Nuclear localization of the CD40 cell membrane receptor bypasses the classic CD40 signalosome and enhances diffuse large B-cell lymphoma growth via direct interaction with the NF-κB pathway.
Upon ligand binding, canonical signaling evoked by cell-surface CD40 follows a multistep cascade requiring cytoplasmic adaptors (called TNF-receptor–associated factors [TRAFs], which are recruited by CD40 in the lipid rafts) and the IKK complex.1 Through NF-κB activation, the CD40 signalosome activates transcription of mutiple genes involved in B-cell growth and survival. Because the CD40 signalosome is active in aggressive lymphoma and contributes to tumor growth, immunotherapeutic strategies directed against CD40 are being designed and currently tested in clinical trials.2,3
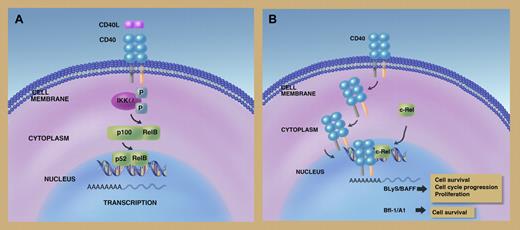
In this issue of Blood, Zhou and colleagues have identified a novel pathway by which CD40 can enhance transcription of genes promoting lymphomagenesis. In addition to its location in the cell membrane, the CD40 receptor is also able to enter the nucleus where it exerts important functions. Upon binding to c-Rel, a member of the NF-κB family, nuclear CD40 promotes transcription of the BLys/BAFF and Bfl-1/A1 genes in lymphoma cells. Cell survival, cell-cycle progression, and proliferation of lymphoma cells ensue. Importantly, results observed in lymphoma cell lines are also convincingly reproduced in lymphoma cells from bioptic specimens, ruling out potential artifacts due to in vitro culture. The fact that nuclear CD40 complexes with c-Rel in diffuse large B-cell lymphoma is remarkable because c-REL amplification is detectable in a sizeable proportion of cases, although the true biological significance of these amplification events is still under investigation.4
In localizing to the nucleus, CD40 follows a pattern displayed by some cell-surface receptors that was unexpected until recently, and that is rapidly expanding and consolidating.5 At least 2 other membrane receptor molecules, the epidermal growth factor receptor and the fibroblast growth factor receptor, have been found in the nucleus, revealing a novel mode of action for these receptors.
Diffuse large B-cell lymphoma is recognized as a highly heterogeneous disease. The pivotal study by Zhou and colleagues does not address the issue of whether the nuclear interaction between CD40 and c-Rel is a consistent finding of the disease, or, rather, clusters with specific biological and clinical subgroups of diffuse large B-cell lymphoma. Also, the degree of specificity for neoplastic lymphoma cells of the nuclear crosstalk between CD40 and c-Rel is still to be defined. Experiments addressing the nuclear transcription function of CD40 and c-Rel in normal germinal center B cells will fill the gap.
It is too early to say whether nuclear CD40 may pave the way to novel target therapies for lymphoma. Because of its subcellular compartmentalization, nuclear CD40 appears to be out of reach for conventional immunotherapeutic strategies exploiting anti-CD40 antibodies. Proteosome inhibition would not be a rational approach either, because, at variance with the canonical CD40 signalosome pathway, nuclear CD40 bypasses proteosome blockade and interacts with c-Rel further downstream in the signaling cascade. Yet the discovery of nuclear interplay between CD40 and the NF-κB system provides a new spin to the design of innovative molecular inhibitors and to the assessment of the potential benefit of CD40 and/or c-REL RNA silencing in diffuse large B-cell lymphoma.
Canonical CD40 signaling (A) in lymphoma cells follows a multistep cascade from the cell membrane to the nucleus, and involves the NF-κB pathway. Alternatively (B), CD40 may directly localize to the nucleus, where it complexes with c-Rel and activates transcription of genes promoting growth, cell-cycle progression, and cell survival of lymphoma cells. Professional illustration by Marie Dauenheimer.
Canonical CD40 signaling (A) in lymphoma cells follows a multistep cascade from the cell membrane to the nucleus, and involves the NF-κB pathway. Alternatively (B), CD40 may directly localize to the nucleus, where it complexes with c-Rel and activates transcription of genes promoting growth, cell-cycle progression, and cell survival of lymphoma cells. Professional illustration by Marie Dauenheimer.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal