Abstract
Familial hemophagocytic lymphohistiocytosis (FHL) is typically an early onset, fatal disease characterized by a sepsislike illness with cytopenia, hepatosplenomegaly, and deficient lymphocyte cytotoxicity. Disease-causing mutations have been identified in genes encoding perforin (PRF1/FHL2), Munc13-4 (UNC13D/FHL3), and syntaxin-11 (STX11/FHL4). In contrast to mutations leading to loss of perforin and Munc13-4 function, it is unclear how syntaxin-11 loss-of-function mutations contribute to disease. We show here that freshly isolated, resting natural killer (NK) cells and CD8+ T cells express syntaxin-11. In infants, NK cells are the predominant perforin-containing cell type. NK cells from FHL4 patients fail to degranulate when encountering susceptible target cells. Unexpectedly, IL-2 stimulation partially restores degranulation and cytotoxicity by NK cells, which could explain the less severe disease progression observed in FHL4 patients, compared with FHL2 and FHL3 patients. Since the effector T-cell compartment is still immature in infants, our data suggest that the observed defect in NK-cell degranulation may contribute to the pathophysiology of FHL, that evaluation of NK-cell degranulation in suspected FHL patients may facilitate diagnosis, and that these new insights may offer novel therapeutic possibilities.
Introduction
Hemophagocytic lymphohistiocytosis (HLH) is a rare disorder, with both familial and acquired forms.1–4 Familial hemophagocytic lymphohistiocytosis (FHL) has an autosomal recessive inheritance, typically affects infants, has an incidence of 1 in 50 000 births, and is usually fatal unless treated by chemoimmunotherapy and subsequent hematopoietic stem-cell transplantation.5 Disease-causing mutations in genes encoding perforin (PRF1, FHL2), Munc13-4 (UNC13D, FHL3), and syntaxin-11 (Stx11, STX11, FHL4) have been identified in families affected by FHL.6–8 Mutations in PRF1 or UNC13D account for approximately 15% to 50% and 15% to 25% of FHL patients, respectively, depending on geographic region.9 Mutations in STX11 were initially identified in patients of Turkish origin, where they account for approximately 20% of FHL patients.9,10 Viral infections may elicit the onset of disease.11,12 Specific criteria for diagnosis of HLH have been established and were recently revised.13 Typical findings include fever, massive hepatosplenomegaly, cytopenia, and hyperferritinemia.1–5,13 The patients are also characterized by defective natural killer (NK)–cell activity, polyclonal CD8+ T-cell expansions, a lymphohistiocytic infiltration of visceral organs associated with macrophage activation, and systemically elevated concentrations of proinflammatory cytokines such as interferon–γ (IFN-γ), tumor necrosis factor–α (TNF-α), interleukin-6 (IL-6), and IL-18.14–19 In contrast to FHL2 and FHL3 patients, FHL4 patients may display longer periods of disease-free remission in the absence of treatment.10 Other autosomal recessive syndromes associated with clinical manifestations similar to FHL, but additionally presenting hypopigmentation, include Griscelli syndrome type 2, Chédiak-Higashi syndrome, and Hermansky-Pudlak syndrome type 2 that result from mutations in genes encoding Rab27a (RAB27A), Lyst (LYST), and adaptor protein 3 (AP-3, ADTB3A), respectively.20–22 Generally, molecular defects in all these genes impair the secretory lysosome-dependent activity of cytotoxic lymphocytes.23,24
The effector function of cytotoxic lymphocytes is crucial for immune defense and homeostasis. Engagement of the T-cell receptor, as well as the FcRγIIIA (CD16) and receptors for natural cytotoxicity on NK cells, induces directed release of secretory lysosomes. These contain effector molecules such as perforin, granzymes, TNF-related apoptosis-inducing ligand (TRAIL), and Fas ligand (FasL) that induce target cell death through distinct pathways.23,25 Perforin-dependent cytotoxicity is an important pathway for immune surveillance of infected and transformed cells, as well as immune homeostasis.24,26–28 Death receptor engagement by ligands such as FasL contributes to immune homeostasis. Hereditary mutations in death receptor pathways may lead to lymphoproliferative diseases.29
Genes identified in FHL and related syndromes have been ascribed different roles in secretory lysosome biogenesis, polarization to the immune synapse, and degranulation. Lyst deficiency interferes with lysosome biogenesis and degranulation,30 whereas AP-3 deficiency impairs secretory lysosome movement along microtubules, polarization, and release at the immune synapse.31 Deficiency of either Rab27a or Munc13-4 impairs docking and degranulation of secretory lysosomes.7,20,32,33 How syntaxin 11-deficiency leads to FHL is less clear.26,34
We demonstrate here that Stx11 is expressed in human cytotoxic T cells and NK cells. Using freshly isolated lymphocytes from FHL4 patients, we show that a deficiency in Stx11 abrogates cytotoxic lymphocyte degranulation. Interestingly, stimulation with IL-2 partially restores degranulation by lymphocytes from such FHL4 patients. The present data suggest that defective NK-cell degranulation contributes to the pathophysiology of FHL4, that evaluation of NK-cell degranulation in patients with suspected FHL may facilitate diagnosis of specific FHL subtypes, and that regimens to either activate NK cells in vivo or reconstitute NK-cell activity in patients by infusion of allogeneic donor NK cells may be of therapeutic value. Furthermore, the results may provide an explanation for the less severe disease progression observed in FHL4 patients, compared with FHL2 and FHL3 patients.
Patients, materials, and methods
Patients and healthy control donors
This study was approved by The Regional Ethical Review Board in Stockholm (approval number 2006/228-31/3, 2006/229-31/3, and 2006/230-31/3). Peripheral blood was obtained with informed consent in accordance with the Declaration of Helsinki from parents of infants with suspected HLH. Genetic analysis was subsequently performed as described under “Mutation analysis.” As controls, blood was obtained with informed consent from healthy adults and infants (< 3 years old [range, 2-25 months; average, 10 months], without hematologic malignancies) from the Karolinska University Hospital. Patients were on HLH-2004 therapy at time of analysis, unless noted otherwise.
Cells
Peripheral blood mononuclear cells (PBMCs) were separated from whole blood or cord blood by density gradient centrifugation (Lymphoprep; Axis-Shield, Dundee, Scotland). Mononuclear cells were removed by plastic adherence. Peripheral blood lymphocytes (PBLs) were maintained in complete medium (RPMI 1640 medium supplemented with 2 mM l-glutamine and 10% fetal calf serum [all Invitrogen, Carlsbad, CA]). For IL-2 stimulation, PBLs cultured at 37°C for the indicated time in medium supplemented with 400 IU/mL recombinant human IL-2 (Chiron, Emeryville, CA). Human NK-cell and CD8+ T-cell populations were isolated from PBMCs by negative selection (Miltenyi Biotec, Auburn, CA). These cells were more than 98% CD3−CD56+ or CD3+CD8+, respectively, as determined by flow cytometry. The human erythroleukemia cell line K562 and the murine mastocytoma cell line P815 (both American Type Culture Collection, Manassas, VA) were maintained in complete medium.
Mutation analysis
Genomic DNA was isolated from peripheral blood or cultured fibroblasts by standard procedures. Primers were designed for amplification and direct DNA sequencing of each coding exon and flanking regions of PRF1,35 STX11,10 and UNC13D (primer sequences and sequencing conditions provided upon request). All sequencing reactions were done using BigDye terminators v3.1 Cycle Sequencing Kit (Applied Biosystems, Foster City, CA). Sequencing reactions were run on a ABI 3730 (Applied Biosystems) and analyzed in SeqScape (Applied Biosystems).
Antibodies
Monoclonal antibodies (Mabs) used for staining were anti-CD3 (UCHT1), anti-CD4 (SK3), anti-CD8 (SK1), anti-CD14 (MφP9), anti-CD16 (3G8), anti-CD56 (NCAM 16.2), anti-CD62L (Dreg56), anti-CD63 (H5C6), anti-CD107a (H4A3), anti-CCR7 (3D12), anti-granzyme A (CB9), and antiperforin (δG9) (all Becton Dickinson, Lincoln Park, NJ). For redirected antibody-dependent cellular cytotoxicity (ADCC) and plate-coated mAb assays, the following purified mouse mAbs were used: anti-CD3 (OKT3 and UCHT1); anti-CD16 (3G8); anti-CD56 (B159); anti-DNAM-1 (DX11); anti-NKG2D (1D11; all BD Bioscience, San Jose, CA); anti-2B4; anti-NKp46 (BAB281; both Beckman Coulter, Hialeah, FL); and isotype control (MOPC-21; Sigma, St Louis, MO). For confocal microscopy experiments, cells were stained with Alexa 647-conjugated antiperforin mAb (δG9; BioLegend, San Diego, CA). Cells were thereafter stained with Alexa 633–conjugated goat antimouse secondary antibodies (Invitrogen). For Western blotting, rabbit polyclonal antiactin (Abcam, Cambridge, United Kingdom) and anti-Stx11 (Valdez et al)36 antibodies were used.
RT-PCR
Templates for reverse-transcription–polymerase chain reaction (RT-PCR) reactions were made by oligo(dT) primed reverse transcription of poly(A)+ RNA as previously described.37 Primers to STX11 (forward 5′-CTCGCTCCCAGTCCAGGCAAAATG-3′ and reverse 5′-GCACAGGCTGGTTTGCAATTCTTG-3′), UNC13D (forward 5′-GAGGATGCCATTCTGCCCCTGATG-3′ and reverse 5′-GTACTTCCGGATGAGTTCCCG-3′), and GP3DH (forward 5′-CCATGGCACCGTCAAGGCTGAGAA-3′ and reverse 5′-GATGGCATGGACTGTGGTCATGAG-3′) were used.
Western blotting
Whole-cell lysates of primary cells or cell lines were separated by sodium dodecyl sulfate/polyacrylamide gel electrophoresis (SDS/PAGE) gels (12% acrylamide/Bis; Bio-Rad, Hercules, CA). The gels were blotted onto Hybond enhanced chemiluminescence (ECL) nitrocellulose membranes (Amersham, Arlington Heights, IL) and incubated with primary antibodies in phosphate-buffered saline (PBS) with 5% skimmed milk. After washes, the membranes were incubated with a goat anti-rabbit IgG horseradish peroxidase (Amersham) in PBS with 5% skimmed milk. Specific bands were visualized by ECL Western Blotting Detection Kit (Amersham) and luminescent image analyzer (Fujifilm; Fuji, Valhalla, NY).
Degranulation and cytokine assays
For quantification of secretory lysosome exocytosis, 2 × 105 PBLs were mixed with 2 × 105 K562 cells or 2 × 105 P815 cells supplemented with 2.5 mg/mL of the indicated mAbs for stimulation. Cells were incubated for 2 hours at 37°C in 5% CO2. Thereafter, cells were spun down, and cell pellets were resuspended in PBS with 2% fetal bovine serum (FBS) and 2 mM EDTA (ethylenediaminetetraacetic acid) and stained with anti-CD3-PerCP, anti-CD56-PE, and anti-CD107a-FITC (fluorescein isothiocyanate) mAbs, followed by flow cytometric analysis. Data were analyzed with FlowJo software (TreeStar, Ashland, OR). Lymphocytes were gated on forward scatter/side scatter. For analysis of T-cell populations, cells were stained with fluorochrome-conjugated anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD16, anti-CD62L, anti-CD107a, and anti-CCR7 and analyzed by flow cytometry (CyAn ADP). For intracellular staining, cells were spun down, and cell pellets were resuspended in PBS with 2% FBS and stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. Cells were fixed with 4% formaldehyde (Sigma) in PBS, permeabilized with PBS with 2% FBS and 0.5% saponin (Sigma), and stained with fluorochrome-conjugated anti-CD63, anti-CD107a, anti-granzyme A, and anti-perforin mAbs, followed by 4-color flow cytometric analysis (FACS Calibur; BD Bioscience). For activation of lymphocytes by plate-coated mAbs, flat-bottomed 96-well tissue culture plates (BD Bioscience) were coated with 50 μg/mL mAbs in PBS. Plates were washed and 2 × 105 PBLs were incubated in plates for 2 hours at 37°C. Thereafter, cells were stained and analyzed by flow cytometry as described above.
Cytotoxicity assays
Standard 4-hour chromium 51 (51Cr)-release assays were performed according to previously established protocols for clinical samples.18 Briefly, 4 × 10451Cr-labeled K562 target cells were incubated in triplicate with PBL effector cells. Effector–to–target cell ratios ranged from 50 to 3 in 200 mL complete medium in 96-well V-bottom plates. The supernatants were measured for 51Cr release on a gamma-counter (Cobra Auto-Gamma; Packard, Palo Alto, CA). Lytic units at 25% lysis were calculated.
Immunostaining and confocal microscopy
A total of 2.5 × 104 purified NK cells alone, or mixed with 2.5 × 104 K562 cells, were resuspended in RPMI medium and allowed to settle on poly(lysine)–coated coverslips (Erie Scientific, Portsmouth, NH) at 37°C for 15 minutes. The cells were fixated with PBS/2% paraformaldehyde and permeabilized with PBS/0.1% saponin (Sigma), nonspecific sites were saturated with PBS/10% FBS/0.1% saponin/0.1% BSA-c (Aurion, Wageningen, the Netherlands), and cells were stained with primary antibodies, followed by wash, blocking with 5% normal goat serum (Jackson ImmunoResearch, Bar Harbor, ME), secondary antibody staining, and mounting in fluorescent medium containing DAPI (Vector Laboratories, Burlingame, CA). Immunofluorescence and polarization of perforin were examined on a confocal microscope (DMIRE2; Leica, Heidelberg, Germany) with a 63×/130 glucerol objective. Images as previously described38 were processed using Leica Confocal Software (version 2.61) and Adobe Photoshop (version 9.0.2).
Statistics
Statistical analysis was performed using unpaired Student t test.
Results
Expression of Stx11 in cytotoxic lymphocytes
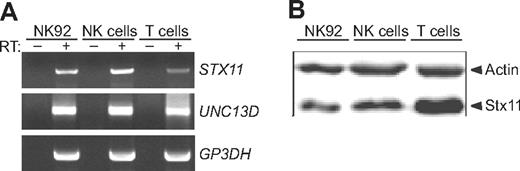
In the initial study that identified mutations in human Stx11 as a cause of FHL4, Stx11 expression was detected in monocytes but not in lymphocytes.8 In contrast, other studies have reported expression of Stx11 in rat primary and secondary lymphoid compartments, such as the spleen, thymus, and lymph nodes as well as in human B lymphocytes.36,39 Perforin and Munc13-4, which are defective in individuals with FHL2 and FHL3, respectively, are expressed in cytotoxic lymphocytes. To determine whether Stx11 is expressed in cytotoxic lymphocytes, RNA from purified, unstimulated CD8+ T cells, and NK cells, as well as the NK-cell line NK92 was prepared and amplified by RT-PCR using primers specific for STX11 and UNC13D. Transcripts of STX11 and of UNC13D were expressed in primary unstimulated NK cells and CD8+ T cells, as well as in NK92 cells (Figure 1A). These cells also expressed transcripts of other genes implicated in exocytosis of secretory lysosomes, such as RAB27A and SNAP23 (data not shown). Furthermore, expression of a 35-kDa protein corresponding to the predicted molecular mass of Stx11 was detected in purified populations of unstimulated NK cells, CD8+ T cells, and NK92 cells by Western blotting with a well-characterized anti-Stx11 polyclonal serum (Figure 1B). The detection of Stx11 in human cytotoxic lymphocytes suggests that this protein could play a role in the process leading to degranulation of secretory lysosomes.
Transcription and expression of Stx11 in cytotoxic lymphocytes. NK cells or CD8+ T cells were isolated from PBMCs by negative selection. (A) cDNA was prepared from cells and cell lines. RT-PCR was performed with gene-specific primers as indicated. (B) Stx11 expression relative to actin control was measured by immunoblotting in lysates derived from purified cell CD8+ T-cell or NK-cell populations, as well as the NK-cell line NK92. Arrowheads indicate bands corresponding to actin and Stx11 immunoreactivity.
Transcription and expression of Stx11 in cytotoxic lymphocytes. NK cells or CD8+ T cells were isolated from PBMCs by negative selection. (A) cDNA was prepared from cells and cell lines. RT-PCR was performed with gene-specific primers as indicated. (B) Stx11 expression relative to actin control was measured by immunoblotting in lysates derived from purified cell CD8+ T-cell or NK-cell populations, as well as the NK-cell line NK92. Arrowheads indicate bands corresponding to actin and Stx11 immunoreactivity.
Degranulation of lymphocyte subsets
A technical difficulty in studies of FHL is the limited amount of peripheral blood available from infants. Therefore, a recently developed assay40 was adopted to measure degranulation of cells freshly isolated from a small amount of peripheral blood from infants by assessing induction of CD107a surface expression. CD107a (LAMP-1) is a transmembrane protein in cytotoxic granules, which is exposed at the cell surface after degranulation. Surface CD107a can be quantified on peripheral blood lymphocytes (PBLs) by flow cytometry. Here, stimulation of peripheral blood mononuclear cells depleted of adherent cells was achieved by reverse antibody-dependent cellular cytotoxicity (ADCC) after incubation of mouse FcR+ P815 cells with anti-CD3 or anti-CD16 mAbs, or by mixing with K562 cells. Cells were analyzed by flow cytometry and lymphocytes were gated on forward scatter/side scatter characteristics and further identified on the basis of expression of the lineage markers CD3 and CD56 (Figure 2A). CD107a was induced on subsets of lymphocytes after incubation with P815 cells with anti-CD3 or anti-CD16 mAbs, or after incubation with K562 cells (Figure 2B). Gating on CD3+ T cells revealed CD107a surface expression on up to 10% of T cells after incubation with P815 cells and anti-CD3 or anti-CD16 mAbs, but not with K562 cells (Figure 2B). Gating on CD3−CD56+ NK cells revealed CD107a surface expression on approximately 20% of NK cells after incubation with K562 cells, and after incubation with P815 cells and anti-CD16 mAb, but not with P815 cells and anti-CD3 mAb (Figure 2B). None of these stimuli induced much CD107a surface expression on CD3−CD56− lymphocytes (Figure 2B). In summary, analysis of CD107a cell surface expression enables identification of patients with deficiencies in lymphocyte degranulation. Notably, this method can be used to identify degranulation defects limited to specific receptors or lymphocyte subsets.
Methodological platform and relative degranulating capacity of lymphocyte subsets. (A-D) Freshly isolated, resting PBLs alone or mixed with target cells and mAbs as indicated were incubated for 2 hours at 37°C. (A,B) Thereafter, cells were stained with fluorochrome-conjugated anti-CD3, -CD56, and -CD107a mAbs and analyzed by flow cytometry. (A) Lymphocytes were gated on forward scatter/side scatter plots, followed by gating of CD3+, CD3−CD56+, and CD3−CD56− cell subsets. (B) Induced CD107a surface expression (ΔCD107a+) was calculated as the percentage of CD107a+ cells after indicated stimulation subtracted from the percentage of CD107a+ cells after incubation of PBLs alone. For gating on CD3+ cells, a fluorochrome-conjugated anti-CD3 mAb was used. Values represent the mean (± SD) of 6 donors. (C,D) After indicated stimulation, cells were stained with fluorochrome-conjugated anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD16, anti-CD62L, anti-CD107a, and anti-CCR7 mAbs. (C) T cells were gated on forward scatter/side scatter plots for lymphocytes (R1 gate), followed by forward scatter/forward area plots (R2 gate), and gated on CD3+CD14− cells (R3 gate). Contour plots show CD3+CD14− T-cell populations (R3 gate) overlayed with dots representing CD3+CD14−CD107a+ T cells (R4 gate). The frequencies of degranulating T cells are indicated. Numbers indicate the percentage of degranulating T cells within each quadrant. (D) Induced CD107a surface expression was determined for indicated CD3+CD14− T-cell subsets. Values represent the mean (± SD) of 5 donors. Numbers indicate mean frequencies of CD3+CD14− T-cell subsets and their SD. (E) Resting PBLs were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD16-, anti-CD56, and anti-CD62L mAbs, followed by fixation, permeabilization, and intracellular staining with anti-CD107a, antiperforin, antigranzyme A, or anti-CD63 mAbs. Values represent relative mean fluorescence intensity (R-MFI), where MFI values for indicated staining have been subtracted for MFI values for isotype control mAbs. Values represent mean (± SD) of 5 donors.
Methodological platform and relative degranulating capacity of lymphocyte subsets. (A-D) Freshly isolated, resting PBLs alone or mixed with target cells and mAbs as indicated were incubated for 2 hours at 37°C. (A,B) Thereafter, cells were stained with fluorochrome-conjugated anti-CD3, -CD56, and -CD107a mAbs and analyzed by flow cytometry. (A) Lymphocytes were gated on forward scatter/side scatter plots, followed by gating of CD3+, CD3−CD56+, and CD3−CD56− cell subsets. (B) Induced CD107a surface expression (ΔCD107a+) was calculated as the percentage of CD107a+ cells after indicated stimulation subtracted from the percentage of CD107a+ cells after incubation of PBLs alone. For gating on CD3+ cells, a fluorochrome-conjugated anti-CD3 mAb was used. Values represent the mean (± SD) of 6 donors. (C,D) After indicated stimulation, cells were stained with fluorochrome-conjugated anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD16, anti-CD62L, anti-CD107a, and anti-CCR7 mAbs. (C) T cells were gated on forward scatter/side scatter plots for lymphocytes (R1 gate), followed by forward scatter/forward area plots (R2 gate), and gated on CD3+CD14− cells (R3 gate). Contour plots show CD3+CD14− T-cell populations (R3 gate) overlayed with dots representing CD3+CD14−CD107a+ T cells (R4 gate). The frequencies of degranulating T cells are indicated. Numbers indicate the percentage of degranulating T cells within each quadrant. (D) Induced CD107a surface expression was determined for indicated CD3+CD14− T-cell subsets. Values represent the mean (± SD) of 5 donors. Numbers indicate mean frequencies of CD3+CD14− T-cell subsets and their SD. (E) Resting PBLs were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD16-, anti-CD56, and anti-CD62L mAbs, followed by fixation, permeabilization, and intracellular staining with anti-CD107a, antiperforin, antigranzyme A, or anti-CD63 mAbs. Values represent relative mean fluorescence intensity (R-MFI), where MFI values for indicated staining have been subtracted for MFI values for isotype control mAbs. Values represent mean (± SD) of 5 donors.
Lymphocytes display functional heterogeneity. Only a small proportion of NK cells or T cells degranulated in response to activating stimuli, in spite of uniform expression of activating receptors. While it is known that NK cells expressing inhibitory receptors for self-MHC are more responsive to activation signals,41–43 which could explain the selective degranulation by a subset of NK cells, it is not clear what dictates T-cell responsiveness. Therefore, we examined degranulating T cells by 8-color flow cytometry for a number of lineage and activation markers such as CD4, CD7, CD8, CD16, CD25, CD27, CD45RA, CD57, CD62L, and CCR7 to phenotypically characterize degranulating cells. Of these markers, generally all but CD62L and CCR7 were observed on degranulating T cells (data not shown). After triggering with P815 cells and anti-CD3 mAb, the majority of CD107a+ cells were CD3+CD4−CD8+CD62L−CCR7− (Figure 2C,D), corresponding to effector T cells.44 T cells that degranulated in response to P815 cells and anti-CD16 mAb were CD16+ (data not shown). In response to anti-CD16 mAb, CD107a+ T cells were mainly CD4−CD8+CD62L−CCR7− effector cells and CD4−CD8−CD62L−CCR7− cells (Figure 2C,D).
Since the assay used here to determine lymphocyte degranulation relies on CD107a expression, and was used to address degranulation in different subsets of total PBLs, it prompted an evaluation of intracellular CD107a expression. Whereas intracellular CD107a was detected in all PBLs, the intensity of CD107a staining varied among individual subsets (Figure 2E). CD107ahigh expression correlated with expression of perforin and granzyme A (Figure 2E). CD56dim NK cells, which express high levels of perforin, had a 5-fold higher fluorescence intensity of CD107a than perforin-negative CD4 or naive CD8+CD62L+ T cells. Notably, expression of another lysosomal protein, CD63, did not correlate with perforin expression (Figure 2E). Therefore, CD107ahigh expression marks cytotoxic lymphocytes, consistent with the extensive colocalization of CD107a and perforin observed by confocal microscopy in NK cells.38
Defective NK-cell degranulation and cytotoxicity in FHL4 patients
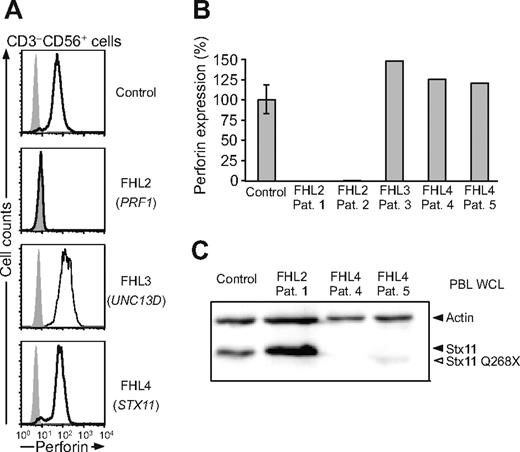
Next, degranulation and cytotoxicity were examined in lymphocytes of FHL patients. Five FHL patients, fulfilling clinical criteria of FHL and with defined mutations in the 3 known FHL-linked genes, were identified (Table 1). Patient 1 was homozygous for a nucleotide substitution in PRF1 (FHL2, 666C>A) with a predicted H222Q amino acid change. Patient 2 carried compound heterozygote mutations in PRF1 (949G>A and 1288G>T) with predicted E317R and D430Y amino acid changes, respectively. Two of these 3 PRF1 mutations have been reported previously,45,46 but 1288G>T is a novel mutation. As expected, little or no perforin was detected in lymphocytes of patients 1 and 2 by flow cytometry (Figure 3A-B). Patient 3 was homozygous for a novel nucleotide substitution in UNC13D (FHL3, 1145G>A) with a predicted premature stop codon (W382X). Patient 4 was homozygous for a novel single nucleotide deletion in STX11 (FHL4, 110-1C>G), which leads to a frameshift and an early stop codon (T37fsX62). Patient 5 was homozygous for a previously described nucleotide substitution in STX11 (FHL4, 802C>T),8,10 with a predicted premature stop codon (Q268X) leading to a protein lacking the C-terminal cysteine-rich motif. Western blot analysis of PBL lysates with an anti-Stx11 polyclonal serum revealed either no or a truncated protein in the FHL4 patients (Figure 3C). Perforin was expressed at high levels in lymphocytes from patients with UNC13D and STX11 mutations relative to healthy adult controls, as demonstrated by flow cytometry (Figure 3A,B).
Clinical findings at diagnosis and mutational analysis of the FHL patients
| Characteristics . | Patient no. . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |
| Ethnic origin | Netherlands | Sweden | Pakistan | Lebanon | Turkey |
| Consanguinity | No | No | Yes | Yes | Yes |
| Sex | Female | Male | Male | Male | Male |
| Affected gene | PRF1 | PRF1 | UNC13D | STX11 | STX11 |
| Mutation* | H222Q | E317R + D430Y | W382X | T37FsX62 | Q268X |
| Age at diagnosis, mo | 2 | 0 | 2 | 7 | 72 |
| Fever higher than 38°C | + | + | + | + | + |
| Splenomegaly | + | + | + | + | + |
| Pancytopenia | + | + | + | + | + |
| Hypertriglyceridemia | − | + | + | + | + |
| Hypofibrinogenemia | + | + | + | + | + |
| Ferritin, μg/L | 22000 | 16000 | 20000 | 5000 | 1000 |
| NK cell activity | Absent | Partial restoration by IL-2 | Partial restoration by IL-2 | Partial restoration by IL-2 | Partial restoration by IL-2 |
| Hemophagocytosis | + | − | + | + | + |
| Outcome | Alive and well after SCT | Dead before SCT | Alive and well, SCT planned | Alive and well after SCT | Alive and well after SCT |
| Characteristics . | Patient no. . | ||||
|---|---|---|---|---|---|
| 1 . | 2 . | 3 . | 4 . | 5 . | |
| Ethnic origin | Netherlands | Sweden | Pakistan | Lebanon | Turkey |
| Consanguinity | No | No | Yes | Yes | Yes |
| Sex | Female | Male | Male | Male | Male |
| Affected gene | PRF1 | PRF1 | UNC13D | STX11 | STX11 |
| Mutation* | H222Q | E317R + D430Y | W382X | T37FsX62 | Q268X |
| Age at diagnosis, mo | 2 | 0 | 2 | 7 | 72 |
| Fever higher than 38°C | + | + | + | + | + |
| Splenomegaly | + | + | + | + | + |
| Pancytopenia | + | + | + | + | + |
| Hypertriglyceridemia | − | + | + | + | + |
| Hypofibrinogenemia | + | + | + | + | + |
| Ferritin, μg/L | 22000 | 16000 | 20000 | 5000 | 1000 |
| NK cell activity | Absent | Partial restoration by IL-2 | Partial restoration by IL-2 | Partial restoration by IL-2 | Partial restoration by IL-2 |
| Hemophagocytosis | + | − | + | + | + |
| Outcome | Alive and well after SCT | Dead before SCT | Alive and well, SCT planned | Alive and well after SCT | Alive and well after SCT |
+ indicates presence; −, absence; SCT, stem cell transplantation.
Patient no. 1: H222Q = c.(666C>A) + c.(666C>A) p.H222Q; no. 2: E317R + D430Y = c.(949G>A) + c.(1288G>T) p.E317R + D430Y; no. 3: W382X = c.(1145G>A) + c.(1145G>A) p.W382X; no. 4: T37FsX62 = c.(110delC) + c.(110delC) p.T37FsX62; and no. 5: Q268X = c.(802C>T) + c.(802C>T) p.Q268X.
Expression of perforin and Stx11 in FHL patients. (A) PBLs were stained with fluorochrome-conjugated anti-CD3, and anti-CD56 mAbs. Thereafter, cells were fixed, permeabilized, stained with antiperforin mAb, and analyzed by flow cytometry. Histograms depict perforin expression in CD3−CD56+ NK cells. (B) Quantification of perforin staining by flow cytometry. Values represent relative mean fluorescence of perforin in CD3−CD56+ NK cells as percent of adult control cells. The error bar indicates the SD of normalized perforin expression for 12 healthy controls. (C) Stx11 expression relative to actin control was measured by immunoblotting. Arrowheads indicate bands corresponding to actin and Stx11 immunoreactivity.
Expression of perforin and Stx11 in FHL patients. (A) PBLs were stained with fluorochrome-conjugated anti-CD3, and anti-CD56 mAbs. Thereafter, cells were fixed, permeabilized, stained with antiperforin mAb, and analyzed by flow cytometry. Histograms depict perforin expression in CD3−CD56+ NK cells. (B) Quantification of perforin staining by flow cytometry. Values represent relative mean fluorescence of perforin in CD3−CD56+ NK cells as percent of adult control cells. The error bar indicates the SD of normalized perforin expression for 12 healthy controls. (C) Stx11 expression relative to actin control was measured by immunoblotting. Arrowheads indicate bands corresponding to actin and Stx11 immunoreactivity.
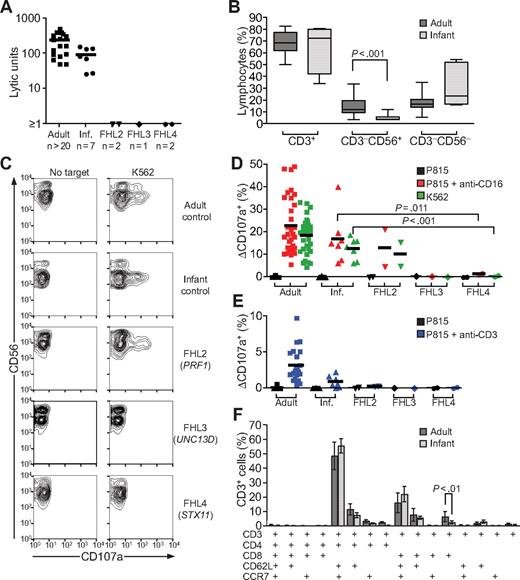
As expected, freshly isolated, unstimulated PBLs from healthy adult and infant donors lysed K562 cells. In contrast, freshly isolated, unstimulated PBLs from FHL2, FHL3, and FHL4 patients did not lyse K562 cells (Figure 4A). Notably, PBLs from healthy infant donors (controls) were relatively less efficient at lysis of K562 cells than PBLs from healthy adult controls (Figure 4A). This could be explained by a significantly larger NK-cell subset, as percentage of total lymphocytes, in adult relative to infant donors (14.2% ± 7.1%; n = 33 [mean ± SD]; and 5.2% ± 3.1%, n = 7, respectively; P < .001; Figure 4B).
Abrogation of degranulation and cytotoxicity by resting PBLs from FHL4 patients. (A) Resting PBLs from healthy adult and infant donors plus FHL2, FHL3, and FHL4 patients were evaluated for cytotoxicity toward K562 cells in 4-hour 51Cr release assays. Lytic units were calculated from specific lysis values. (B) Resting PBLs from adult and infant donors were stained with anti-CD3 and -CD56 mAbs and analyzed by flow cytometry. The percentage of indicated lymphocyte subsets was determined. Bars indicate the mean (±SD) of 7 donors. (C-E) Resting PBLs were incubated alone or with target cells as indicated for 2 hours at 37°C. Thereafter, cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. (C) Lymphocytes were gated on forward scatter/side scatter plots, followed by gating on CD3 versus CD56 plots. Profiles show CD56 versus CD107a mAb staining of CD3−CD56+ NK cells. (D) Induced CD107a surface expression on CD3−CD56+ NK cells after indicated stimulation was plotted. Lines represent mean values. (E) Induced CD107a surface expression on lymphocytes after indicated stimulation was plotted. Lines represent mean values. (F) Resting PBLs from healthy adults or infant donors were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD62L, and anti-CCR7 mAbs. Values represent the mean (±SD) percentage of indicated CD3+CD14− T-cell subsets.
Abrogation of degranulation and cytotoxicity by resting PBLs from FHL4 patients. (A) Resting PBLs from healthy adult and infant donors plus FHL2, FHL3, and FHL4 patients were evaluated for cytotoxicity toward K562 cells in 4-hour 51Cr release assays. Lytic units were calculated from specific lysis values. (B) Resting PBLs from adult and infant donors were stained with anti-CD3 and -CD56 mAbs and analyzed by flow cytometry. The percentage of indicated lymphocyte subsets was determined. Bars indicate the mean (±SD) of 7 donors. (C-E) Resting PBLs were incubated alone or with target cells as indicated for 2 hours at 37°C. Thereafter, cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. (C) Lymphocytes were gated on forward scatter/side scatter plots, followed by gating on CD3 versus CD56 plots. Profiles show CD56 versus CD107a mAb staining of CD3−CD56+ NK cells. (D) Induced CD107a surface expression on CD3−CD56+ NK cells after indicated stimulation was plotted. Lines represent mean values. (E) Induced CD107a surface expression on lymphocytes after indicated stimulation was plotted. Lines represent mean values. (F) Resting PBLs from healthy adults or infant donors were stained with anti-CD3, anti-CD4, anti-CD8, anti-CD14, anti-CD62L, and anti-CCR7 mAbs. Values represent the mean (±SD) percentage of indicated CD3+CD14− T-cell subsets.
To assess degranulation, freshly isolated PBLs were mixed with target cells and CD107a surface expression was evaluated. After incubation with K562 cells, comparable degranulation was observed in NK cells from both healthy adult and infant donors (18.5% ± 7.9%, n = 33 and 12.6% ± 4.8%; n = 7, respectively, Figure 4C,D). Degranulation was similar in the FHL2 patients (patient 1: 15.6%; patient 2: 4.9%, Figure 4C,D). In contrast, no degranulation was observed in FHL3 or FHL4 patients, as assessed by CD107a surface expression on NK cells after incubation with K562 cells (patient 3: 0.1%; patient 4: 0.8%; and patient 5: 0.3%; Figure 4C,D). Importantly, PBLs from all patients expressed normal levels of intracellular CD107a (data not shown). The lack of degranulation in NK cells from FHL3 patients supports recent findings published by Marcenaro et al.47 Similarly, anti-CD16 mAb stimulation induced CD107a surface expression on NK cells from adult and infant donors (22.7% ± 12.4%; n = 33 and 16.9% ± 12.2%; n = 7, respectively), the FHL2 patient (patient 1: 21.3%; patient 2: 4.7%), but not the FHL3 and FHL4 patients (patient 3: 0.4%; patient 4: 1.8%; and patient 5: 1.1%; Figure 4C-D).
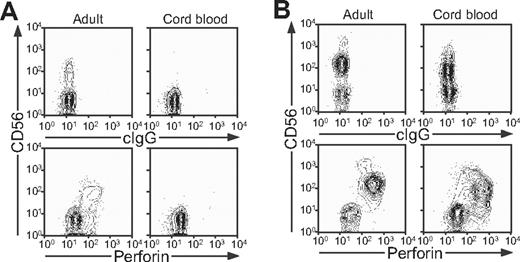
Since T-cell dysfunction has been viewed as a cause of FHL, degranulation by T cells was also evaluated. Compared with adult donors, stimulation of infant T cells with anti-CD3 mAb induced significantly (P < .001) less CD107a surface expression. Negligible CD107a surface expression was observed in FHL2, FHL3, and FHL4 patients (Figure 4E). The latter results can be explained by the fact that infant donors have a reduced frequency of CD8+CD62L−CCR7− effector T cells (P < .01, Figure 4F), consistent with an immature immune system. Therefore, assessment of degranulation by NK cells, rather than T cells, appears to be a more sensitive and consistent marker of dysfunctional degranulation by lymphocytes from infants. At birth, perforin expression is readily detected in cord blood NK cells, but not T cells (Figure 5A,B). The lack of perforin-expressing T cells in cord blood is in accordance with the low frequency of effector T cells in infants. Since FHL appears to be a disease uniquely linked to perforin and perforin release, these data suggest an important role for NK cells in the pathophysiology of FHL in infant patients.
Perforin expression in adult and cord blood NK cells and T cells. Cells from adult peripheral and cord blood donors were stained with fluorochrome-conjugated anti-CD3 and -CD56 mAbs, followed by fixation, permeabilization, and intracellular staining with antiperforin mAb. Lymphocytes were gated on forward scatter/side scatter plots, followed by gating on CD3+ and CD3− cells. Profiles show (A) CD56 versus perforin mAb staining of CD3+ T cells and (B) CD56 versus perforin mAb staining of CD3− cells.
Perforin expression in adult and cord blood NK cells and T cells. Cells from adult peripheral and cord blood donors were stained with fluorochrome-conjugated anti-CD3 and -CD56 mAbs, followed by fixation, permeabilization, and intracellular staining with antiperforin mAb. Lymphocytes were gated on forward scatter/side scatter plots, followed by gating on CD3+ and CD3− cells. Profiles show (A) CD56 versus perforin mAb staining of CD3+ T cells and (B) CD56 versus perforin mAb staining of CD3− cells.
Polarization of perforin in FHL4 NK cells
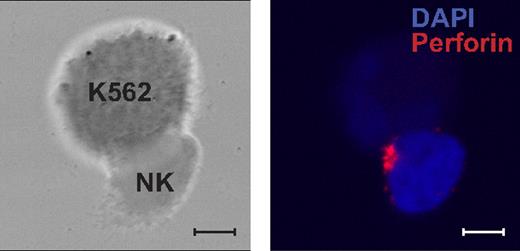
To attain more insight into the functional defects in Stx11-deficient cytotoxic lymphocytes, other readouts of cellular activation were assessed. Cross-linking of CD3 or CD16 on T cells and NK cells, respectively, induced intracellular Ca2+ mobilization in lymphocytes from FHL4 patients. Moreover, to test whether Stx11 is required for a step preceding granule movement, resting NK cells from a FHL4 patient were stained for perforin after mixing with sensitive target cells (Figure 6). NK cells from the FHL4 patient polarized perforin-containing granules toward the K562 target cells to a similar extent as observed with NK cells from healthy donors (11 [48%] of 23 conjugates showed perforin polarized toward K562 target cells in NK cells from FHL4 as opposed 48 [51%] of 95 conjugates in NK cells from healthy controls). Polarization of cytotoxic granules toward target cells in Stx11-deficient cells suggests that Stx11 is required for a late step in vesicle docking or fusion with the plasma membrane. Furthermore, incubation of PBLs of FHL4 patients with K562 cells, or PBL stimulation with anti-CD16 mAb induced TNF-α and IFN-γ production by NK cells, as assessed by intracellular staining after 6 hours of stimulation (data not shown), in accordance with the high cytokine levels measured in serum from FHL patients. Thus, the impairment in FHL4 patients specifically affected degranulation, without apparent perturbation of proximal activation signals or release of cytokines, such as TNF-α and IFN-γ. PBLs from parents of FHL patients, with a single mutated STX11 or UNC13D allele, displayed normal degranulation (data not shown).
Polarization of secretory lysosomes in Stx11-deficient resting NK cells. Resting NK cells from a FHL4 patient mixed with K562 target cells. Cells were fixed, permeabilized, and stained with antiperforin mAb (red) and DAPI (blue). Left panel shows a differential interference contrast (DIC) image. The right panel shows overlay of nuclear staining with DAPI and antiperforin mAb. Scales represent 5 μm.
Polarization of secretory lysosomes in Stx11-deficient resting NK cells. Resting NK cells from a FHL4 patient mixed with K562 target cells. Cells were fixed, permeabilized, and stained with antiperforin mAb (red) and DAPI (blue). Left panel shows a differential interference contrast (DIC) image. The right panel shows overlay of nuclear staining with DAPI and antiperforin mAb. Scales represent 5 μm.
Degranulation and cytotoxicity are partially restored by IL-2 stimulation of FHL4 NK cells
Stimulation of PBLs with IL-2 or mitogens partially restores cytotoxic function in some FHL patients, with the exception of those with NK-cell deficiency subtype 2.18,19 To study this functional restoration in more detail, cytotoxicity and degranulation in PBLs after stimulation with 400 IU/mL IL-2 for 72 hours was quantified (Figure 7A-E). After exposure to IL-2, PBLs from both control adult and infant donors were more efficient at lysing K562 cells than resting PBLs (Figure 7A). Whereas no or some cytotoxicity was observed in FHL2 patients with missense mutations in perforin (patients 1, 2; Figure 7A), cytotoxicity in FHL3 and FHL4 patients was partially restored by IL-2 stimulation (patients 3-5; Figure 7A).
IL-2 partially restores degranulation and cytotoxicity by PBLs from FHL4 patients. (A-E) PBLs from healthy adult and infant donors, plus FHL2, FHL3, and FHL4 patients were stimulated with 400 IU/mL IL-2 for 72 hours. (A) IL-2–stimulated PBLs were evaluated for cytotoxicity toward K562 cells in 4-hour 51Cr release assays. Lytic units were calculated from specific lysis values. (B) PBLs were maintained in medium or medium with IL-2 added for the indicated times. Thereafter, PBLs were incubated alone or with K562 cells as indicated for 2 hours at 37°C. Cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. Induced CD107a surface expression on CD3−CD56+ NK cells after incubation with K562 cells relative to PBLs alone was plotted as a function of stimulation time with IL-2. (C-E) PBLs stimulated with IL-2 for 72 hours were incubated alone or with target cells as indicated for 2 hours at 37°C. Thereafter, cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. (C) Lymphocytes were gated on forward scatter/side scatter plots, followed by gating on CD3 versus CD56 plots. Profiles show CD56 versus CD107a mAb staining of CD3−CD56+ NK cells. (D) Induced CD107a surface expression on CD3−CD56+ NK cells after indicated stimulation was plotted. Lines represent mean values. (E) Induced CD107a surface expression on lymphocytes after indicated stimulation was plotted. Lines represent mean values.
IL-2 partially restores degranulation and cytotoxicity by PBLs from FHL4 patients. (A-E) PBLs from healthy adult and infant donors, plus FHL2, FHL3, and FHL4 patients were stimulated with 400 IU/mL IL-2 for 72 hours. (A) IL-2–stimulated PBLs were evaluated for cytotoxicity toward K562 cells in 4-hour 51Cr release assays. Lytic units were calculated from specific lysis values. (B) PBLs were maintained in medium or medium with IL-2 added for the indicated times. Thereafter, PBLs were incubated alone or with K562 cells as indicated for 2 hours at 37°C. Cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. Induced CD107a surface expression on CD3−CD56+ NK cells after incubation with K562 cells relative to PBLs alone was plotted as a function of stimulation time with IL-2. (C-E) PBLs stimulated with IL-2 for 72 hours were incubated alone or with target cells as indicated for 2 hours at 37°C. Thereafter, cells were stained with fluorochrome-conjugated anti-CD3, anti-CD56, and anti-CD107a mAbs. (C) Lymphocytes were gated on forward scatter/side scatter plots, followed by gating on CD3 versus CD56 plots. Profiles show CD56 versus CD107a mAb staining of CD3−CD56+ NK cells. (D) Induced CD107a surface expression on CD3−CD56+ NK cells after indicated stimulation was plotted. Lines represent mean values. (E) Induced CD107a surface expression on lymphocytes after indicated stimulation was plotted. Lines represent mean values.
In terms of degranulation, IL-2 stimulation augmented the percentage of NK cells that expressed surface CD107a after incubation with K562 cells in both adult and infant donors, and maximal degranulation was observed after 48 to 72 hours of IL-2 stimulation (Figure 7B-D). Comparable augmentation of CD107a surface expression was observed in FHL2 samples (Figure 7C,D). Whereas K562 cells did not induce degranulation by resting FHL3 or FHL4 NK cells, degranulation was detected in FHL4 NK cells upon IL-2 stimulation (Figure 7C,D). Although restoration of degranulation by IL-2 was more pronounced in FHL4 patients relative to the FHL3 patient, a larger group of patients is required to establish this observation as statistically significant. The data indicating enhanced degranulation by IL-2–treated NK cells from FHL3 patients are consistent with another report.47 Degranulation induced by P815 cells with anti-CD3 was enhanced by IL-2 in healthy donors, and also occurred in lymphocytes from FHL4 patients (Figure 7E). The data revealed induction of degranulation and cytotoxicity independent of Stx11 upon IL-2 stimulation of lymphocytes from FHL4 patients, which could provide one explanation for the less severe clinical symptoms in FHL4 patients relative to FHL2 and FHL3 patients.8,10
Discussion
Analysis of patients with defined immunodeficiencies offers a powerful approach to understanding the role of individual genes in immunity and to the pathogenesis of associated diseases.48 In the present study, degranulation of cytotoxic lymphocytes was compared among patients with mutations in each of the 3 genes that have been linked to FHL by genetic analysis, namely FHL2, FHL3, and FHL4. As reported previously, human perforin-deficient (FHL2) lymphocytes degranulate normally, whereas Munc13-4–deficient (FHL3) lymphocytes fail to do so.7,47 Here, we demonstrate that freshly isolated Stx11-deficient (FHL4) NK cells fail to degranulate in response to activation signals. Surprisingly, degranulation by FHL4 cytotoxic lymphocytes was partially restored by exposure to IL-2, possibly accounting for later onset, and longer periods of remission without specific treatment in FHL4 patients, than in FHL2 and FHL3 patients.8,10 In addition, these data give prominence to NK cells as important effectors of cytotoxic responses in infants, whose perforin-expressing effector T cells are still underdeveloped.
One concern regarding interpretation of the results has been the possible influence of medication, including corticosteroids that are part of the HLH-2004 regimen, on NK-cell function in FHL patients. The fact that NK cells from FHL2 patients on HLH-2004 therapy degranulated to a similar extent as age-matched controls argues against a strong suppression of degranulation by medication. NK cells from an infant with secondary HLH (no mutations identified in PRF1, UNC13D, or STX11 and no consanguinity) also degranulated comparable with healthy control infants (Y.T.B., J.-I.H., unpublished data, February 2006). Defects in NK-cell degranulation have so far not been observed in adult-onset secondary HLH. However, in such patients, a trend toward low NK-cell numbers has been observed (Y.T.B., J.-I.H., unpublished).
At birth, NK cells are the major perforin-expressing lymphocyte subset. Notably, perforin-positive T cells were not detected in cord blood, and few effector T cells were present in PBLs of infants. Therefore, lack of proper NK-cell function probably plays a prominent role in the pathogenesis of FHL, a disease that usually has its onset during infancy and early childhood and that may even develop, and be treated, in utero.4,49 In animal models of HLH, an HLH-like disease can be induced in perforin-deficient mice by infection with lymphocytic choriomeningitis virus or murine cytomegalovirus (CMV).50,51 These models have established a requirement for CD8+ T cells, IFN-γ, and TNF-α in the pathogenesis of the disease.50,51 Pathology was apparently not driven by high viral loads, but rather excessive cytokine production due to increased abundance of antigen-presenting cells.50,51 Thus, perforin is thought to play an important role in immune regulation. Depletion of NK cells from granzyme-deficient mice exacerbated pathology upon viral infection,51 indicating an important role for NK cells eliminating activated effector cells. In healthy infants, perforin-dependent NK-cell cytotoxicity might play an important role in maintaining immune homeostasis, through direct elimination of either antigen-presenting cells or activated T cells.
The regulation of adaptive immune responses through killing of dendritic cells by NK cells is well appreciated,52–54 but how NK cells might contribute to killing of activated T cells is less clear. Only a few patients with selective NK-cell deficiencies have been described in the literature.55 Two patients with NK-cell deficiency reportedly died from herpes virus infections.56,57 Interestingly, a primary immunodeficiency was recently described with reduced frequency of circulating NK cells.58 In addition to recurrent viral infections, the index case patient in this study presented with HLH-like symptoms.58 We would predict that NK-cell deficiencies should predispose for HLH and be characterized by excessive immune activation upon pathogenic challenge.
The present results also have implications for diagnosis and therapy of FHL2, FHL3, and FHL4. Diagnostically, the CD107a assay for degranulation, combined with evaluation of cytotoxicity, offers a rapid method for distinguishing FHL2 from FHL3 and FHL4, thereby providing guidance for genetic analysis and classification, in line with the discussion by Marcenaro et al47 Therapeutically, our results suggest that reagents that would specifically activate NK cells or CD8+ T cells may be beneficial in the treatment of FHL4. Further, as we argue that NK cells are the major perforin-expressing cell population in infants, an alternative therapeutic approach could be NK-cell donor lymphocyte infusion to FHL recipients lacking cytotoxic activity. Donor NK cells might stabilize patients prior to transplantation, by contributing to restoration of immune homeostasis.
The mechanism whereby Stx11 deficiency leads to pathogenesis in FHL was suggested to not involve intrinsic defects in lymphocytes, but rather to indirectly impair lymphocyte function through interactions with antigen-presenting cells.8 We describe transcription of STX11 and protein expression in freshly isolated NK cells and CD8+ T cells, and in the NK-cell line NK92. The anti-Stx11 polyclonal serum used to probe for Stx11 in PBLs did not detect a mutant Stx11 with an early frameshift and stop codon (patient 4), but did detect a truncated Stx11 lacking a C-terminal segment (patient 5). Moreover, Stx11-deficient lymphocytes did not degranulate. Together, these results point to an intrinsic defect in lymphocyte function. The exact molecular interactions underlying disease remain to be elucidated. Stx11 is a protein of 287 amino acids containing a Qa soluble N-ethylmaleimide-sensitive factor attachment protein receptor (SNARE) motif from amino acids 201 to 277.36,59 Vesicular fusion normally requires the formation of a complex consisting of SNARE proteins, which combine to contribute alpha-helical Qa, Qb, Qc, and R-SNARE domains to a complex that drives membrane fusion.60 Stx11 coprecipitates with SNAP-23 in B cells.36 The ubiquitously expressed SNAP-23 contains both Qb and Qc SNARE motifs interspaced by a palmitoylated, membrane targeting sequence. In other cell types, Stx11 has been localized to punctuate intracellular structures, which correspond to late endosomes and the trans-Golgi network, as determined by colocalization with mannose-6-phosphate receptor.36,39,59 More detailed studies of Stx11 localization and function in cytotoxic lymphocytes are required to resolve how this protein facilitates exocytosis of secretory lysosome content.
Unlike other syntaxins with carboxy-terminal hydrophobic residues that insert posttranslationally into the membrane, Stx11 carries carboxy-terminal cysteine residues that may be palmitoylated.36 These carboxy-terminal cysteine residues are not required for membrane targeting of Stx11.36,39 However, the C-terminal segment of Stx11 appears to be required for degranulation, because lymphocytes from patient 5 expressing a truncated Stx11 protein (albeit at lower levels) lacking the 20 C-terminal amino acids failed to degranulate. This functional defect accounts for the prevalence of FHL in children homozygous for this mutation,8,10 but how the C-terminal sequence facilitates degranulation has yet to be determined.
IL-2 has long been known to enhance NK cell cytotoxicity.61 Data show that IL-2 induces a greater proportion of cytotoxic lymphocytes to degranulate to any given stimulus, compared with resting lymphocytes. Since degranulation and killing capacity were partially restored in FHL4 PBLs, degranulation can occur independently of Stx11. How IL-2 provides compensation for the lack of Stx11 is still unknown. Modulation or up-regulation of other SNARE proteins could be a potential mechanism. The IL-2–induced Stx11–independent mechanism of degranulation may also lower the cells' intrinsic threshold for degranulation relative to resting cells, thereby facilitating a greater frequency of degranulating cells and augmenting lymphocyte cytotoxicity. In support of this hypothesis, IL-2–stimulated, Stx11-deficient FHL4 lymphocytes degranulated in response to P815, a stimulus that did not induce degranulation by resting lymphocytes from infant donors or FHL4 patients. Furthermore, degranulation induced by engagement of CD3, CD16, or combinations of receptors for natural cytotoxicity, by plate-coated mAbs was enhanced in IL-2–stimulated relative to resting lymphocytes (Y.T.B., unpublished). A final test of this hypothesis would be to determine whether plate-coated mAbs can induce degranulation of IL-2–stimulated, but not resting, lymphocytes from FHL4 patients. Unfortunately, the limited availability of cells from FHL4 patients has precluded such experiments. Clinically, Stx11-independent degranulation and cytotoxicity induced by proinflammatory cytokines such as IL-2 may provide one explanation for the milder disease of FHL4 patients, compared with FHL2 and FHL3 patients. Of note, some but less marked restoration of degranulation and cytotoxicity was observed in the FHL3 patient who was analyzed here relative to the FHL4 patients. Redundancy in the function of Munc13-4 after IL-2 activation may be less than for Stx11 for degranulation. Compared with FHL2, FHL3 has a later onset, indicating a relatively less severe disease progression.62 Taken together, nonsense mutations leading to deficiency of genes implicated in FHL might be expected to lead to the following order of disease severity: FHL2>FHL3>FHL4. More patients need to be accumulated to better correlate the outcome of different genetic mutations with each other. Furthermore, the present findings should stimulate cell biologic approaches to better understand the role of Stx11 in lymphocyte cytotoxicity.
An Inside Blood analysis of this article appears at the front of this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
Y.T.B is supported by the National Institutes of Health–Karolinska Institute Graduate Partnership Program. This research was supported by the Swedish Research Council, Swedish Cancer Society, and Swedish Children's Cancer Foundation (H.-G.L. and J.-I.H.), Swedish Foundation for Strategic Research (H.-G.L.), the Cancer and Allergy Foundation of Sweden, Stockholm County Council, and Märta and Gunnar V Philipson Foundation (J.-I.H.) and the Intramural Research Program of the National Institutes of Health, National Institute of Allergy and Infectious Diseases (E.O.L.).
We thank A. Killander for collection of peripheral blood from infants; and A. Hoffman for assistance with microscopy.
National Institutes of Health
Authorship
Contribution: Y.T.B. designed and performed most of the experimental work, analyzed and interpreted data, and drafted the paper; E.R. designed and performed genetic analysis, assisted by C.Z., J.E., and D.M., under the supervision of M.N.; C.Z. and S.M.W. designed and performed Western blot experiments; A.G.B., J.J.B., T.C., R.A.F., J.W., and J.-I.H. recruited study subjects; K.H. and P.A.R. designed research; J.-I.H., E.O.L., and H.-G.L. designed research, analyzed and interpreted data, and drafted the paper. J.-I.H., E.O.L., and H.-G.L. contributed equally to this work.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jan-Inge Henter, Childhood Cancer Research Unit, Department of Woman and Child Health, Karolinska Institutet, Karolinska University Hospital Solna, S-17176, Sweden; e-mail: jan-inge.henter@ki.se; Eric O. Long, Laboratory of Immunogenetics, National Institute of Allergy and Infectious Diseases, National Institutes of Health, Rockville, MD 20852; e-mail: elong@nih.gov; Hans-Gustaf Ljunggren, Center for Infectious Medicine, Department of Medicine, Karolinska Institutet, Karolinska University Hospital Huddinge, S-14186 Stockholm, Sweden; e-mail: hans-gustaf.ljunggren@ki.se.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal