40L levels. Consistent with previous reports, MΦs derived from MRL/lpr mice secreted significantly less IL-6 than MΦs from C57BL/6 mice (Figure 4C).26,27 We were unable to detect sCD40L in CM from C57BL/6 or MRL/lpr MΦs by ELISA; however, it was detectable by immunostaining. Unstimulated C57BL/6 and MRL/lpr MΦs showed negligible CD40L staining (data not shown). In contrast, LPS stimulation induced 55% of the C57BL/6 MΦs to express CD40L compared with 18% of MRL/lpr MΦs (data not shown). Most importantly, the amount of CD40L staining by LPS-stimulated MRL/lpr MΦs was 3-fold lower than LPS-stimulated C57BL/6 MΦs (Figure 4D). We further examined MΦ defects by comparing the repressive ability of CM from ex vivo MΦ isolated from C57BL/6, C57BL6.lpr, MRL, and predisease and postdisease MRL/lpr mice. As shown in Figure 4E, C57BL/6.lpr MΦ CM repressed Ig secretion comparable with C57BL/6 MΦ CM (1.1- vs 1-fold). In contrast, MRL MΦ CM was less repressive (0.64-fold). MRL/lpr MΦ CM derived from predisease and postdisease mice were equally defective at repressing Sm-specific Ig secretion (0.71- vs 0.72-fold). Thus, the data indicate that the MΦ defects are associated with the MRL background and that regardless of disease status, defects in regulating autoreactive B cells occur coincident with failure to secrete soluble mediators that repress terminal differentiation.
Repression of Ig secretion by MRL/lpr MΦs is defective coincident with a failure to secrete soluble mediators. Sm-specific B cells (1 × 105) were stimulated with LPS (30 μg/mL) and cocultured with the indicated number of C57BL/6 (n = 5) or MRL/lpr (n = 9) BMMΦs (A) or LPS-activated BMMΦ CM from C57BL/6 (n = 5) or MRL/lpr (n = 9) mice (B) for 4 days. IgMa/κ was determined by ELISA. LPS-stimulated B cells (100%) secreted 1.2-20 μg/mL. (C) IL-6 levels in LPS-activated BMMΦ CM from C57BL/6 (○) or MRL/lpr (●) MΦs were determined by ELISA. (D) MΦs derived from C57BL/6 and MRL/lpr mice were LPS stimulated (15 μg/mL) for 3 hours then stained with anti-CD40L. The quantitative data from 5 experiments (100 cells/experiment) is shown. The absolute number of LPS-stimulated C57BL/6 MΦs that expressed CD40L averaged 55%. (E) Sm-specific B cells (1 × 105) were stimulated with LPS (30 μg/mL) and cocultured with LPS-activated MΦ CM from ex vivo C57BL/6 (n = 6), C57BL/6.lpr (n = 6), MRL (n = 5), predisease MRL/lpr (n = 5), or postdisease MRL/lpr (n = 6) mice for 4 days. IgMa/κ was determined by ELISA. LPS-stimulated B cells (100%) secreted 1-4 μg/mL. Each circle represents an individual mouse. The horizontal bars mark the mean secretion. Statistical analysis was performed using the exact Wilcoxon rank-sum test to compare all experimental groups to LPS-stimulated C57BL/6 MΦs (panels A-C) or the one-sample t test to compare unstimulated cultures to stimulated cultures (panel D) or experimental groups to C57BL/6 MΦ CM (panel E) (*P ≤ .05).
Repression of Ig secretion by MRL/lpr MΦs is defective coincident with a failure to secrete soluble mediators. Sm-specific B cells (1 × 105) were stimulated with LPS (30 μg/mL) and cocultured with the indicated number of C57BL/6 (n = 5) or MRL/lpr (n = 9) BMMΦs (A) or LPS-activated BMMΦ CM from C57BL/6 (n = 5) or MRL/lpr (n = 9) mice (B) for 4 days. IgMa/κ was determined by ELISA. LPS-stimulated B cells (100%) secreted 1.2-20 μg/mL. (C) IL-6 levels in LPS-activated BMMΦ CM from C57BL/6 (○) or MRL/lpr (●) MΦs were determined by ELISA. (D) MΦs derived from C57BL/6 and MRL/lpr mice were LPS stimulated (15 μg/mL) for 3 hours then stained with anti-CD40L. The quantitative data from 5 experiments (100 cells/experiment) is shown. The absolute number of LPS-stimulated C57BL/6 MΦs that expressed CD40L averaged 55%. (E) Sm-specific B cells (1 × 105) were stimulated with LPS (30 μg/mL) and cocultured with LPS-activated MΦ CM from ex vivo C57BL/6 (n = 6), C57BL/6.lpr (n = 6), MRL (n = 5), predisease MRL/lpr (n = 5), or postdisease MRL/lpr (n = 6) mice for 4 days. IgMa/κ was determined by ELISA. LPS-stimulated B cells (100%) secreted 1-4 μg/mL. Each circle represents an individual mouse. The horizontal bars mark the mean secretion. Statistical analysis was performed using the exact Wilcoxon rank-sum test to compare all experimental groups to LPS-stimulated C57BL/6 MΦs (panels A-C) or the one-sample t test to compare unstimulated cultures to stimulated cultures (panel D) or experimental groups to C57BL/6 MΦ CM (panel E) (*P ≤ .05).
Discussion
Balancing tolerance and immunity requires strict regulation of Ig production by B lymphocytes. During T-dependent humoral immune responses, the binding of foreign antigen promotes immunity through Ig secretion. In contrast, persistent ligation of self-reactive BCRs ensures tolerance by desensitizing autoreactive receptors to subsequent stimulation. Likewise, innate immune responses require that autoreactive B cells remain unresponsive during polyclonal B-cell activation. Our data show that DCs and MΦs are key regulators of autoreactive B cells during innate immune responses.8 DCs and MΦs maintain low-affinity Sm-specific B cells in an unresponsive state, in part through the secretion of IL-6. Here we show that in addition to IL-6, MΦs secrete sCD40L to regulate autoreactive B cells. Similar to IL-6, sCD40L selectively repressed Ig secretion by B cells continuously exposed to self-antigen but had no affect on naive B cells. The ability of IL-6 and sCD40L to selectively repress chronically antigen experienced B cells was demonstrated with IgTg and non-Tg B cells, indicating that repression of Ig secretion occurs in mixed cell populations. Repression by sCD40L did not reflect changes in proliferation, but rather a reduction in the number of B cells that differentiated into ASCs. The importance of MΦs in maintaining B-cell tolerance was apparent in lupus prone, MRL/lpr mice. We found that compared with C57BL/6 mice, MΦs from MRL/lpr mice were significantly less efficient at repressing Ig secretion coincident with diminished production of IL-6 and CD40L. The defect was apparent in all MRL/lpr mice, regardless of their disease status. Likewise, defects in MRL/lpr DCs are associated with the MRL background.28 These data indicate that MΦs regulate autoreactive B cells by secreting repressive factors that inhibit the formation of ASCs. This mechanism of tolerance is diminished in lupus-prone mice, suggesting its role in the autoimmunity associated with SLE.
The ability of sCD40L to repress Ig secretion by autoreactive B cells is reminiscent of cell fate decisions in the germinal center. Ligation of CD40 enhances immunity by inducing proliferation, germinal center formation, and class switch recombination; however, CD40 signal transduction also inhibits differentiation of B cells and reduces Ig secretion by B-cell hybridomas.24,29-33 Furthermore, sustained CD40 signaling in germinal center B cells selects for memory cell formation by inhibiting differentiation of ASCs.6,24,25,34 The findings that sCD40L represses autoantibody production during innate immune responses expands our understanding of the pleiotropic nature of CD40L by identifying that CD40/CD40L interactions are important in more than T-dependent immune responses. Thus, molecules that have historically been thought to promote immunity also protect from autoimmunity by differentially regulating Ig secretion.
The diverse roles of CD40L in adaptive and innate immune responses suggest that other receptors influence the outcome of CD40 signal transduction. Our data showed that repression of Ig secretion by IL-6 and sCD40L selectively occurred in B cells continuously exposed to self-antigen. This suggests that the BCR regulates the outcome of signal transduction through other receptors (Figure 2).8 Although the BCR-derived signals remain to be elucidated, others have reported that persistent BCR stimulation induces Fas-mediated apoptosis, whereas cells undergoing transient stimulation remain refractory to Fas stimulation.7 In addition, persistent BCR stimulation regulates TLR9-mediated Ig secretion in an Erk-dependent manner.35 Cross-talk between the BCR and CD40 also lowers the signaling threshold for B cell activation and regulates BCR-mediated apoptosis.36-42 Thus, some aspects of B- cell fate are directed by the ability of persistent BCR ligation to alter the expression of Fas receptor or the outcome of CD40, TLR9, and IL-6 receptor ligation.
Activation of the innate immune response induces MΦs to secrete multiple soluble mediators that repress autoantibody secretion. Although IL-6 and sCD40L contribute to repression, either is sufficient, indicating that IL-6 and sCD40L possess redundant function in repressing autoantibody production (Figure 1D). Our data also showed that B cell subsets are differentially susceptible to repression by each of the soluble mediators (Figure 2D). This indicates that IL-6 and sCD40L possess specialized function wherein MΦs partially repress MZ B cells, whereas DCs and MΦs repress FO B cells. MZ B cells play an important role in T-independent immunity and express Ig receptors that recognize multiple antigens, including self-antigens. Although the identity of the MΦ subtype that represses Ig secretion remains undefined, it is interesting to speculate that the cells required for retention of MZ B cells in the spleen43 might regulate MZ B cell activation. Studies are under way to characterize repression of other B-cell subsets, including the pre-plasma cells that become dysregulated in lupus-prone mice.18 It is also noteworthy that the magnitude of MΦ-mediated repression of 2-12H MZ B cells (40%) was less than FO cells (87%), indicating that MZ B cells may be regulated by additional mechanisms (Figure 2D). Because 2-12/Vκ8 B cells do not have a significant population of MZ B cells, they are efficiently repressed by MΦs (96%) (Figure 4A). However, another possibility is that B cells expressing different affinity receptors may be more or less susceptible to DC/MΦ-mediated tolerance. This is evident when repression of HEL-specific B cells is compared with Sm-specific B cells. Both of these models contain comparable percentages of MZ B cells, yet IL-6 and sCD40L repress approximately 55% of Ig secretion by high-affinity HEL-specific B cells and 70% of secretion by low-affinity 2-12H/Vκ8 B cells (Figures 1C, 2A).8 Thus, our data identify DC/MΦ-mediated tolerance as a mechanism that regulates autoreactive B cells during innate immune responses and reveals that multiple factors differentially regulate unique B-cell subsets, raising the possibility that the location determines the factors required to regulate Ig secretion.
The identification of MΦs as regulatory cells that maintain B- cell tolerance raises the possibility that defects in MΦ function predisposes to autoimmunity and possibly SLE. Here, we show that MΦs from autoimmune-prone mice are deficient in the production of IL-6 and sCD40L. Previous studies identified that MΦs exhibit defects in IL-6 secretion that is triggered by apoptotic cells.26,27,44 Coincidently, MΦs from autoimmune mice and SLE patients are defective in phagocytosis of apoptotic cells.45,46 Thus, the diminished clearance of apoptotic cells may chronically suppress the secretion of tolerogenic factors, such as IL-6 and sCD40L. Reduced secretion of tolerogenic factors during an innate immune response would allow activation and terminal differentiation of autoreactive B cells. Alternatively, failure of lupus-prone B cells to be reprogrammed, such that CD40 and IL-6 receptor ligation does not repress Ig secretion, may lead to autoimmunity. Whether these mechanisms are dysregulated in vivo remains to be determined.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We thank Paul Carnathan, Jennifer Rutan, and Diane Carnathan for excellent technical assistance; Dr Robert Bagnell, Department of Pathology, Director of Microscopy Services Laboratory, for help with Qdot imaging and analysis; Dr Daniel Conrad for the CD40 ligand transfected cells; Dr Tom Tedder for the HEL-specific IgMa; Dr Marc Monestier for the nucleosome-specific IgG; and Dr Steve Clarke for critically reviewing the manuscript.
This work was supported by US Public Health Service grants AI53266 (to B.J.V.) and AI033613 (to L.J.W.). M.A.K. was supported by training grant AR07417 from the National Institute of Arthritis & Musculoskeletal & Skin Diseases and by Postdoctoral Fellowship grant PF04056 from the American Cancer Society.
Authorship
Contribution: M.A.K. directed and performed experiments, analyzed data, and wrote the manuscript; N.J.W. performed experiments and analyzed data; A.L.G. performed experiments; L.L. performed statistical analysis; K.A. and L.J.W. provided mice; and B.J.V. codirected experiments and wrote the manuscript.
Conflict-of-interest disclosure: B.J.V. and M.A.K. are among the inventors of a pending patent related to the work that is described in the present study. The remaining authors declare no competing financial interests.
Correspondence: Barbara Vilen, CB# 7290, University of North Carolina, Chapel Hill, NC 27599; e-mail: barb_vilen@med.unc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal