Abstract
2B4 (CD244) and its ligand, CD48, are expressed on all natural killer (NK) cells. In studies using 2B4-deficient, CD48-deficient, or wild-type NK cells with blocking antibodies, we found that in the absence of 2B4-CD48 interactions, activated murine NK cells kill each other. We also show that NK-NK fratricide in the absence of 2B4-CD48 interaction is dependent on perforin both in vitro and in vivo. 2B4 has been reported to have activating, costimulatory, and inhibitory functions on murine NK cells. 2B4-mediated inhibition of NK-cell fratricide explains some of the paradoxes of 2B4 function reported in studies of murine NK cells. We show that in the absence of 2B4 signaling, activated NK cells have defective cytotoxicity and proliferation because of fratricide and not due to the absence of a 2B4-dependent activation signal.
Introduction
2B4 is expressed by all natural killer (NK) cells as well as a subset of memory CD8+ αβ T cells, γδ T cells, basophils, and monocytes.1 The ligand to 2B4, CD48, is a glycophosphatidylinositol-linked molecule expressed on all nucleated hematopoetic cells, including NK cells themselves.2 Murine 2B4 has been reported to have activating and inhibitory activities on NK cells.3–8 These studies raise questions of how triggering the same 2B4 receptor on NK cells can lead to variable functional outcomes. Here we show that 2B4 can inhibit NK-NK fratricide and that fratricide can explain some of the apparent dual functions of 2B4 on murine NK cells
Materials and methods
Mice
Wild-type (WT) C57BL/6 (B6), rag knockout (KO), β2m KO, and perforin KO mice were purchased from Jackson Laboratories (Bar Harbor, ME). 2B4 KO mice were generated in B6-derived embryonic stem (ES) cells as previously described.8 CD48−/− cells were generously provided by Dr Arlene Sharpe (Harvard University, Boston, MA).9 The mice were maintained at the University of Chicago in a pathogen-free animal housing facility. The mouse protocols were reviewed and approved by the Institutional Animal Care and Use Committee (IACUC) at the University of Chicago. All KO mice were derived or crossed onto the B6 background and were used at 5 to 10 weeks of age for experiments.
NK LAK preparation
NK lymphokine-activated killer (LAK) cells were prepared as described previously.10
Antibodies and fluorescence-activated cell sorter (FACS) analysis
Anti-2B4, anti-CD48, and anti-CD16/32 blocking antibodies were produced by 2B4, HM48–1, and 2.4G2 hybridoma cell lines, respectively. Fluorescently labeled monoclonal antibodies (mAbs) purchased from BD Biosciences (San Jose, CA) are the following: anti-2B4 (2B4), anti-CD48 (HM48–1), anti-CD3 (145–2C11), and anti-DX5 (DX5). Fluorescently labeled anti-NK1.1 (PK136) mAb was purchased from eBioscience (San Diego, CA). Apoptosis was detected using BD Pharmingen (San Jose, CA) Annexin V-FITC Apoptosis Kit I.
In vitro cytotoxicity assay and spontaneous release assay
Target LAK cells were labeled with 100 μL of sodium chromate (51Cr) for 1 hour at 37°C, washed, and then plated at 2000 cells per well. Effector LAK cells were added at the indicated ratios in triplicates. After 6 hours of incubation at 37°C, supernatants were collected for analysis, and percent lysis was calculated using standard methods. For fratricide assays using blocking mAb, LAK cells were labeled with 51Cr for 1 hour at 37°C. LAK cells were incubated alone at 5E4 cells per well in the presence of 10 μg/mL 2.4G2 plus indicated blocking mAb and incubated at 37°C for 6 hours. Percent specific lysis was calculated using the following equation: % specific lysis=([cpm in the presence of blocking mAb] − [spontaneous release without ab])/([CPM with 0.5% Triton X] − [spontaneous release without ab]).
Proliferation assay
LAK proliferation was measured by 3H-thymidine incorporation as described previously.10
BLT ester assay
Plates were coated overnight with 15μg/mL αNK1.1 mAb. Coated plates were used to stimulate 3 × 105 LAK cells per well in the presence of 10 μg/mL 2.4G2 mAb with or without 10 μg/mL αCD48 mAb or α2B4 mAb. After 6 hours of incubation at 37°, 50 μL supernatant was analyzed from triplicate samples for N-α-benzyloxycarbonyl-L-lysine thiobenzyl (BLT) esterase activity as previously described by Cho et al.11 The % specific esterase release = (experimental esterase release − spontaneous release)/(maximum release with Triton X − spontaneous release).
In vivo NK stimulation and analysis
Mice were injected with 100 μg CpG 1826 (Coley Pharmaceutical, Wellesley, MA) in 100 μL PBS intraperitoneally. Five days after injection, NK cells from the blood, liver, and spleen were enumerated using Sphero AccuCount Blank Particles, 10.2 μm (Spherotech, Lake Forrest, IL) and NK1.1+, CD3- fluorescent antibody staining. The fold expansion of blood NK cells was calculated by dividing the number of NK per milliliter in CpG-injected mice by that of noninjected mice. The fold expansions of spleen- and liver-derived NK cells were calculated by dividing the total number of NK cells per spleen or liver of CpG-injected mice by that of noninjected mice.
Results and discussion
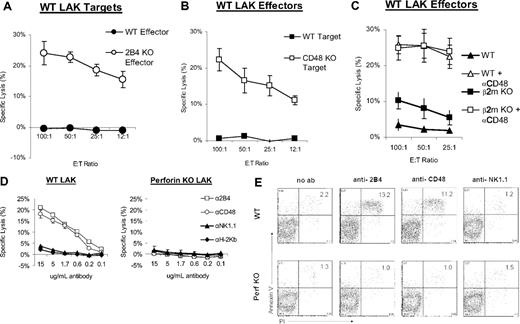
2B4 inhibits NK cytotoxicity against CD48-expressing tumor cells and T-cell blasts.8,12,13 Because all nucleated hematopoetic cells express CD48, we hypothesized that 2B4 can also inhibit NK-mediated lysis of other nontransformed leukocyte subsets, including NK cells themselves. To determine if 2B4 inhibits NK-NK fratricide, killing assays were performed using LAK cells as both targets and effectors. WT and 2B4 KO LAK cells were used as effectors against WT LAK targets in chromium release assays. As expected, WT LAK cells failed to kill other WT cells. However, LAK cells from 2B4 KO mice killed WT LAK cells effectively (Figure 1A). Thus, in the absence of 2B4-mediated inhibition, NK cells can kill each other. To confirm that 2B4-CD48 interactions are required for the inhibition of fratricide, WT LAK were used to kill CD48 KO and WT LAK targets in chromium release assays. WT LAK cells kill CD48 KO LAK cells but not WT LAK cells (Figure 1B), showing that 2B4-CD48 interactions are required for the inhibition of fratricide. We next compared the role of major histocompatibility complex (MHC)–dependent and 2B4-dependent inhibition in regulating fratricide. To test this, we used WT LAK cells to kill β2m KO and WT LAK targets with and without anti-CD48 blocking antibodies. β2m KO LAK cells, which have low MHC class I expression, are killed slightly more than WT LAK cells, indicating that the presence of self class I MHC can inhibit fratricide (Figure 1C). However, if CD48 on the β2m KO or WT LAK targets are blocked with anti-CD48 antibody, a much higher level of fratricide occurs, indicating that class I–dependent inhibition is not as potent as CD48-dependent inhibition in controlling LAK fratricide (Figure 1C).
In the absence of 2B4-CD48 interactions, activated NK cells undergo perforin-dependent fratricide. LAK cells activated with IL-2 in vitro were used as effectors and targets in cytotoxicity assays. (A) WT and 2B4 KO LAK cells were used as effectors against WT LAK cells. (B) WT LAK cells were used as effectors against WT and CD48 KO LAK cells. (C) WT LAK cells were used as effectors against untreated, or anti-CD48 antibody–coated WT, or β2m KO LAK cells. To coat LAK targets, cells were incubated for 15 minutes in 10 μg/mL anti-CD48 antibody at room temperature. Coated cells were then washed and used in killing assays. (D) WT and perforin KO LAK cells were loaded with chromium and incubated in the presence of anti-CD16/CD32 blocking antibody plus anti-2B4, anti-CD48, anti-NK1.1, or anti-H-2Kb antibody. Chromium release, which indicates lysis due to fratricide, was measured after 6 hours of incubation. (E) WT and perforin KO LAK cells were incubated in the presence of anti-CD16/CD32 blocking antibody plus anti-2B4, anti-CD48, or anti-NK1.1 antibody. Annexin V and PI staining was measured after 6 to 7 hours of incubation with blocking antibody. The percents of Annexin V+, PI+ apoptotic cells are noted in the upper right quadrants of the FACS dot plots. Results are representative of 4 independent experiments. Error bars are standard deviation (SD).
In the absence of 2B4-CD48 interactions, activated NK cells undergo perforin-dependent fratricide. LAK cells activated with IL-2 in vitro were used as effectors and targets in cytotoxicity assays. (A) WT and 2B4 KO LAK cells were used as effectors against WT LAK cells. (B) WT LAK cells were used as effectors against WT and CD48 KO LAK cells. (C) WT LAK cells were used as effectors against untreated, or anti-CD48 antibody–coated WT, or β2m KO LAK cells. To coat LAK targets, cells were incubated for 15 minutes in 10 μg/mL anti-CD48 antibody at room temperature. Coated cells were then washed and used in killing assays. (D) WT and perforin KO LAK cells were loaded with chromium and incubated in the presence of anti-CD16/CD32 blocking antibody plus anti-2B4, anti-CD48, anti-NK1.1, or anti-H-2Kb antibody. Chromium release, which indicates lysis due to fratricide, was measured after 6 hours of incubation. (E) WT and perforin KO LAK cells were incubated in the presence of anti-CD16/CD32 blocking antibody plus anti-2B4, anti-CD48, or anti-NK1.1 antibody. Annexin V and PI staining was measured after 6 to 7 hours of incubation with blocking antibody. The percents of Annexin V+, PI+ apoptotic cells are noted in the upper right quadrants of the FACS dot plots. Results are representative of 4 independent experiments. Error bars are standard deviation (SD).
NK-mediated lysis of most tumor targets requires perforin.14 To determine whether NK fratricide in the absence of 2B4-CD48 interactions is also dependent on perforin, fratricide among perforin KO and WT LAK cells was compared. WT and perforin KO LAK cells were labeled with 51Cr and incubated in the presence of anti-2B4, anti-CD48, or control blocking antibodies. An increase of chromium release from WT LAK cells occurs in the presence of anti-2B4 or anti-CD48 blocking antibodies in a dose-dependent manner, whereas control antibodies against H-2Kb and NK1.1 failed to increase the lysis of NK cells (Figure 1D). No chromium release could be detected from perforin KO LAK cells with any blocking antibody, indicating that NK-NK cell lysis is perforin dependent (Figure 1D).
Perforin-dependent NK fratricide in the presence of anti-2B4 or anti-CD48 blocking antibodies was also confirmed via annexin V and PI staining. The percentage of apoptotic WT, but not perforin KO NK cells, is higher in the presence of anti-2B4 and anti-CD48 blocking antibodies compared with control (anti-NK1.1) antibody (Figure 1E), confirming that NK fratricide and apoptosis occurs in the absence of 2B4-CD48 interactions. To rule out the possibility of NK cell death due to antibody-dependent cellular cytotoxicity (ADCC), FcR blockage using anti-CD16/32 antibody was performed in all experiments using blocking antibodies. That such blockade was effective in preventing ADCC is supported by the data showing that no cell death could be detected with anti-NK1.1 control antibodies (Figure 1D,E).
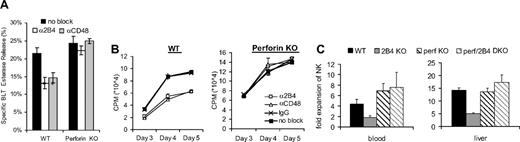
We and others have previously reported that 2B4-CD48 interactions among NK cells are required for optimal NK functions.5,10 There are 2 distinct mechanisms that can explain these results: (1) 2B4-CD48 signaling may activate optimal NK functions, or (2) 2B4-CD48 signaling may inhibit NK fratricide to allow for optimal NK function. To determine if 2B4-mediated activation or inhibition is required for optimal NK functions, the cytotoxicities of WT and perforin KO NK cells in the absence of 2B4-CD48 interactions were compared. Because perforin KO LAK cells have impaired cytolytic function,14 the cytolytic ability of LAK cells stimulated by anti-NK1.1 antibody–coated plates was measured indirectly by using the BLT esterase assay to detect granzyme secretion.11 Consistent with previous reports,5 WT LAK cells have decreased esterase release in the presence of anti-2B4 or anti-CD48 blocking antibodies (Figure 2A). In contrast, perforin KO LAK cells do not exhibit a decrease in esterase activity in the presence of anti-2B4 or anti-CD48 blocking antibodies (Figure 2A).
Perforin-dependent fratricide causes defective NK function in the absence of 2B4-CD48 interactions. (A) WT and perforin KO LAK cells were stimulated with anti-NK1.1 mAb–coated plates in the presence of anti-2B4 or anti-CD48 blocking antibodies. After 6 hours of stimulation, culture supernatants were measured for granzyme secretion via the BLT esterase assay. *P < .05. Results are representative of 3 independent experiments. (B) WT and perforin KO NK cells were cultured in complete media supplemented with IL-2 in the presence of anti-2B4 or anti-CD48 blocking antibodies. Thymidine incorporation was measured at different times during culture. Results are representative of 3 independent experiments. (C) Five days after injection with CpG, NK cells from WT, 2B4 KO, perf KO, and perf/2B4 DKO mice were counted. Fold expansions were calculated by dividing NK numbers of mice injected with CpG by NK numbers in noninjected control mice; n=3 mice per group, and data are representative of 2 independent experiments. Error bars are SD.
Perforin-dependent fratricide causes defective NK function in the absence of 2B4-CD48 interactions. (A) WT and perforin KO LAK cells were stimulated with anti-NK1.1 mAb–coated plates in the presence of anti-2B4 or anti-CD48 blocking antibodies. After 6 hours of stimulation, culture supernatants were measured for granzyme secretion via the BLT esterase assay. *P < .05. Results are representative of 3 independent experiments. (B) WT and perforin KO NK cells were cultured in complete media supplemented with IL-2 in the presence of anti-2B4 or anti-CD48 blocking antibodies. Thymidine incorporation was measured at different times during culture. Results are representative of 3 independent experiments. (C) Five days after injection with CpG, NK cells from WT, 2B4 KO, perf KO, and perf/2B4 DKO mice were counted. Fold expansions were calculated by dividing NK numbers of mice injected with CpG by NK numbers in noninjected control mice; n=3 mice per group, and data are representative of 2 independent experiments. Error bars are SD.
In the absence of 2B4-CD48 interactions, NK cells have also been found to have defective proliferation.5 To determine if defective proliferation in the absence of 2B4-CD48 interactions was due to a lack of an activation signal or a lack of inhibition signal, the proliferation of NK cells from WT and perforin KO mice in the presence of anti-2B4, anti-CD48, or control antibody was measured by 3H-thymidine following culture with IL-2. Blocking 2B4-CD48 interactions among WT but not perforin KO NK cells decreased proliferation in IL-2 (Figure 2B). These data indicate that the 2B4-CD48 interactions among LAK cells do not activate optimal NK proliferation but inhibit perforin-mediated fratricide. Together these data show that in the absence of 2B4 signaling, activated NK cells have defective function in vitro due to a lack of inhibition leading to fratricide.
To determine if perforin-dependent NK fratricide in the absence of 2B4-CD48 interactions affects NK proliferation in vivo, WT, 2B4 KO, perforin/2B4 (perf/2B4) double knockout (DKO), and perforin (perf) KO mice were injected with CpG intraperitoneally. NK proliferation in the blood, liver, and spleen of these mice was compared 5 days after CpG injection. The numbers of NK cells in untreated WT, 2B4 KO, perf/2B4 DKO, and perf KO mice are similar8 (Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). The degree of NK expansion in the blood and livers of 2B4 KO mice was less than that of the WT mice (Figure 2C). This defect in expansion of NK cells was not seen in perf KO mice or perf/2B4 DKO mice (Figure 2C). These data indicate that the decrease in expansion of NK cells in the blood and livers of 2B4 KO mice compared with the WT mice is perforin dependent. NK proliferation in the spleen and migration out of the spleen can be detected during infection15 or under lymphopenic conditions16 with different kinetics. However, 5 days after injection with CpG, expansion of activated NK cells in the spleens of WT and 2B4 KO could not be detected, possibly due to NK migration into other tissues at this time point. Therefore, 2B4-dependent inhibition of fratricide could not be detected in the spleen 5 days after injection with CpG (Figure S1).
Unlike in NK cells from B6 mice, 2B4-CD48 interactions predominantly activate human NK cells.17 The molecular bases for the different functions of 2B4 across different species are unclear and are currently under investigation in our laboratory. Here we restricted our examination of 2B4 function to B6 murine NK cells. Some studies of B6 mouse models indicate that 2B4-CD48 interactions augment NK-cell functions,5–7,10 while other studies indicate that 2B4 inhibits functions.3,4,8,12 The data here show that 2B4 can inhibit NK-cell fratricide among activated NK cells and that fratricide provides an explanation for some of these conflicting findings. We demonstrate that in the absence of 2B4, activated NK cells have defective proliferation and cytotoxicity due to fratricide and not the absence of an activation signal. In addition to blocking fratricide, 2B4-CD48 interactions among NK cells can also inhibit IFN-γ production (Figure S2). Together these data suggest that there are activation signals between NK cells that can stimulate cytotoxicity and cytokine production, which can be inhibited by 2B4. Because 2B4 inhibition of fratricide is only apparent on NK cells activated with IL-2 in vitro or CpG in vivo, another possibility is that activated NK cells constitutively degranulate, and adhesion among NK cells is sufficient to induce granule polarization18 leading to fratricide. In any case, the specific receptor and/or adhesion molecule interactions that 2B4 inhibits among NK cells are unknown and warrant more studies.
In addition to providing an explanation to some of the conflicting findings in studies of 2B4, this work provides evidence for a non-MHC—related mechanism of self-tolerance required for activated NK cells. Because CD48 is expressed on all nucleated hematopoetic cells, we hypothesize that 2B4-mediated inhibition may play an important role in activated NK-cell tolerance of other leukocyte subsets as well.
The online version of this manuscript contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the Molecular and Cellular Biology Training Grant (3T32GM007183–31S1).
We thank the National Institutes of Health (NIH) AIDS Research and Reagent Program, Division of AIDS, National Institute of Allergy and Infectious Diseases (NIAID), NIH, for the generous gift of h-rIL-2. We also thank Megan McNerney for helpful comments on this work and manuscript.
National Institutes of Health
Authorship
Contribution: R.T.T. designed and executed experiments, analyzed and interpreted data, and wrote the paper; D.G. designed and executed the thymidine proliferation experiments and analyzed and interpreted these data; V.K. provided guidance for the progress of the project and wrote the paper; and all authors checked the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Ruth T. Taniguchi, University of Chicago Department of Pathology, Committee on Immunology; 5812 S Ellis Ave, Rm S-315, MC 3083, Chicago, IL 60637; e-mail: rutht@uchicago.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal