An international group of experts has pooled their data and produced an important new staging system for mycosis fungoides and Sézary syndrome.
Lymphoid malignancies, including non-Hodgkin lymphomas, Hodgkin disease, myeloma, and acute and chronic lymphoid leukemias, occur in more than 90 000 people each year in the United States. However, about 38 or so distinct diseases are derived from lymphoid cells, and the vast majority of patients have 1 of about 6 of those diseases. Thus, most types of lymphoid malignancies are rare, a fact that has led to some diagnostic and therapeutic confusion. Given that no single center is likely to see sufficient numbers of patients with each disease to make dramatic advances, the road forward in the diagnosis and management of these rare forms of lymphoma is paved by international cooperation.
The international cooperative effort in lymphoma has been led by a focus on defining the histopathology, molecular pathology, immunologic phenotype, and clinical features of the distinct disease entities. The World Health Organization (WHO) Classification of Lymphoid Malignancies1 was among the first steps in this cooperation. Subsequently, the special features of cutaneous lymphomas were recognized, and a new WHO–European Organisation for Research and Treatment of Cancer (EORTC) classification of cutaneous lymphomas was developed.2
Progress has been made in understanding the distinctive behavior of the various types of lymphoid tumors involving the skin. Accordingly, the staging system for mycosis fungoides and Sézary syndrome that was developed in the mid-1970s has been found to be imperfect at separating patients into distinct prognostic subsets. Therefore, another international cooperative effort has been made to update and modify the staging system for mycosis fungoides and Sézary syndrome to take into account the information that has emerged on this disease over the past 30 years. The report of the new system appears in this issue of Blood.
The new system reflects several important improvements. The notion of a suspicious lesion (T0) has been eliminated; now only patients with confirmed malignancy are staged. The definition of patch and plaque involvement of the skin has been more precisely defined (T1 or T2). Nodal involvement is assessed first on the basis of node size (> 1.5 cm, not > 1 cm, the criterion used for “abnormal” in other lymphomas). Nodes less than 1.5 cm are not biopsied. If one biopsies a larger node, its N grade is determined by the extent of involvement of cells with cerebriform nuclei; no such cells is N1, presence of such cells that do not efface the nodal architecture is N2, and partial or complete effacement of architecture by abnormal cells is N3. N1 and N2 are subgrouped based on the absence “a” or presence “b” of clonal cells. Visceral involvement is documented either by imaging (liver or spleen) or by biopsy (any other organ) and is considered unlikely in the absence of node and blood involvement. Blood involvement is assessed as absent (< 5% cells with cerebriform nuclei; B0), present in > 5% of peripheral blood lymphocytes but less than 20% (or a total of < 1000 cells with cerebriform nuclei/μL; B1), or extensive (≥ 1000 cells with cerebriform nuclei/μL; B3).
These new criteria are not likely to be the end of the changes that are required. Histologic features such as large-cell transformation and growth in a hair-centered (folliculotropic) pattern affect prognosis but are not yet incorporated into the new staging system. We still do not know whether patch disease that progresses through plaque and tumor stages is different from disease that presents as plaque disease. The detection of clonal T-cell receptor gene rearrangements in node and blood also exert an as-yet-undefined effect on disease progression. Methods of assessing the presence of such rearrangements and quantifying them need to be standardized. As data on these questions are collected, modifications of the new staging scheme will likely be needed.
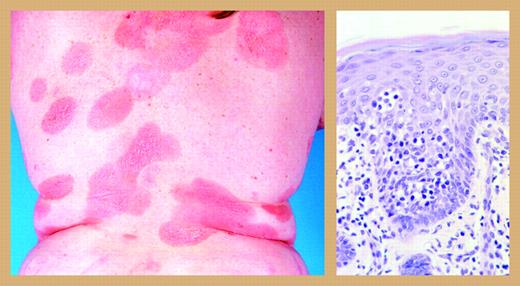
However, the existence of a critical mass of clinicians, pathologists, and laboratory scientists who are working together and thinking about the disease spells trouble for mycosis fungoides, the cancer that sounds like a fungal infection but kills like a cancer. By working together, unanswered questions will be answered more quickly and novel approaches to treatment can be explored collaboratively much faster than in the early days, when individual centers were working on their own. Mycosis fungoides responds to a wide range of treatments3 ; topical and systemic chemotherapy, electron beam radiation therapy, psoralens and ultraviolet light, and retinoids. Newer treatments with novel mechanisms of action, like vorinostat, a histone deacetylase inhibitor, denileukin diftitox, an IL-2 receptor targeting agent, and T-cell–specific monoclonal antibodies are active and may be able to be assembled into rational combinations with even greater efficacy. This disfiguring and disabling disease (see figure) is a superb candidate for eradication, and it seems that the in-ternational community now has the strategy, weapons, and tactics at hand to mount a well-planned and well-executed attack.
Mycosis fungoides. Reprinted from Willemze et al2 with permission.
Mycosis fungoides. Reprinted from Willemze et al2 with permission.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■
REFERENCES
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal