Abstract
Within the broad problem of host immune surveillance versus tumor immune evasion, a most intriguing question is how the cellular immunity can cope with cancerous cells that have gotten rid of the classical antigen-presenting machinery. One such option stems from (1) the fact that HLA loss is often attended with expression of Hsp70 on the tumor cell surface, and (2) our findings that human lymphocytes express a protein Tag7 (also known as PGRP-S) capable of tight and specific interaction with cognate Hsp70. Here we show that a subpopulation of human CD4+CD25+ lymphocytes, obtained either in culture as lymphokine-activated killers or directly from healthy donors, carry Tag7 and FasL on their surface and can indeed kill the HLA-negative tumor-derived cells K562 and MOLT-4 that expose Hsp70 and Fas. The primary binding of lymphocyte Tag7 to target-cell Hsp70 is very specific (eg, it is blocked by preincubating either cell with minimal peptides from the “partner” protein), and secures cell contact indispensable for subsequent FasL/Fas-triggered apoptosis. Unrelated to natural killer cell action or the putative role of Hsp as an antigen-presenting substitute, this novel mechanism is rather a backup analog of orthodox (CD8+) target recognition (Tag7 acting as built-in T-cell receptor and Hsp70 itself as ligand).
Introduction
The classical agents of cellular immunity and antitumor defense—natural killer (NK) cells and cytotoxic T lymphocytes (CTLs)—destroy their targets by a direct cell-cell contact mechanism, which must include recognition of a distinctive element on the target cell by a corresponding lymphocyte receptor, formation of a contact zone between the two cells, and induction of cytolysis. At the latter stage, all known cytotoxic lymphocytes use either perforin/granzyme secretion or FasL-Fas interaction;1 this issue raises no debate
By contrast, the first and decisive step of the process—target recognition—depends on mechanisms specific for particular types of target and killer cell. Normally, CD8+ T cells detect specific pathogen- or tumor-related peptide antigens presented in the context of major histocompatibility complex (MHC) or HLA class I molecules. However, cancerous cells (as well as certain viruses) broadly use a manifold strategy known as immune escape or evasion. The vast literature on this problem, together with the known countermeasures that the host immunosurveillance system can take to cope with it, has been comprehensively reviewed.2–4 In one of the most radical modes, tumor cells may completely lose the MHC antigen-presenting system. The very existence of HLA-lacking tumors proves that this trick is not easily tackled by the antitumor defense system. It would thus be reasonable to look for additional components and interactions that may be involved in recognition of HLA-negative cells. The present work has actually developed from a serendipitous finding that brought us into a new field of research.
Our previous studies concerned the possible functions of Tag7, a 20-kDa protein product of the tag7 gene discovered in this laboratory more than a decade ago.5 Tag7 is classed (as PGRP-S) with the family of evolutionarily conserved peptidoglycan recognition proteins (PGRPs), components of innate immunity against microbes in mammals as well as in insects.6,7 What is more pertinent, murine and human Tag7 have been implicated in some kind of antitumor activity,8 and application of Tag7-transformed cells against melanoma is currently in the second phase of clinical trials.9
When we initially tried to isolate Tag7 from cultured lymphocytes, it consistently copurified with another proteinaceous entity, which on closer inspection proved to be the major heat shock protein Hsp70. Further work confirmed that Tag7 can make a stable 1:1 complex with Hsp70 in solution, and demonstrated that the same complex is formed in and secreted by CTLs upon contact with certain tumor-derived cells, being cytotoxic for other tumor cell lines.5
In parallel, a broader search through literature revealed that expression of heat shock proteins (Hsp's), including Hsp70, on the surface of HLA-deficient tumor cells is quite a widespread phenomenon.10–13 Moreover, tumor cells with Hsp70 exposed on the plasma membrane appear to be more susceptible to NK cells and CTLs.13–16 In the case of T cells, this phenomenon is usually ascribed to the ability of Hsp70 to present antigen peptides inducing a T-cell response, which may account for the therapeutic effect of Hsp70 preparations from tumor cells.17–19 In regard to NK cells, it has been asserted that surface Hsp70 can be important for tumor cell recognition.20,21 However, neither the particular step of the cytolytic process nor particular interactions that might involve Hsp's have to our knowledge been elucidated or even sought after. On the other hand, the selectivity and affinity of cognate Tag7-Hsp70 interaction that we directly observed5 gave us grounds to suppose that Tag7, if exposed on the lymphocyte surface, could be the agent recognizing Hsp70 on the surface of an “evasive” cancerous cell. If such is the case, we could envisage an alternative or supplementary means of reinforcing the immune surveillance.
Here we demonstrate, in vitro and ex vivo, that lymphocyte-borne Tag7 can really participate in target recognition and binding indispensable for FasL/Fas-mediated contact killing of HLA-negative tumor cells.
Materials and methods
Cell cultivation and sorting
Human erythroblastoid K562 and lymphoblastoid MOLT-4 cells were cultured in RPMI 1640 with 2 mM l-glutamine and 10% fetal calf serum (all from Invitrogen, Carlsbad, CA). Peripheral blood mononuclear cells (PBMCs) were isolated and activated as described earlier.22 Cell sorting was performed with commercially available magnetic bead isolation kits (Dynal Biotech ASA, Oslo, Norway) by the manufacturer's protocols; specific antibodies to FasL, Tag7, Fas, or Hsp70 were attached to magnetic beads coated with anti-mouse and anti-rabbit IgG.
Proteins and antibodies
Tag7, Hsp70, and its fragments, as well as rabbit antibodies to Tag7 and the anti-Hsp70 used in cytotoxicity tests and immunoadsorption were obtained as previously.5 Tryptic peptides of Tag7 were produced by standard digestion, and the Hsp-reactive peptides were isolated by adsorption on Hsp70 Sepharose. Polyclonal antibodies to the N-terminus of Fas (N-18) and the C-terminal part of FasL (C-178) were from Santa Cruz Biotechnology (Santa Cruz, CA); mouse anti-Hsp70 monoclonal antibody (MA3-009) was from Affinity Bioreagents (Golden, CO); monoclonal antibody to CD94 (clone HP-3D9, NK cell–inhibitory) was from BD Biosciences (Pharmingen, San Diego, CA); FITC-labeled anti-FasL was from BD Biosciences; R-phycoerythrin–labeled antibodies to CD3, CD4, CD8, CD25, and CD56 and FITC-labeled antibodies to CD3 and CD2 were from Caltag Laboratories, Burlingame, CA; FITC- and Cy3-labeled secondary antibodies were from Sigma (St Louis, MO). Proteins were solubilized from the cell surface with 1 M KCl.23 Cell membranes were isolated as previously.5 Protein concentration was determined with the Bradford reagent (Sigma).
Biotinylation and cross-linking
Labeling and chemical cross-linking of cell-surface–associated proteins essentially followed a published protocol.24 rHsp70 was labeled with biotin N-hydroxysuccinimide ester (BHE; Pierce, Rockford, IL) at a 1:100 molar ratio for 2 hours at room temperature and dialyzed in PBS for 18 hours at 4°C. Lymphokine-activated killer (LAK) or K562 (each 2.5 × 107 cells/mL) were labeled with 0.5 mg/mL BHE in PBS (pH 8.0) for 30 minutes at room temperature, and were washed twice with ice-cold PBS before cross-linking. Biotinylated Hsp70 (40 ng) was incubated with LAK cells (5 × 107) in 1 mL of PBS with 50 mM HEPES (pH 8.3), 0.2 mM bis(sulfosuccinimidyl)suberate (BS3; Pierce) for 30 minutes at 4°C. LAK and K562 cells (either kind surface-labeled) were coincubated in RPMI 1640 at 37°C for 3 hours and cross-linked as described. For reversible cross-linking, BS3 was replaced with DTSSP (Pierce). The cells were washed twice in PBS, and their membrane proteins were isolated.
Immunoadsorption and blotting
Rabbit antibodies against rTag7 and rHsp70 (affinity-purified on rTag7 and rHsp70)5 were coupled to cyanogen bromide–activated Sepharose according to the standard protocol. The columns were checked for cross-reactivity: Hsp70 did not bind to anti-Tag7 Sepharose, and Tag7 did not bind to anti-Hsp70 Sepharose. For controls, preimmune rabbit IgG were likewise coupled to Sepharose. Cell membrane proteins after cross-linking were applied onto Ab-Sepharose, washed with PBS, then with PBS plus 0.5 M NaCl, and eluted with 0.25 M triethylamine (pH 12).
Polyacrylamide gel elecrophoresis (PAGE) was conducted as previously,5 and biotinylated products were visualized on nitrocellulose blots with horseradish peroxidase–conjugated streptavidin and an ECL Plus kit (all from GE Healthcare, Vienna, Austria).
Cytotoxicity
K562 cells were cultured in 96-well plates at a density of 3 × 104 cells/well, then lymphocytes (20:1) were added in 100 μL and incubated for 3 hours at 37°C. In inhibition tests, antibodies were used at 20 μg/mL, and Hsp70, its fragments, and Tag7 were used at 70 nM (tryptic peptides, 0.5 μg/mL). Cell death was determined with a Cytotox 96 Assay kit (Promega, Madison, WI), always subtracting the percentage of dead cells in the control (within 5%). Cytoxicity of the soluble rTag7-rHsp70 complex was assayed as previously.5
Microscopy
Apoptosis of K562 after incubation with LAKs was verified with a Dead End Fluorometric TUNEL Kit (Promega) using a Leica DMR fluorescence microscope (Leica, Heidelberg, Germany). To visualize cell contacts, Fas+ K562 cells and CD4+CD25+ CTLs (1:2) were incubated in RPMI 1640 for 30 minutes, washed twice with PBS, and fixed with 4% formaldehyde (Sigma) for 20 minutes at 4°C. Then, cells were washed and stained in PBS with rabbit anti-Tag7 antibodies followed by FITC-labeled goat anti-rabbit IgG (Sigma), and with phycoerythrin-labeled anti-CD95 (anti-Fas) antibody (Caltag Laboratories). After washing with 50 mM NH4Cl, stained material was bound to polylysine-treated coverslips. Specimens were mounted in Movioli (Sigma). Fluorescence images were obtained with a Leica TCS SP2 confocal microscope, analyzed with Leica confocal software, and prepared in Photoshop CE (Adobe Systems, San Jose, CA).
Flow cytometry
The cells were fixed with 1% paraformaldehyde25 (Sigma) and stained with appropriate antibodies at room temperature. The samples (at least 104 cells each) were analyzed with an Epics Elite flow cytometer (Coulter, Marseille, France) in the logarithmic channel of fluorescence. The data were processed with Immuno-4 (version 4.02; Coulter) for single-parameter histograms or with EXPO32 software (Applied Cytometry Systems, Sheffield, United Kingdom).
Results
System validation and layout of the work
To have at our disposal sufficient amounts of cytotoxic lymphocytes, we incubated human PBMCs with IL-2. The surface expression of standard markers CD3, CD4, CD8, and CD56 on the fourth and sixth days of evolution of the LAK population is compared in Figure 1. About two-thirds of the cell population were represented by CD3+ lymphocytes, and the portion of CD4+ cells declined insignificantly, while that of CD8+ slightly increased (their sum closely accounting for the CD3+ total), whereas CD56+ (NK) cells were clearly observable on the fourth day but had virtually disappeared by the sixth day. See Figures S1, S2 (available on the Blood website; see the Supplemental Materials link at the top of the online article) for the pertinent flow cytometry data.
Dynamic composition of lymphokine-activated killer-cell culture. PBMCs were isolated from donor blood and activated with IL-2. The abundance of specified cell markers was determined by flow cytometry. Results are shown for the fourth (□) and sixth (▩) days of incubation (mean ± SEM, n=3-5).
Dynamic composition of lymphokine-activated killer-cell culture. PBMCs were isolated from donor blood and activated with IL-2. The abundance of specified cell markers was determined by flow cytometry. Results are shown for the fourth (□) and sixth (▩) days of incubation (mean ± SEM, n=3-5).
Such reproducible albeit mixed 6-day NK-free LAK-cell populations were used in most experiments, and the main results were further checked ex vivo.
The target cells, unless specified otherwise, were human tumor–derived, HLA-negative K562; our cell line was additionally checked to be devoid of surface MHC class I antigens A, B, C, and E (A. E. Berezhnoi et al, manuscript in preparation). From earlier studies, we knew that these cells were partly susceptible to 6-day LAK, but could be protected with antibodies against either FasL or Fas.26 On the other hand, K562 were insensitive to the Tag7-Hsp70 complex secreted by these CTLs5 (Figure 2A), and thus were suitable for studying the cell-to-cell contact interactions. Furthermore, K562 were not affected by LAK granzymes A and B (L.P.S., unpublished data, November 1997).
Contrasted susceptibility of target cells to soluble Tag7-Hsp70 and to FasL/Fas-mediated contact killing. (A) K562 cells were incubated for 18 hours with various concentrations of the in vitro assembled 1:1 complex of recombinant Tag7 and Hsp70 (■) and the analogous complex secreted by LAK cells (▩); cytotoxicity of the same recombinant complex toward L929 cells (□) is shown for comparison. (B) Cytotoxicity of CTLs (6-day LAK culture) and their FasL+ and FasL− fractions toward K-562 cells and their Fas+ and Fas− fractions (all separations with antibody-coated magnetic beads), coincubation for 3 hours at 37°C. All data are presented as means (± SEM) in at least 5 independent assays.
Contrasted susceptibility of target cells to soluble Tag7-Hsp70 and to FasL/Fas-mediated contact killing. (A) K562 cells were incubated for 18 hours with various concentrations of the in vitro assembled 1:1 complex of recombinant Tag7 and Hsp70 (■) and the analogous complex secreted by LAK cells (▩); cytotoxicity of the same recombinant complex toward L929 cells (□) is shown for comparison. (B) Cytotoxicity of CTLs (6-day LAK culture) and their FasL+ and FasL− fractions toward K-562 cells and their Fas+ and Fas− fractions (all separations with antibody-coated magnetic beads), coincubation for 3 hours at 37°C. All data are presented as means (± SEM) in at least 5 independent assays.
As shown in Figure 2B, more than one-third of K562 cells were killed in 3 hours by CTLs (taken at a 20:1 excess with allowance for the fact that only a fraction of the lymphocyte population may be active in this respect).
In view of the functional heterogeneity of both CTL and target cell populations, we used magnetic beads with appropriate antibodies to separate LAKs into FasL+ and FasL− fractions and to separate K562 cells into Fas+ and Fas− fractions. Only FasL+ LAKs proved cytotoxic, and only Fas+ K562 cells proved susceptible (Figure 2B). Thus, in all cases, the reported cytotoxicity represented Fas/FasL-mediated contact killing. The cells died apoptotically as evidenced by fluorimetric transferase-mediated dUTP nick-end labeling (TUNEL) analysis (data not shown).
From earlier studies, we also knew that our CTLs express Tag75 and that K562 cells have membrane-associated Hsp70.27 To test our idea of the alternative/supplementary recognition mechanism (“Introduction”), we obviously had to ascertain (1) whether these proteins were exposed on the surface of the respective cells, (2) whether they could bind with each other upon cell contact, and (3) whether such interaction had any functional significance in regard to cytotoxicity. With positive answers to these questions, we certainly had (4) to verify the principal results with lymphocytes directly obtained from human blood; it was also important (5) to specify the CTL markers correlating with this function.
Target cells have surface Hsp70, CTLs have surface Tag7
Specific antibodies indeed revealed Hsp70 on the surface of a sizable portion of K562 cells; flow cytometry showed that it was considerably more abundant in the Fas+ fraction (Figure 3A).
Hsp70 and Fas are exposed on K562, Tag7, and FasL are exposed on CTLs. Specified 6-day LAK subpopulations were isolated using antibody-coated magnetic beads. (A) Flow cytometry of Fas− and Fas+ K562 for the presence of Hsp70 on their surface. (B) Biotinylated Tag7 isolated from surface-labeled FasL− and FasL+ CTLs (streptavidin-peroxidase blotting). (C) Flow cytometry for Tag7 exposure on FasL− and FasL+ LAKs. (D) Flow cytometry for Tag7 and FasL exposure on purified CD4+CD25+ cells. Top right quadrants (B2) contain double positives.
Hsp70 and Fas are exposed on K562, Tag7, and FasL are exposed on CTLs. Specified 6-day LAK subpopulations were isolated using antibody-coated magnetic beads. (A) Flow cytometry of Fas− and Fas+ K562 for the presence of Hsp70 on their surface. (B) Biotinylated Tag7 isolated from surface-labeled FasL− and FasL+ CTLs (streptavidin-peroxidase blotting). (C) Flow cytometry for Tag7 exposure on FasL− and FasL+ LAKs. (D) Flow cytometry for Tag7 and FasL exposure on purified CD4+CD25+ cells. Top right quadrants (B2) contain double positives.
CTLs were incubated with biotin ester under conditions when it labeled only the cell-surface proteins.24 Subsequent isolation of the peripheral membrane proteins and purification on anti-Tag7 Sepharose yielded biotinylated Tag7 (data not shown). To check whether the presence of Tag7 correlated with the presence of FasL on the CTL surface, the same procedure was run on FasL+ and FasL− fractions. Tag7 proved to be far more abundant on the surface of FasL+ lymphocytes (Figure 3B[b]). In line with this, bivariate flow cytometry showed that the portion of CTLs with surface Tag7 detected by antibodies was 4 times larger in the FasL+ fraction (Figure 3C).
Tag7 on a CTL interacts with Hsp70 on target cell
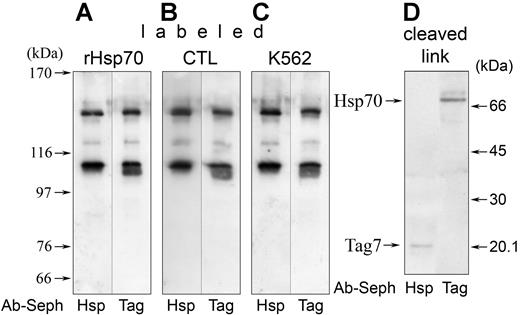
First, we ascertained whether the supposed contact between Tag7 and Hsp70 could be fixed with cross-linking reagents. Indeed, upon incubation of CTLs with biotinylated Hsp70 in the presence of BS,3 the membrane protein material retained on either anti-Hsp70 or anti-Tag7 Sepharose contained 2 major labeled products electrophoretically corresponding to approximately 110 and 140 kDa (Figure 4A). Exactly the same patterns, quite discrete even in heavily loaded gels, were obtained when CTLs were incubated with surface-labeled target cells and vice versa (Figure 4B,C). No biotinylated material was bound on control columns with preimmune rabbit IgG (data not shown).
Tag7 on CTLs interacts with Hsp70 on K562. (A-C) Streptavidin-peroxidase blotting of biotinylated products resolved by PAGE after coincubation and irreversible cross-linking of (A) CTLs with labeled soluble Hsp70, (B) surface-labeled CTLs with K562, and (C) CTLs with surface-labeled K562 followed by adsorption of membrane material on Sepharose-coupled rabbit polyclonal antibodies (Ab-Seph; antigen specified under the lanes). (D) The same procedure performed after reversible cross-linking of surface-labeled CTLs with K562 (left), and CTLs with surface-labeled K562 (right); the complex was cleaved during PAGE sample processing.
Tag7 on CTLs interacts with Hsp70 on K562. (A-C) Streptavidin-peroxidase blotting of biotinylated products resolved by PAGE after coincubation and irreversible cross-linking of (A) CTLs with labeled soluble Hsp70, (B) surface-labeled CTLs with K562, and (C) CTLs with surface-labeled K562 followed by adsorption of membrane material on Sepharose-coupled rabbit polyclonal antibodies (Ab-Seph; antigen specified under the lanes). (D) The same procedure performed after reversible cross-linking of surface-labeled CTLs with K562 (left), and CTLs with surface-labeled K562 (right); the complex was cleaved during PAGE sample processing.
Finally, cell coincubation (either CTL or K562 surface biotinylated) was conducted in the presence of a reversible cross-linking agent DTSSP. After immunoadsorption of cross-linked complexes and subsequent dissociation, labeled Tag7 was found in the material from anti-Hsp70 Sepharose, and labeled Hsp70 in the material from anti-Tag7 Sepharose (Figure 4D), both at proper positions in PAGE.
Thus, Tag7 on the CTL surface and Hsp70 on the K562 surface at least come into close proximity upon cell contact.
Tag7-Hsp70 binding is prerequisite to induction of cytolysis in half of FasL-responsive targets
To ascertain whether Tag7 and Hsp70 are involved in the action of CTLs on K562 cells, we ran a series of cytotoxic assays with specific blocking and interfering/competing agents. Pretreatment of CTLs with anti-Tag7 antibodies or pretreatment of target cells with anti-Hsp70 antibodies protected about half of the total number of cells susceptible to FasL-induced cytolysis (Figure 5A,B).
Half of the FasL-Fas cytotoxicity requires Tag7-Hsp70 interaction. (A) Effects of Tag7 blocking on CTL (30-minute preincubation) with antibodies, rHsp70, its peptide-binding domain (PBD; aa 385-539), or 14-mer peptide (aa 450-463); ineffectiveness of the Hsc70-derived 14-mer analog; and the effect of removing the Hsp-exposing targets. (B) Effects of Hsp70 blocking on K562 (30-minute preincubation) with antibodies, rTag7, and its tryptic peptides (TPs); ineffectiveness of anti-CD94; and the effect of removing the Tag-bearing CTLs; and (C) Cytotoxicity of 4-day LAK culture (containing NK cells) and the effects of antibodies. All data are presented as means (± SEM) in at least 5 independent assays.
Half of the FasL-Fas cytotoxicity requires Tag7-Hsp70 interaction. (A) Effects of Tag7 blocking on CTL (30-minute preincubation) with antibodies, rHsp70, its peptide-binding domain (PBD; aa 385-539), or 14-mer peptide (aa 450-463); ineffectiveness of the Hsc70-derived 14-mer analog; and the effect of removing the Hsp-exposing targets. (B) Effects of Hsp70 blocking on K562 (30-minute preincubation) with antibodies, rTag7, and its tryptic peptides (TPs); ineffectiveness of anti-CD94; and the effect of removing the Tag-bearing CTLs; and (C) Cytotoxicity of 4-day LAK culture (containing NK cells) and the effects of antibodies. All data are presented as means (± SEM) in at least 5 independent assays.
Likewise, the overall cytotoxicity was about halved upon preincubation of CTLs with rHsp70, its peptide-binding domain, or just the principal Tag7-binding tetradecapeptide5 (Figure 5A), whereas the tetradecapeptide analog from Hsc7020 that does not bind to Tag75 was ineffective.
Reciprocally, preincubation of K562 cells with either soluble Tag7 or with its Hsp70-reactive tryptic peptides also protected half of the FasL-susceptible cells (Figure 5B).
Cytolysis was insensitive to the antibody against CD94 known to suppress NK-cell activity21 (Figure 5B), confirming that we indeed worked with CTLs. For comparison, the LAK population on the fourth day of stimulation with IL-2, containing NK cells (our earlier work22 and Figure 1), exerted cytotoxicity that was largely suppressible with anti-Hsp70 and anti-CD94 but not with anti-Tag7 antibodies (Figure 5C). (The latter is likely to mean that the CD4+ cells in the “younger” culture, though carrying Tag7 and FasL [Table 1; Figures S1, S2], either represented a different population22 or were not yet cytotoxic.)
Surface exposure of Tag7 and FasL in the LAK culture
| Marker . | Tag7, % . | FasL, % . | ||
|---|---|---|---|---|
| 4 days . | 6 days . | 4 days . | 6 days . | |
| CD3 | 5.1 | 9.6 | 9.1 | 6.9 |
| CD4 | 3.0 | 4.9 | 4.4 | 3.1 |
| CD8 | 3.7 | 4.9 | 4.9 | 5.1 |
| CD56 | 0.8 | NA | 1.4 | NA |
| Marker . | Tag7, % . | FasL, % . | ||
|---|---|---|---|---|
| 4 days . | 6 days . | 4 days . | 6 days . | |
| CD3 | 5.1 | 9.6 | 9.1 | 6.9 |
| CD4 | 3.0 | 4.9 | 4.4 | 3.1 |
| CD8 | 3.7 | 4.9 | 4.9 | 5.1 |
| CD56 | 0.8 | NA | 1.4 | NA |
Data are percentages of double positives (row × column) revealed by flow cytometry of the total LAK culture (the composition of the latter is shown in Figure 1); days indicate the ″culture age″ (time of incubation with IL-2).
NA indicates not applicable.
Thus, half of the CTL-sensitive K562 cells were subject to contact killing only upon functional interaction between Tag7 and Hsp70 on the cell surfaces.
Importantly, all these effects (blocking, shielding, or interference) practically coincide at about half of the overall cytotoxicity for the simple reason that they actually represent complete suppression of the mechanism under study (Figure 5A,B; the rightmost bars show the assays where either Hsp70-exposing or Tag7-exposing cells were removed from the population): indeed, the other half of the target cells were killed via a process unrelated to Tag7 or Hsp70 (next section).
Tag7/Hsp70-conditioned cytolysis is executed by CD4+CD25+ lymphocytes
Next, the total LAK population was fractionated using magnetic beads with antibodies to standard lymphocyte markers. In full accord with the data in Figure 1, the CTLs were predominantly represented by CD8+ and CD4+ cells, with nearly equal apparent contributions to cytotoxicity (Table 2). Note the nice balance in the CD4+/CD8− and CD4−/CD8+ activities as well as in their sum versus overall cytotoxicity, and the fair agreement between the activities of either subpopulation obtained by positive and by negative selection.
Cytotoxicity of lymphokine-activated and freshly obtained T cells and their sensitivity to specified antibodies
| Group . | Cytotoxicity . | ||||
|---|---|---|---|---|---|
| Basal . | + anti-Tag . | + anti-Hsp . | + anti-FasL . | + anti-Fas . | |
| LAK cells | |||||
| Total | 38 ± 3 | 17 ± 3 | 18 ± 2 | 4 ± 0.4 | 2.5 ± 0.3 |
| Total* | 42 ± 0.4 | 20 ± 2 | 19 ± 2 | 3 ± 0.9 | 3 ± 0.9 |
| CD4−CD8− | 4 ± 0.4 | ND | ND | ND | ND |
| CD4− | 24 ± 2 | 23 ± 3 | 25 ± 3 | 3 ± 0.4 | ND |
| CD8− | 21 ± 2 | 3 ± 0.3 | 4 ± 0.6 | 4 ± 0.4 | ND |
| CD4+ (ps) | 18 ± 2 | 4 ± 0.4 | 3 ± 0.3 | 5 ± 0.7 | ND |
| CD4+ (ps)* | 20 ± 2 | 3 ± 0.4 | 3 ± 0.2 | 4 ± 0.4 | ND |
| CD4+ (ns) | 16 ± 2 | 3 ± 0.3 | 3 ± 0.3 | 4 ± 0.6 | 3.5 ± 0.7 |
| CD8+ (ps) | 17 ± 2 | 15 ± 1 | 16 ± 1 | 3.5 ± 0.5 | ND |
| CD8+ (ns) | 19 ± 2 | 17 ± 2 | 19 ± 2 | 4 ± 0.6 | ND |
| CD4+CD25− | 3 ± 0.3 | ND | ND | ND | ND |
| CD4+CD25+ | 17 ± 2 | 3 ± 0.4 | 4 ± 0.9 | 4 ± 0.7 | 4 ± 0.4 |
| CD4+CD25+* | 19 ± 3 | 2 ± 0.3 | 4 ± 0.8 | 3 ± 0.7 | ND |
| CD4+CD25+Tag− | 4 ± 0.5 | ND | ND | ND | ND |
| Donor PBMCs | |||||
| Total | 25 ± 2 | 4 ± 0.4 | 3 ± 0.9 | 5 ± 0.6 | 3 ± 0.6 |
| Total* | 27 ± 2 | 5 ± 0.5 | 3 ± 0.9 | 4 ± 0.6 | ND |
| CD8− | 24 ± 2 | 3 ± 0.3 | 5 ± 0.5 | 4 ± 0.6 | ND |
| CD8+ | 5 ± 0.9 | ND | ND | ND | ND |
| CD4− | 6 ± 0.7 | ND | ND | ND | ND |
| CD4+ | 27 ± 2 | 4 ± 0.4 | 4.5 ± 0.5 | 3 ± 0.3 | ND |
| CD4+CD25− | 9 ± 1 | 2 ± 0.1 | 1.5 ± 0.1 | 3 ± 0.3 | ND |
| CD4+CD25+ | 26 ± 2 | 3 ± 0.6 | 5 ± 0.5 | 4 ± 0.4 | ND |
| CD4+CD25+* | 27 ± 3 | 4 ± 0.4 | 3 ± 0.7 | 4 ± 0.9 | 3 ± 0.9 |
| Group . | Cytotoxicity . | ||||
|---|---|---|---|---|---|
| Basal . | + anti-Tag . | + anti-Hsp . | + anti-FasL . | + anti-Fas . | |
| LAK cells | |||||
| Total | 38 ± 3 | 17 ± 3 | 18 ± 2 | 4 ± 0.4 | 2.5 ± 0.3 |
| Total* | 42 ± 0.4 | 20 ± 2 | 19 ± 2 | 3 ± 0.9 | 3 ± 0.9 |
| CD4−CD8− | 4 ± 0.4 | ND | ND | ND | ND |
| CD4− | 24 ± 2 | 23 ± 3 | 25 ± 3 | 3 ± 0.4 | ND |
| CD8− | 21 ± 2 | 3 ± 0.3 | 4 ± 0.6 | 4 ± 0.4 | ND |
| CD4+ (ps) | 18 ± 2 | 4 ± 0.4 | 3 ± 0.3 | 5 ± 0.7 | ND |
| CD4+ (ps)* | 20 ± 2 | 3 ± 0.4 | 3 ± 0.2 | 4 ± 0.4 | ND |
| CD4+ (ns) | 16 ± 2 | 3 ± 0.3 | 3 ± 0.3 | 4 ± 0.6 | 3.5 ± 0.7 |
| CD8+ (ps) | 17 ± 2 | 15 ± 1 | 16 ± 1 | 3.5 ± 0.5 | ND |
| CD8+ (ns) | 19 ± 2 | 17 ± 2 | 19 ± 2 | 4 ± 0.6 | ND |
| CD4+CD25− | 3 ± 0.3 | ND | ND | ND | ND |
| CD4+CD25+ | 17 ± 2 | 3 ± 0.4 | 4 ± 0.9 | 4 ± 0.7 | 4 ± 0.4 |
| CD4+CD25+* | 19 ± 3 | 2 ± 0.3 | 4 ± 0.8 | 3 ± 0.7 | ND |
| CD4+CD25+Tag− | 4 ± 0.5 | ND | ND | ND | ND |
| Donor PBMCs | |||||
| Total | 25 ± 2 | 4 ± 0.4 | 3 ± 0.9 | 5 ± 0.6 | 3 ± 0.6 |
| Total* | 27 ± 2 | 5 ± 0.5 | 3 ± 0.9 | 4 ± 0.6 | ND |
| CD8− | 24 ± 2 | 3 ± 0.3 | 5 ± 0.5 | 4 ± 0.6 | ND |
| CD8+ | 5 ± 0.9 | ND | ND | ND | ND |
| CD4− | 6 ± 0.7 | ND | ND | ND | ND |
| CD4+ | 27 ± 2 | 4 ± 0.4 | 4.5 ± 0.5 | 3 ± 0.3 | ND |
| CD4+CD25− | 9 ± 1 | 2 ± 0.1 | 1.5 ± 0.1 | 3 ± 0.3 | ND |
| CD4+CD25+ | 26 ± 2 | 3 ± 0.6 | 5 ± 0.5 | 4 ± 0.4 | ND |
| CD4+CD25+* | 27 ± 3 | 4 ± 0.4 | 3 ± 0.7 | 4 ± 0.9 | 3 ± 0.9 |
Data are mean percentages plus and minus the SEM (n = 3-5). Cytotoxicity is given as the percentage of K562 cells killed within 3 hours of standard incubation.
ps indicates positive mode of cell selection with antibody-coated magnetic beads; ns, negative mode of cell selection with antibody-coated magnetic beads; and ND, not determined.
MOLT-4 as target cells.
Cytotoxic assays in the presence of appropriate antibodies clearly demonstrated that both CD4+ and CD8+ CTLs used the FasL-mediated mechanism, but the CD4+ action required Tag7-Hsp70 interaction, whereas the CD8+ action did not (Table 2), although Tag7 (as well as FasL) was quite comparably represented on both kinds of cell (Table 1; Figures S1, S2).
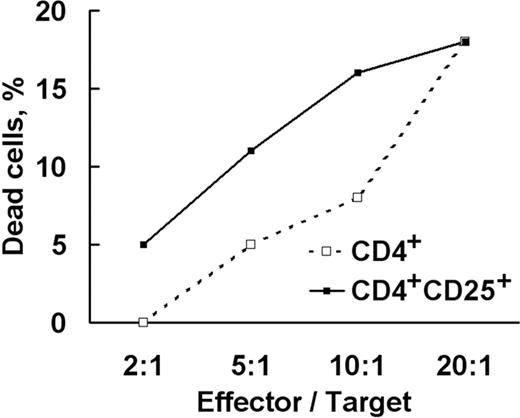
Further analysis revealed that the CD4+ cytotoxicity was entirely associated with surface exposure of CD25 and Tag7 (Table 2). Quite in line with this, flow cytometry demonstrated the much higher occurrence of FasL and Tag7 on the surface of purified CD4+CD25+ cells (Figure 3D), with virtually coincident distribution patterns, just as it should be when both agents are carried by the same cell. Furthermore, comparison at different effector–target cell ratios (Figure 6) revealed that CD4+CD25+ had a “specific cytotoxicity” 2 times higher than the CD4+ subset.
Cytolytic activity increases with purification. The CD4+ population was isolated from the 6-day LAK culture with antibody-coated magnetic beads, and the CD4+CD25+ subset was similarly isolated from CD4+. Both were incubated with K562 in standard assays at different effector–target cell ratios.
Cytolytic activity increases with purification. The CD4+ population was isolated from the 6-day LAK culture with antibody-coated magnetic beads, and the CD4+CD25+ subset was similarly isolated from CD4+. Both were incubated with K562 in standard assays at different effector–target cell ratios.
Assays with another human tumor–derived HLA-negative cell line, MOLT-4, yielded very similar results (asterisks in Table 2), corroborating the general nature of the observed phenomena.
Direct visualization of Tag7 in the region of cell contact
The confocal micrographs in Figure 7 show a CD4+CD25+ lymphocyte bound to a K562 cell. The contact zone contained several protein lumps stained for Tag7 (Figure 7A). Within the same area, there was a distinct cluster stained for Fas (Figure 7B). Superposition of the two images revealed an arrangement suggesting that the Fas site was encircled by Tag7 (Figure 7C). Thus, confocal microscopy confirmed the existence of cell contacts involving Tag7.
Visualization of Tag7 and Fas at the cell contact region by confocal microscopy. The larger oval cell in the center is the K562 target, and the smaller cell at the top right is the CD4+CD25+ lymphocyte (10.0×/1.40 NA oil objective). (A) Decoration with anti-Tag7 antibodies and FITC-labeled anti-IgG. (B) Decoration with phycoerythrin-labeled anti-Fas antibodies. (C) Image superposition.
Visualization of Tag7 and Fas at the cell contact region by confocal microscopy. The larger oval cell in the center is the K562 target, and the smaller cell at the top right is the CD4+CD25+ lymphocyte (10.0×/1.40 NA oil objective). (A) Decoration with anti-Tag7 antibodies and FITC-labeled anti-IgG. (B) Decoration with phycoerythrin-labeled anti-Fas antibodies. (C) Image superposition.
Cytotoxicity of lymphocytes freshly obtained from donor blood
In every third sample of donor blood (collected officially from 10 healthy Moscow residents, 20-40 years old), PBMCs proved cytotoxic to K562 and MOLT-4 cells. (Maybe coincidentally, the same samples proved virtually devoid of NK-cell activity.) The averaged data for these donors are given in Table 2. Flow cytometry showed about 3% of CD4+CD25+ cells. The overall cytotoxicity was practically blocked by antibodies against FasL or Fas as with LAK cells, but this time antibodies against Tag7 or Hsp70 also blocked it completely. Quite expectedly, removal of “normal” CD8+ T cells did not appreciably decrease the cytotoxicity toward both HLA-negative tumor cell lines, whereas removal of either CD4+ or CD25+ cells completely eliminated it. Conversely, the cells positively selected on magnetic beads with antibodies to CD4 and/or CD25 were highly cytotoxic.
Thus, nonstimulated CD4+CD25+ lymphocytes of healthy donors exhibited exactly the same features as those obtained in vitro by lymphokine activation.
Discussion
The data presented here allow at least 2 main novel conclusions: (1) there are CD4+CD25+ T lymphocytes that can cause apoptosis in some tumor-derived cell lines lacking HLA; and (2) they kill such cells through a FasL/Fas-mediated contact mechanism that, however, requires (prior) interaction between Tag7 and Hsp70 on their respective surfaces.
Quite similar results have been obtained in vitro (with LAKs) and ex vivo (with PBMCs freshly obtained from healthy donors), which testifies to the physiologic significance of these findings as well as confirms the validity of the LAK model.
The CD4 and CD25 markers are present on the surface of regulatory cells such as T helpers (responsible for cytokine production)28 and specialized T regulatory (Treg) or suppressor cells. The literature does not support any cytotoxic action of CD4+ helpers; by contrast, cytotoxicity of Treg cells has been suggested as one of the possible mechanisms of their suppressive action.29 We cannot yet say whether our CD4+CD25+ Tag7+ cells are related to Treg cells. From Figure 3D, it is clear that only 1 in 5 or 6 CD4+CD25+ LAK cells carries Tag7 and FasL, so in any case they are a specialized subgroup; again, the presence of the Treg markers may be just an ostensible similarity.
Most recently, we have managed to directly observe appreciable amounts of CD4+CD25+, FasL+Tag7+ cells in a fresh blood sample as well as among LAKs, though technical difficulties do not yet allow a quantitative statement.
We assayed the CTL action on K562 and MOLT-4 cells devoid of the conventional antigen-presenting machinery. Such cells are commonly regarded as classical targets for NK cells. It has been reported that NK cells more efficiently kill allogeneic tumor cells that, along with HLA components, have Hsp70 on their surface.21 Our data obtained by way of control (Figure 5C shows a “younger” 4-day LAK culture) are quite consistent with these findings, but we show that the 6-day population is devoid of functional NK cells (Figures 1, 5B) but has clearly distinct T cells that use Tag7 for recognition of and binding to HLA-negative but Hsp-exposing cells.
Indeed, with a set of independent techniques (cell sorting, cytotoxicity blocking and interference, chemical cross-linking), we have proved that Tag7 on the surface of a CTL comes into immediate contact with Hsp70 on the surface of a target cell, and that such interaction is necessary for the CD4+ FasL+ cells to induce apoptosis in roughly one-half of the LAK-susceptible Fas+ tumor cell population. The other half is apparently killed by CD8+ cells in a FasL/Fas-mediated but Tag7/Hsp70-independent way (Table 2).
Analysis of the aggregate data does not reveal any trivial correlation between Tag7 exposure and cytotoxicity, either among subsets of the same cell batch or in the dynamics of LAK development. Thus, we can only state that the presence of this protein on the CTL surface is a necessary but not a sufficient condition for implementation of the novel immunosurveillance mechanism suggested here. Likewise, one should not expect a direct correlation between the amount of Tag7 found on a particular cell and the cytolytic activity exhibited by this lymphocyte.
A very important point is that although a Tag7-Hsp70 complex must be formed between a CD4+CD25+ CTL and a target K562 or MOLT-4 cell for the contact killing mechanism to come into effect, such a complex has no cytotoxic function of its own in this case (as contrasted to the already reported5 action of the CD8+ LAK-secreted Tag7-Hsp70 complex on L929 and some other tumor-derived cells). Indeed, neither Fas− K562 cells are killed by total CTLs (Figure 2B), though a detectable fraction of these targets carries Hsp70 (Figure 3A), nor do FasL− CTLs show any cytotoxicity (Figure 2B), though some of them carry Tag7 (Figure 3C); unfortunately, the shares of such cells proved too small for reliable isolation. To add, preincubation of the targets with soluble Tag7 not only does not kill them but, on the contrary, fully prevents the action of the CD4+ CTLs (Figure 5B). Most plausibly, the main role of the surface Tag7 is to (1) “apprehend” an HLA-deficient, evasive cancerous cell by its exposed Hsp70 and (2) anchor the CTL fast to the target around the site of further FasL-Fas interaction, as directly corroborated by our microscopic data (Figure 7).
Note that the secreted5 and the anchoring complexes are apparently not identical. The cross-linked products (110 and 140 kDa; Figure 4B-C) are larger than the 1:1 Tag7-Hsp70 complex5 (90 kDa), suggesting that here we have extra Tag7 units or, more likely, some other protein(s) of lymphocyte origin (indeed, cross-linking of CTLs with soluble Hsp70 [Figure 4A] gives the same bands, which means that there is no other contribution from the target). Work along this line is under way.
It is noteworthy that only a little more than one-third of the total K562 population is killed by total LAKs; even in the Fas+ fraction, half of the cells remain unsusceptible (Figure 2B). Among the susceptible cells, the CD4+ and the CD8+ CTLs kill different subpopulations, as evidenced by the additivity of their action (eg, Table 2). The heterogeneity of target cells (including long-established genetically homogeneous lines) in their sensitivity to cytotoxic effectors (NK cells, CTLs) is discussed in the literature and is usually associated with the loss of surface receptors (which is in line with the higher mortality among Fas+ K562 that we observed) and/or impaired intracellular relay of the apoptotic signal.30,31 We have recently reported5 that limit dilution yields L929 clones with broadly varying susceptibility to the secreted Tag7-Hsp70 complex (from 8%-70%), but this characteristic is quite unstable. Much the same has been observed with some TNFα-resistant clones.32 As previously, we chose to work with a stable culture in which the overall effect was partial but the results were well reproducible.
Summing up, we dare claim (in surprise) some advance along three different directions. As stated in the introduction, tumor cells may get rid of HLA to evade immune surveillance. This feature is critically important for metastasis. Indeed, it is only by immune evasion that a cancerous cell can survive in the circulation; and again, the existence of HLA-negative metastatic colonies in an immunocompetent organism has already been documented.33 Here we enlarge the set of cells that can deal with such “missing self” neoplasms. Along with NK, (invariant) NK T, and γδ T cells,34 the host defense system has Tag7-bearing, Hsp70-detecting CD4+CD25+ CTLs that do not depend on the MHC class II presentation held to be necessary for the “orthodox” CD4+ lymphocytes. The physiologic/clinical value of this mechanism is yet to be elucidated. Intriguingly, another subpopulation of target cells in our cell culture experiments was concurrently killed by CD8+ CTLs that had been activated in the absence of a specific antigen (whereas the “normal” CD8+ T cells ex vivo proved helpless against the HLA-negative tumor cells). Some special features of cytotoxicity and regulation of LAK-derived, non–MHC-restricted CTLs have been considered by other authors.35 Whether and how such “unconventional” cells can arise in the organism, and what recognition mechanism(s) they use, are some more questions for further studies.
Our data also further expand the already quite broad list of duties for the well-known heat shock protein. Recently, we have demonstrated that intracellular Hsp70 and Tag7 make a secretable “binary weapon” used by CD8+ lymphocytes.5 Here, we show that interaction between the same proteins residing on the surfaces of different cells gives rise to a somewhat different complex with entirely different function (ie, recognition and anchoring instead of cytotoxicity). Note that this role of surface Hsp70 has nothing to do with presenting antigenic peptides instead of the MHC machinery or otherwise priming the acquired immunity system; rather, Hsp70 itself acts as the marker and ligand, the “antigen of HLA loss.”
Likewise, another angle is added to our point of departure, Tag7. Indeed, the members of the PGRP family have for some time been known as factors of innate immunity6,7 ; particularly, human PGRP-S (Tag7), most abundant in neutrophil granules, acts synergistically with lysozyme to kill intra- and extracellular bacteria.36 Different researchers also ascribe to these bactericidal proteins a more general function of pattern recognition.37–39 By the preceding study5 and this work, we show that Tag7 is still more versatile, being involved together with Hsp70 in 2 other mechanisms serving the same purpose of host defense. Note again a striking example of convergence in function between absolutely unrelated cell components: instead of the orthodox CD8+ T-cell receptor, the CD4+CD25+“backup sentries” carry a Tag7-centered recognizer, searching for cancerous cells that evade the acquired immunity system.
To conclude, functional analogies strike the eye. We see PGRP cooperation with a lytic enzyme in antimicrobial activity36 —and Tag7 cooperation with the lymphocyte's own Hsp70 in direct cytotoxicity.5 We see recognition of a prokaryotic cell-wall pattern that entails a signal relay or lytic cascade6,7 —and recognition of a tumor cell-surface Hsp70 that entails contact-mediated apoptosis (this study). In other words, the same small protein can help the organism sense and fight both the alien and the inner foe. Again we see how diverse functions are attained through combining a limited set of elements.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Moscow Anticancer Program, the Russian Foundation for Basic Research, RAS program for Molecular and Cellular Biology, and International Centre for Genetic Engineering and Biotechnology (ICGEB).
Authorship
Contribution: L.P.S. designed and performed research, controlled and analyzed data, and wrote the paper; E.A.D., Y.V.S., and D.V.Y. performed research and analyzed data; T.I.L., O.D.K., E.A.R., and S.V.K. performed research; A.V.G. controlled and analyzed data and wrote the paper; N.V.G. analyzed data; and G.P.G. supervised the project, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Lidia P. Sashchenko, Institute of Gene Biology, Russian Academy of Sciences, 34/5 Vavilova St, Moscow 119334, Russia; e-mail: sashchenko@genebiology.ru.






This feature is available to Subscribers Only
Sign In or Create an Account Close Modal