Abstract
Studies of detergent-resistant membrane (DRM) rafts in mature erythrocytes have facilitated identification of proteins that regulate formation of endovacuolar structures such as the parasitophorous vacuolar membrane (PVM) induced by the malaria parasite Plasmodium falciparum. However, analyses of raft lipids have remained elusive because detergents interfere with lipid detection. Here, we use primaquine to perturb the erythrocyte membrane and induce detergent-free buoyant vesicles, which are enriched in cholesterol and major raft proteins flotillin and stomatin and contain low levels of cytoskeleton, all characteristics of raft microdomains. Lipid mass spectrometry revealed that phosphatidylethanolamine and phosphatidylglycerol are depleted in endovesicles while phosphoinositides are highly enriched, suggesting raft-based endovesiculation can be achieved by simple (non–receptor-mediated) mechanical perturbation of the erythrocyte plasma membrane and results in sorting of inner leaflet phospholipids. Live-cell imaging of lipid-specific protein probes showed that phosphatidylinositol (4,5) bisphosphate (PIP2) is highly concentrated in primaquine-induced vesicles, confirming that it is an erythrocyte raft lipid. However, the malarial PVM lacks PIP2, although another raft lipid, phosphatidylserine, is readily detected. Thus, different remodeling/sorting of cytoplasmic raft phospholipids may occur in distinct endovacuoles. Importantly, erythrocyte raft lipids recruited to the invasion junction by mechanical stimulation may be remodeled by the malaria parasite to establish blood-stage infection.
Introduction
The mature erythrocyte is a terminally differentiated, nonendocytic cell in nature. Membrane invagination is uncommon in mature healthy erythrocytes. However, these cells are readily invaded by malaria parasites, which involute the red cell1,2 to generate a host-derived parasitophorous vacuolar membrane (PVM). This process is central to the establishment of the blood-stage infection that is responsible for all symptoms and pathologies of this major human disease.3
Recently, we and others have shown that erythrocytes contain detergent-resistant membranes (DRMs).4–10 These highly buoyant, lipid-rich complexes have also been isolated from various other cell types and appear to be enriched for proteins and lipids present in lipid rafts of cellular membranes. The leading definition of membrane rafts suggests that they may consist of small dynamic domains in the plasma membrane stabilized by cholesterol11 and in response to various stimuli coalesce into a larger, less mobile zone, as shown in T cells.12,13
When human erythrocytes are invaded by the malaria parasite Plasmodium falciparum, selected DRM proteins (major proteins flotillin-1 and flotillin-2, and at least 13 additional proteins) are exclusively internalized into the newly formed PVM. Other DRM proteins, such as stomatin, and nonraft cytoskeletal proteins are not.4 Host cholesterol is also detected in the PVM, and depletion of erythrocyte cholesterol (which disrupts DRM complexes but has no effect on membrane deformation or cellular ATP levels) blocks infection. Since DRM proteins are not detected in large membrane domains at the erythrocyte surface, it is presumed that they exist in small dynamic rafts, and a subset of these components is recruited into the PVM in response to parasite stimuli.
Although the erythrocyte membrane is generally refractory to endovacuolation and infection by most pathogens, it can be readily induced to exo- and endocytose by chemical stimuli. Calcium induces exocytosis of microvesicles that show selective enrichment for stomatin and exclusion of flotillins.9 Likewise, formation of endovesicles by amphipathic drugs like primaquine is well described.14 There are no reports of clathrin-coated endocytosis in mature erythrocytes. Receptor-mediated endocytosis is documented in fetal erythrocytes, but is a feature of circulating reticulocytes in fetal blood and not of mature erythrocytes.15 However, stimulation by primaquine induces active endocytosis in mature erythrocytes, presumably by destabilizing the membrane, but virtually nothing is known about endovesicle protein and lipid composition and whether, like the malarial vacuole, they selectively enrich for DRM components. Likewise, while the prevailing data suggest that vacuole formation induced by malaria parasites in erythrocytes involves DRM proteins and thus is likely to be raft-based, how this compares to nonpathogenic, non–receptor-mediated, raft-associated endocytosis in the erythrocyte membrane is not known.
Here, we isolated and characterized primaquine-induced endovesicles and determined that chemical perturbation alone is sufficient to selectively enrich for DRM proteins in membrane domains that have characteristics of rafts. The phospholipid composition of these vesicles was determined by structural methods and used to develop probes to investigate lipids associated with the newly formed PVM. In addition to finding that drug-induced endovesicles show all protein and cholesterol characteristics of rafts, we report the vesicles also contain phosphatidylserine (PS) and phosphatidylinositol (4,5) bisphosphate (PIP2). Notably, PIP2 is a major phosphoinositide (PI) in erythrocyte membranes and influences membrane junctional complex stability. To evaluate the presence of raft lipids PIP2 and PS in the malarial vacuole, we tagged them with specific fluorescently labeled protein reagents in resealed erythrocyte ghosts whose signaling properties and infection by malaria parasites are comparable to normal intact erythrocytes.16
Unexpectedly, we found that PIP2 is excluded from the vacuole, while PS is detected in newly formed PVM, thus providing the first evidence for erythrocyte phospholipid remodeling on the cytoplasmic face of the malarial vacuole. Thus, we show that PIP2 is differently remodeled in distinct raft-based endovesicles/vacuoles induced in the erythrocyte. The data strongly suggest that major raft proteins and lipids may enrich in endovesicles upon simple mechanical perturbation of the bilayer achieved by ampipathic drugs. However, in pathogen-induced endovacuolation such as malarial infection, specific raft proteins and lipids are actively excluded from the vacuole, suggesting a new model for erythrocyte raft movement in invasion.
Materials and methods
Antibodies, immunoblotting, and indirect immunofluorescence assays
Formation and purification of primaquine-induced endovesicles and white erythrocyte membranes
Cells were washed free of serum in Hanks buffer, diluted to 25% hematocrit (Hct), and chilled to 4°C. In some cases, fluorescent markers (Lucifer yellow [LY] or 70-kDa fluoroscein isothiocyanate [FITC]–dextran; both 0.5-3.0 mg/mL final) were added to chilled cells. Endovesiculation was induced by addition of primaquine to a final concentration of 4 mM and incubation at 37°C for 30 to 45 minutes with rotation as described.18 Cells were subsequently washed three times with phosphate-buffered saline containing 11 mM D-glucose (PBS-G) at 25°C and analyzed for endovesiculation or subjected to sucrose density gradient centrifugation.
To isolate endovesicles, primaquine-treated cells were resuspended to 50% Hct in chilled sucrose solution (210 mM sucrose, 10 mM EDTA, 5 mM KH2PO4/K2HPO4 [pH 7.4]) and placed on ice. Cells were passaged through a cold 28 G needle 3 times, then sonicated 5 times for 5 seconds each using a Heat Systems sonicator XL (Heat Systems/Misonix, Farmingdale, NY); liberation of endovesicles was monitored by light and fluorescence microscopy. Homogenized material was mixed with an equal volume of 80% sucrose in Tris-buffered saline (TBS), loaded into an appropriate 37-mL capacity ultracentrifuge tube, overlaid with 3 parts of 35% sucrose and 2 parts of 5% sucrose, and centrifuged at 103 000g for 18 hours at 4°C in a SW28 rotor (Beckman Coulter, Fullerton, CA). All sucrose gradient solutions were diluted in TBS (25 mM Tris/150 mM NaCl [pH 7.4]). The gradient was centrifuged at 103 000g for 18 hours at 4°C in a SW28 rotor. Fractions were taken from the top of the 37-mL gradient as follows: fraction 1, 10.5 mL; fraction 2, 5 mL (5%-35% interface); fraction 3, 11.5 mL; fraction 4, 4.5 mL (35%-40% interface); and fraction 5, 5.5 mL. When multiple tubes were used to obtain large quantities of endovesicles, multiple tubes containing fractions 2 or 3 were combined and overlaid on a 1-mL 40% sucrose button and concentrated by centrifugation at 100 000g for 2 hours. The protein and cholesterol concentrations of each fraction were estimated as described.4 White membranes were purified as described19 and used to determine reference cholesterol-to-protein ratios.
Lipid mass spectrometry on endovesicles and erythrocyte membranes
Phospholipids were extracted using a modified Bligh and Dyer procedure.20 In this method, erythrocyte or endovesicle fractions were extracted with equal parts of 0.1 N HCl:MeOH (1:1) and CHCl3. As an example, a 2-mL sample was extracted with 2 mL HCl:MeOH and 2 mL CHCl3. The samples were vortexed (1 minute) and centrifuged (5 minutes at 18 000g). The lower phase was then isolated and evaporated (Labconco Centrivap Concentrator; Kansas City, MO) followed by reconstitution with 80 μL MeOH:CHCl3 (9:1). Full-scan mass spectrometric analysis was performed on a Finnigan TSQ Quantum Ultra triple quadrupole mass spectrometer (ThermoFinnigan, San Jose, CA) equipped with a Harvard Apparatus (Halliston, MA) syringe pump and an electrospray source. Samples were analyzed at an infusion rate of 10 μL/minute in both positive and negative ionization modes over the range of 400 to 1200 mass-charge ratio (m/z). Data were collected with the Xcalibur software package (ThermoFinnigan). Identification of the individual glycerophospholipids from both vesicles and controls was accomplished by electrospray ionization–tandem mass spectrometry (ESI-MS/MS) using an Applied Biosystems 4000 Qtrap hybrid triple quadrupole/linear ion trap mass spectrometer (Applied Biosystems, Foster City, CA). Each peak of interest was subjected to collision-induced dissociation, and the structure of the phospholipid molecular species was determined by their product ion spectrum.21–23 Results are represented for distinct phospholipid species as sample mass-charge (m/z) ratios, and control measurements were made on hemoglobin-free erythrocyte membranes. A total of 4 independent experiments with triplicate samples per experiment were analyzed in this manner. Data were acquired in negative mode and are represented as mass-charge ratios for phosphatidic acid (PA), phosphatidylglycerol (PG), PI, phosphatidylethanolamine (PE), and PS lipid species, and in positive- and negative-mode ESI-MS/MS for the phosphatidylcholine (PC) species.
Purification of recombinant PLCδ1 PH domain proteins and loading into erythrocyte ghosts
The human PLCδ1 pleckstrin homology (PH) domain was amplified by polymerase chain reaction (PCR; forward primer 5′-TACGGGATCCATGGACTCGGGCCGGGACTT-3′; reverse primer 5′-CGATCCCGGGGGAGCCTGAGTGGTGGATGAT-3′) and inserted between the BamHI and XmaI sites of pRSETA-BFP-GFP24 to form the pRSETA-PH-GFP construct. The PH domain sequence was also amplified using the above forward primer and reverse primer (5′-CGATAAGCTTGGAGCCTGAGTGGTGGATGAT-3′) and inserted into pTrcHisA between the BamHI and HindIII sites, forming the parallel non-GFP PH domain construct. Full-length PLCδ1 in pRSETA was constructed previously, as described.24 All constructs were transformed into Escherichia coli BL21 (DE3) pLys competent cells (Strategene, La Jolla, CA) and purified by affinity chromatography as previously described.25 Purified recombinant proteins were loaded into erythrocyte ghosts using recently described ghost-loading methods.16
Protease protection and in vitro liposome association assays
The orientation of the GFP-PLCδ1 PH domain in loaded erythrocyte ghosts was assessed by protease protection assays as previously described.16 The association of GFP PLCδ1 PH domain with phospholipids was measured by vesicle-binding assay as described.26–28 Appropriate amounts of DMPC (1,2-dimyristoyl-sn-glycero-3-phosphocholine) and phospholipids (PIP2, PI4P, PA) dissolved in chloroform-methanol (2:1) were mixed and dried under argon gas. The dried materials were resuspended in 1 mL of 20 mM HEPES (pH 7.3) and 170 mM sucrose. After vigorous vortexing, the suspension was kept overnight at −80°C. After 3 cycles of freezing (−80°C) and thawing (37°C), the suspension was passed through 100-nm membranes using a Mini Extruder (Avanti Polar Lipids, Alabaster, AL). The resulting large unilamellar vesicles (800 μL) were diluted with 0.2 M KCl and 20 mM HEPES (pH 7.3) buffer (800 μL) and collected by centrifugation at 20 000g for 1 hour. Vesicles were resuspended in 100 mM KCl and 20 mM HEPES (pH 7.3). Fixed amounts of GFP PLCδ1 PH domain were added to 50 μL of vesicle suspension in 200 μL of 100 mM KCl and 20 mM HEPES (pH 7.3) and allowed to bind at 25°C for 1 hour. The vesicles were collected by centrifugation at 100 000g at 4°C for 30 minutes. The pelleted vesicles were dissolved in SDS-PAGE buffer. The supernatants and pellets were analyzed by electrophoresis followed by immunoblotting with anti-GFP antibodies.
Parasite culture and invasion assays
P. falciparum strain 3D7 was cultured and synchronized by standard techniques.29,30 For invasion assays, mature schizont-stage cultures were synchronized using Percoll density gradients. Uninfected ghosts were incubated in RPMI supplemented with 10% human serum at 2% to 5% Hct with 1% to 3% synchronized schizonts in multiwell plates under standard conditions. Giemsa-stained thin blood smears were made at 0 and 12 to 18 hours after invasion to determine parasitemias; parasitemias were also determined by flow cytometry on selected samples.
Analysis of drug-induced endovesicles and parasitemia by flow cytometry
To measure endovesiculation, cells subjected to primaquine treatment were washed three times in PBS-G, resuspended to 1% Hct in PBS-G, and scored immediately for FITC-dextran or LY uptake using a FACSCalibur flow cytometer (Becton Dickinson, San Jose, CA). To measure parasitemia, malaria-infected erythrocytes or their ghosts were washed three times in PBS-G, resuspended to 1% Hct in PBS-G, treated with 5 nM Syto13 (Invitrogen, Carlsbad, CA) for 5 minutes at 25°C to stain parasite nuclei, washed and resuspended in PBS-G, and scored for Syto13 fluorescence in the FL1 channel using the FACSCalibur Flow cytometer.
Measurement of membrane mechanical stability of hemoglobin-free erythrocyte ghosts loaded with the GFP-PLCδ1 PH domain
Hemoglobin-free erythrocyte ghosts containing recombinant GFP-PLCδ1 PH domain (10-50 μM final) were prepared as previously described.31 Membrane mechanical stability was measured using previously described methods.32 Ghosts were resuspended in dextran (40 kDa, 50% wt/vol) and examined using an ektacytometer under a shear stress of 750 dyne/cm2 as described.33 Data are represented as change in the deformability index (DI) over time. DI values decrease as ghosts fragment and lose membrane surface area; thus, the rate of decline describes membrane mechanical stability. The time required for the DI to reach half-maximum (T50) is a quantitative measure of decreased stability; the T50 was determined for erythrocyte membranes treated with various levels of GFP-PLCδ1 PH domain.
Loading and localization of annexin V in erythrocyte ghosts
Washed erythrocytes were resuspended to 50% Hct in PBS-G containing Alexa-Fluor 594–labeled annexin V (2.5 μM final concentration; Invitrogen) and loaded into dialysis cassettes. Ghosts were subsequently prepared by dialysis and resealing using the methods of Murphy et al.16 To assess PS localization (following invasion assays), cells were washed in PBS-G, stained with Hoechst 33342, and imaged live or fixed 20 minutes in 3% paraformaldehyde in PBS, washed, stained with Hoechst 33342, and imaged in 0.5% paraformaldehyde in PBS.
Microscopy
Cell morphology and ghost-loading efficiency were assessed by light and fluorescence microscopy, respectively. Parasitemias were determined by counting Giemsa-stained thin blood smears. In experiments with ghosts, parasitemias were corroborated by counting Hoechst 33342-stained nuclei of fluorescently labeled ghosts. Fluorescence microscopy and digital imaging were performed on an Olympus IX inverted fluorescence microscope with a Photometrix cooled CCD camera and DeltaVision software (Applied Precision, Seattle, WA) as described.6
Results
Production of detergent-free endovesicles with raft characteristics by drug-induced endocytosis in erythrocytes
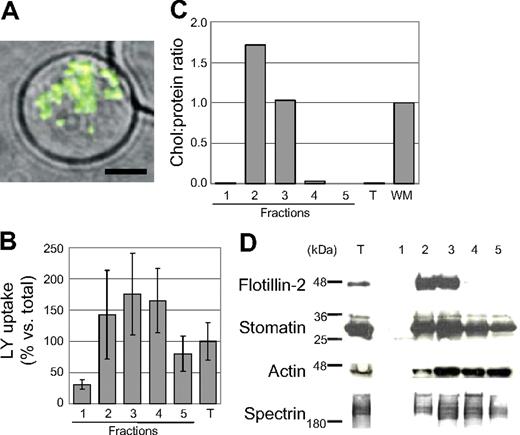
After primaquine treatment of erythrocytes in the presence of LY, more than 75% of cells showed punctuate LY signal by fluorescence microscopy (Figure 1A). Endovesicles were isolated from sucrose density gradients as described in “Materials and methods, Formation and purification of primaquine-induced endovesicles and white erythrocyte membranes.” As measured by fluorescence spectrometry, LY was enriched in gradient fractions 2 through 4 but was depleted from fractions 1 and 5 (Figure 1B), suggesting that endovesicles floated and equilibrated at the 5% to 35% sucrose interface at fraction 2. With respect to buoyancy, endovesicles were similar to DRM complexes prepared at 4°C in low levels of detergents such as Triton X-100.4 They contained relatively low protein but high cholesterol content compared with hemoglobin-free erythrocyte membranes (Figure 1C). Lipid raft markers (flotillin-2, band 7/stomatin) were enriched in endovesicles compared with erythrocyte membranes as measured by immunoblotting (Figure 1D). Flotillin-2 was particularly enriched, whereas band 7/stomatin was distributed in fractions 2 through 5. The high cholesterol content and enrichment of raft proteins in endovesicles mirrors that of DRM preparations described by multiple laboratories.4–8,10 Endovesicles also contained the cytoskeletal proteins spectrin and actin (Figure 1D), whose association with DRMs is dependent on detergent-to-protein ratios used to isolate DRMs.4 This suggests that high levels of detergents may abrogate links between cytoskeleton and vesicular rafts prepared without detergent. Together, the data suggest that primaquine-induced endovesicles bear density, cholesterol, protein, and cytoskeletal similarities to DRMs. Nonetheless, they are distinct from malarial vacuoles, which are enriched for flotillin-1 and flotillin-2, but contain neither stomatin nor cytoskeletal components (Table 1).
Characteristics of primaquine-induced endovesicles. (A) Micrograph of a live mature erythrocyte containing LY+ primaquine-induced endovesicles. Single merged optical section of differential interference contrast (DIC) and green fluorescent (LY) channels; 60×/1.42 oil objective (Olympus). Scale bar equals 3 μm. (B) Association of LY with membrane fractions 1 through 5 from primaquine-treated erythrocytes separated by sucrose density centrifugation and quantified by immunoblotting with anti-LY antibodies and densitometry. Fraction numbers are indicated (1, top; 5, bottom). Bars show total LY signal in each fraction divided by total LY signal in the starting material (T) expressed as a percentage. Error bars indicates standard deviation of duplicate measurements. (C) Cholesterol-protein ratios of endovesicle fractions 1 through 5. Cholesterol and protein levels were measured and ratios calculated as described for erythrocyte DRMs4 ; hemoglobin-free erythrocyte membranes were used as reference values. T indicates starting material; WM, hemoglobin-free white membranes. (D) Immunblotting of erythrocyte endovesicle membrane fractions using specific antisera for the indicated human proteins (flotillin-2, stomatin, actin, spectrin) and specific secondary antibodies. T indicates total lysate. Fractions 2 and 3 were enriched in the raft protein flotillin-2, mildly enriched for human stomatin, and contained lesser amounts of cytoskeletal proteins actin and spectrin.
Characteristics of primaquine-induced endovesicles. (A) Micrograph of a live mature erythrocyte containing LY+ primaquine-induced endovesicles. Single merged optical section of differential interference contrast (DIC) and green fluorescent (LY) channels; 60×/1.42 oil objective (Olympus). Scale bar equals 3 μm. (B) Association of LY with membrane fractions 1 through 5 from primaquine-treated erythrocytes separated by sucrose density centrifugation and quantified by immunoblotting with anti-LY antibodies and densitometry. Fraction numbers are indicated (1, top; 5, bottom). Bars show total LY signal in each fraction divided by total LY signal in the starting material (T) expressed as a percentage. Error bars indicates standard deviation of duplicate measurements. (C) Cholesterol-protein ratios of endovesicle fractions 1 through 5. Cholesterol and protein levels were measured and ratios calculated as described for erythrocyte DRMs4 ; hemoglobin-free erythrocyte membranes were used as reference values. T indicates starting material; WM, hemoglobin-free white membranes. (D) Immunblotting of erythrocyte endovesicle membrane fractions using specific antisera for the indicated human proteins (flotillin-2, stomatin, actin, spectrin) and specific secondary antibodies. T indicates total lysate. Fractions 2 and 3 were enriched in the raft protein flotillin-2, mildly enriched for human stomatin, and contained lesser amounts of cytoskeletal proteins actin and spectrin.
Summary comparison of DRM protein recruitment to primaquine (drug)–induced vesicles compared with malaria-induced vacuoles
| Protein . | Drug-induced vesicles . | Malaria-induced vesicles . |
|---|---|---|
| Flotillin-1, flotillin-2 | + | + |
| Stomatin | + | − |
| Actin | + | − |
| Spectrin | + | − |
| Protein . | Drug-induced vesicles . | Malaria-induced vesicles . |
|---|---|---|
| Flotillin-1, flotillin-2 | + | + |
| Stomatin | + | − |
| Actin | + | − |
| Spectrin | + | − |
Phospholipidomic analysis of drug-induced erythrocyte endovesicles
While erythrocyte raft proteins have been isolated and characterized, this has not been done for phospholipids of erythrocyte rafts, largely because DRMs used to characterize rafts contain detergents that interfere with mass spectral lipid analysis. Since drug-induced endovesicles are prepared without detergents, we compared their phospholipid content to that of control erythrocyte membranes by ESI-MS/MS.
In agreement with previous studies on whole-erythrocyte membranes,34 control erythrocyte membranes contained a variety of phospholipids, including PA, PG, PS, PC, and PE (Table 2; Figure S1, available on the Blood website; see the Supplemental Materials link at the top of the online article). Low-abundance PIs were not detected in control membranes. PA, PS, and PC were all found in primaquine-induced vesicles (Tables 2, S1,S2) with no difference in the degree of unsaturation or the chain length of fatty acids associated with control or drug-induced endovesicle membranes. In contrast, PE and PG phospholipids were depleted from endovesicle preparations compared with controls (Tables 2, S1,S2). No PGs were detected in any endovesicle preparations, while only 1 PE species (766 m/z; 16:0, 22:4, or 18:0, 20:4; chain length: degree of unsaturation is used throughout to identify fatty acids of a given phospholipid) was detected (compared with at least 9 PEs in controls). Since PE and PG are relatively abundant in control membranes, this suggested that endovesicles were specifically depleted of these lipids compared with control membranes. Finally, PIs were detected only in drug-induced endovesicles (Tables 2, S1), suggesting significant enrichment relative to the starting material where low-abundance PIs could not even be detected. The PIs included a small amount with chain lengths and unsaturation of 16:0, 18:1 and larger quantities of 16:1, 22:4; 18:1, 20:4; 18:0, 20:4; and 18:1, 20:3 species. The association of PI lipids (especially PIP2) with rafts has been debated, with some authors suggesting that PIP2 is raft-based,35,36 while others claim it is not.37 In our cholesterol-rich, detergent-free erythrocyte endovesicles, PIs are indeed enriched as determined by mass spectrometry.
Comparison of phospholipid classes present relatively enriched, or absent in starting erythrocyte ghost membranes compared to DIV membranes
| Phospholipid class . | Erythrocyte ghosts . | DIV membranes . |
|---|---|---|
| PC | + | + |
| PE | + | Trace |
| PG | + | − |
| PA | + | + |
| PS | + | + |
| PI | − | + |
| Phospholipid class . | Erythrocyte ghosts . | DIV membranes . |
|---|---|---|
| PC | + | + |
| PE | + | Trace |
| PG | + | − |
| PA | + | + |
| PS | + | + |
| PI | − | + |
DIV indicates drug-induced vesicle.
Localization and specificity of recombinant GFP-PLCδ1 PH domain fusion protein in loaded ghosts
The major PI lipid species in erythrocyte membranes is PIP2,34,38 suggesting that the PI lipid species found in drug-induced vesicles may be PIP2. We estimated approximately 50 000 PIP2 molecules per erythrocyte (approximately 1 μM). Since PIP2 influences membrane remodeling,35 we wanted to independently determine if PIP2 was specifically enriched in endovesicles. We also wanted to follow the fate of PIP2 in the malarial vacuole, but since the malarial PVM cannot be isolated from the infected cell membrane without contamination, mass spectrometric analysis of isolated lipids could not be carried out directly. Thus, we used a fluorescent protein probe (PH domain of human PLCδ1) to tag PIP2. This probe is a widely used marker of PIP2 and IP3;39,40 membrane-bound PH domain is associated predominantly with PIP2.
Erythrocyte ghosts loaded with purified recombinant GFP-PLCδ1 PH domain displayed a pattern of predominantly peripheral fluorescence, suggestive of a membrane-bound state (Figure 2A). The protein was associated with membrane rather than cytoplasmic fractions as determined by immunoblotting (Figure 2B) similar to that observed for the membrane-associated raft protein flotillin-1 (data not shown). Like flotillin-1, the PH domain protein in loaded ghosts was also protected from digestion by exogenous protease (Figure 2C), suggesting a cytoplasmic orientation (where PIs typically reside). Finally, the specificity of recombinant GFP-PLCδ1 PH domain for PIP2 was confirmed by liposome-binding assays. PC liposomes (supplemented with PIP2, PI4P, or PA) exposed to purified GFP-PLCδ1 PH domain were isolated by centrifugation and assayed for GFP signal by immunoblotting; the GFP-PLCδ1 PH domain protein preferentially bound PIP2-containing liposomes compared with those containing PI4P or PA (Figure 2D).
The GFP-PLCδ1 PH domain preferentially binds PIP2 on the cytoplasmic face of the plasma membrane of loaded-erythrocyte ghosts. (A) Single optical sections of fluorescence micrographs (60×/1.42 oil objective [Olympus]) of erythrocyte ghosts loaded with recombinant GFP-PLCδ1 PH domain (5 μM) protein. Scale bar equals approximately 5 μm. (B) Membrane association of GFP-PLCδ1 PH domain in loaded ghosts. Ghosts loaded with GFP-PLCδ1 PH domain were lysed and fractionated into membrane and cytoplasmic fractions by centrifugation, separated by SDS-PAGE, and analyzed for recombinant protein by anti-GFP immunoblotting. T, total PLCδ1 PH domain–loaded ghost lysate; C, cytoplasmic fraction; M, membrane fraction; 5 × 107 cells per lane; protein loading equal by Ponceau staining. Purified GFP-PLCδ1 standard is shown at right; nanogram scale. (C) Protease protection assay of GFP-PLCδ1 PH domain in loaded ghosts. Ghosts loaded with GFP-PLCδ1 PH domain were incubated in buffer with or without proteinase K and/or 1% Triton X-100 detergent, then separated and analyzed as in panel B. In addition to anti-GFP immunoblotting, control antibodies to erythrocyte flotillin-1 (flot1; completely protease protected) were also used. C, control; P, proteinase K; T, Triton X-100; P/T, proteinase K and Triton X-100; 2 × 107 cells per lane; protein loading equal by Ponceau staining. (D) In vitro liposome association of GFP PLCδ1 PH domains. Recombinant GFP PLCδ1 PH domain fusion protein was incubated with purified liposomes containing PIP2, PI4P, or PA, separated into a liposome-containing pellet (P) and liposome-depleted supernatant (S) fractions by ultracentrifugation, and immunoblotted for GFP as described in the other panels. Numbers represent the relative ratios of DMPC to PIP2, PI4P, or PA in liposomes (0.1 = 1:20; 0.5 = 1:40; 0.025 = 1:80). *Lane with most PH domain binding of PIP2-containing liposomes.
The GFP-PLCδ1 PH domain preferentially binds PIP2 on the cytoplasmic face of the plasma membrane of loaded-erythrocyte ghosts. (A) Single optical sections of fluorescence micrographs (60×/1.42 oil objective [Olympus]) of erythrocyte ghosts loaded with recombinant GFP-PLCδ1 PH domain (5 μM) protein. Scale bar equals approximately 5 μm. (B) Membrane association of GFP-PLCδ1 PH domain in loaded ghosts. Ghosts loaded with GFP-PLCδ1 PH domain were lysed and fractionated into membrane and cytoplasmic fractions by centrifugation, separated by SDS-PAGE, and analyzed for recombinant protein by anti-GFP immunoblotting. T, total PLCδ1 PH domain–loaded ghost lysate; C, cytoplasmic fraction; M, membrane fraction; 5 × 107 cells per lane; protein loading equal by Ponceau staining. Purified GFP-PLCδ1 standard is shown at right; nanogram scale. (C) Protease protection assay of GFP-PLCδ1 PH domain in loaded ghosts. Ghosts loaded with GFP-PLCδ1 PH domain were incubated in buffer with or without proteinase K and/or 1% Triton X-100 detergent, then separated and analyzed as in panel B. In addition to anti-GFP immunoblotting, control antibodies to erythrocyte flotillin-1 (flot1; completely protease protected) were also used. C, control; P, proteinase K; T, Triton X-100; P/T, proteinase K and Triton X-100; 2 × 107 cells per lane; protein loading equal by Ponceau staining. (D) In vitro liposome association of GFP PLCδ1 PH domains. Recombinant GFP PLCδ1 PH domain fusion protein was incubated with purified liposomes containing PIP2, PI4P, or PA, separated into a liposome-containing pellet (P) and liposome-depleted supernatant (S) fractions by ultracentrifugation, and immunoblotted for GFP as described in the other panels. Numbers represent the relative ratios of DMPC to PIP2, PI4P, or PA in liposomes (0.1 = 1:20; 0.5 = 1:40; 0.025 = 1:80). *Lane with most PH domain binding of PIP2-containing liposomes.
Recombinant PIP2–specific GFP-PLCδ1 PH domain fusion protein and PS-specific annexin V do not affect malarial infection
Before using the PLCδ1 PH domain to study lipid localization of PIP2, we wanted to ascertain that loading PLCδ1 PH domain in ghosts did not alter either drug-induced endocytosis or malarial invasion. To quantitatively monitor drug-induced endocytosis, we loaded ghosts with nonfluorescent PLCδ1 PH domain (5-15 μM), induced endocytosis in the presence of FITC-conjugated dextran, and measured endocytosis by flow cytometry. The PLCδ1 PH domain did not alter the degree of endocytosis in PLCδ1 PH domain-loaded ghosts versus controls (Figure S2A). Similarly, there was no change in malarial invasion of PLCδ1 PH domain-loaded ghosts (Figure S2B), even at PIP2-saturating concentrations (> 1 μM).
Since PIP2 influences the structural organization of cellular membranes and in light of recent data that PIP2 alters the erythrocyte spectrin-actin-protein 4.1R junctional complex formation,41,42 we measured membrane mechanical properties of PLCδ1 PH domain–loaded hemoglobin-free ghosts. The loaded PLCδ1 PH domain protein associated with the membrane of hemoglobin-free ghosts by microscopy (data not shown), but loading (up to 10 μM) did not alter membrane mechanical stability (Figure S2C). At 25 μM, a small decrease in mechanical stability was observed, so subsequent assays were carried out at 5 to 10 μM.
In summary, our results indicate that the PLCδ1 PH domain does not grossly affect erythrocyte membrane properties during drug-induced endovesiculation or malarial invasion. Hence, this probe was suitable to monitor the localization of PIP2 in subsequent studies. We also monitored the distribution of a second lipid, PS, in malarial infection. Erythrocyte PS is a second cytoplasmic lipid recruited into drug-induced vesicles and can be monitored using Alexa-Fluor 594–labeled annexin V. Like the PH domain, labeled annexin V (up to 2.5 μM) did not affect malarial invasion (Figure S2D), so it was used to track PS during malarial infection.
PIP2 is enriched in drug-induced endovesicles but is excluded from the malarial vacuole
Erythrocyte ghosts were loaded with GFP-PLCδ1 PH domain protein (5 μM final concentration), endocytosis was induced with primaquine in the presence rhodamine-labeled dextran, and localization of fluorescent dextran and membrane probes were assessed by microscopy. Rhodamine dextran used to track primaquine-induced endocvesicles showed diverse morphologies within individual cells: some endovesicles occupied much of the erythrocyte cytoplasm, whereas others had less internalized membrane. In every case, regardless of the degree of endocytosis observed, GFP-labeled PLCδ1 PH domain colocalized with the rhodamine dextran signal (Figure 3A-D), establishing that PIP2 was specifically enriched on drug-induced endovesicle membranes. In conjunction with the aforementioned depletion of PEs and PGs from these vesicles, the data argues that complex but ordered membrane lipid sorting occurs during primaquine-induced endocytosis (Figure 3E).
The GFP-PLCδ1 PH domain accumulates on drug-induced endovesicles. (A-D) Fluorescence micrographs (60×/1.42 oil objective [Olympus]) of erythrocyte ghosts loaded with GFP- PLCδ1 PH domain (panel B; green) and treated with primaquine in the presence of 70-kDa rhodamine dextran (panel C; red). Rhodamine dextran accumulated inside endovesicles, and the GFP-PLCδ1 PH domain colocalized to the same region as rhodamine dextran. Merge shown in panels A (bar equals 10 μm) and D. Dashed box in panel A denotes cell magnified in panels B through D. Scale bar equals 3 μm (panels B-D). (E) Model of erythrocyte lipid uptake and exclusion in drug-induced vesicles (DIVs). Based on mass spectrometry and immunofluorescence results, DIVs appear to undergo complex but ordered lipid sorting during their primaquine-induced formation. In particular, whereas PE and PG are absent from isolated endovesicles, PS and especially PI phospholipids traffic to the endovesicle membranes. With respect to PIP2, this is quantitatively apparent by mass spectrometry and fluorescence microscopy. DIVs may consist of smaller, dynamic raft microdomains containing PIP2 and PS. The extent to which the ratio of cytoskeletal proteins such as spectrin and actin are changed in DIV as well as the presence of junctional complex proteins like 4.1 in DIV have not yet been established.
The GFP-PLCδ1 PH domain accumulates on drug-induced endovesicles. (A-D) Fluorescence micrographs (60×/1.42 oil objective [Olympus]) of erythrocyte ghosts loaded with GFP- PLCδ1 PH domain (panel B; green) and treated with primaquine in the presence of 70-kDa rhodamine dextran (panel C; red). Rhodamine dextran accumulated inside endovesicles, and the GFP-PLCδ1 PH domain colocalized to the same region as rhodamine dextran. Merge shown in panels A (bar equals 10 μm) and D. Dashed box in panel A denotes cell magnified in panels B through D. Scale bar equals 3 μm (panels B-D). (E) Model of erythrocyte lipid uptake and exclusion in drug-induced vesicles (DIVs). Based on mass spectrometry and immunofluorescence results, DIVs appear to undergo complex but ordered lipid sorting during their primaquine-induced formation. In particular, whereas PE and PG are absent from isolated endovesicles, PS and especially PI phospholipids traffic to the endovesicle membranes. With respect to PIP2, this is quantitatively apparent by mass spectrometry and fluorescence microscopy. DIVs may consist of smaller, dynamic raft microdomains containing PIP2 and PS. The extent to which the ratio of cytoskeletal proteins such as spectrin and actin are changed in DIV as well as the presence of junctional complex proteins like 4.1 in DIV have not yet been established.
To monitor PIP2 localization in malarial infection, erythrocyte ghosts were loaded with 5 μM GFP-PLCδ1 PH domain protein, incubated with schizont-stage parasites until new rings formed (3-12 hours after addition of schizonts), and analyzed for PH domain localization by microscopy. In striking contrast to enrichment seen in drug-induced endovesicles, the PVM of young, ring-stage parasites was completely devoid of GFP fusion protein fluorescence (Figure 4A,B). Comparatively, in ghosts loaded with fluorescent annexin V, red fluorescence was associated with young ring-stage parasites (Figure 4C,D), suggesting that erythrocyte PS is carried into the new vacuolar membrane.
PIP2 is absent from malaria-induced vacuoles. (A,B) Fluorescence micrographs of a GFP-PLCδ1 PH domain–loaded ghost (green) infected by a young ring-stage malaria parasite. The parasite nucleus is stained with blue Hoechst 33342 as shown in the merged image. Scale bar equals 2 μm. (C,D). Erythrocyte ghost loaded with Alexa-Fluor 594–labeled annexin V (red) containing a Hoechst-stained intracellular ring-stage parasite as shown in the merged image. Scale bar equals 2 μm. (E,F). Merozoite (blue) attached to the surface (as shown by light microscopy) (E) and indenting (arrowhead) the membrane of an erythrocyte ghost loaded with GFP-PLCδ1 PH domain (F). Scale bar equals 2 μm. (G-H). An erythrocyte ghost loaded with GFP-PLCδ1 PH domain (G) containing a recently invaded young ring-stage parasite (H) (merge with Hoechst stain). Images acquired with a 100×/1.35 oil objective (Olympus).
PIP2 is absent from malaria-induced vacuoles. (A,B) Fluorescence micrographs of a GFP-PLCδ1 PH domain–loaded ghost (green) infected by a young ring-stage malaria parasite. The parasite nucleus is stained with blue Hoechst 33342 as shown in the merged image. Scale bar equals 2 μm. (C,D). Erythrocyte ghost loaded with Alexa-Fluor 594–labeled annexin V (red) containing a Hoechst-stained intracellular ring-stage parasite as shown in the merged image. Scale bar equals 2 μm. (E,F). Merozoite (blue) attached to the surface (as shown by light microscopy) (E) and indenting (arrowhead) the membrane of an erythrocyte ghost loaded with GFP-PLCδ1 PH domain (F). Scale bar equals 2 μm. (G-H). An erythrocyte ghost loaded with GFP-PLCδ1 PH domain (G) containing a recently invaded young ring-stage parasite (H) (merge with Hoechst stain). Images acquired with a 100×/1.35 oil objective (Olympus).
To further characterize PIP2 sorting at the earliest times of infection, we analyzed cells from culture 30 minutes after addition of schizonts. Here, we detected PH domain labeling immediately beneath extracellular merozoite stage parasites attached to and even apparently indenting the erythrocyte membrane (Figure 4E,F). However, in newly formed intracellular rings (quantitatively formed within 1 hour after addition of schizonts), the PH domain had cleared from the intravacuolar parasites (Figure 4G,H). The data suggest that early interactions of merozoites with erythrocytes that lead to indentation do not result in clearance of PIP2. Rather, PIP2 is cleared at a later step of vacuole formation. Together, these data suggest that the parasite actively sorts host raft PIP2 out of the invasion junction.
Discussion
Here, we investigated drug-induced endovesicles derived from mature erythrocytes for their protein and lipid compositions. Although the process of drug-induced endocytosis is well established, a detailed characterization of the resultant vesicles has not been undertaken. Indeed, we find that they show hallmark characteristics of buoyancy, high cholesterol-to-protein ratios, and protein enrichment characteristic of DRM complexes thought to represent components of rafts. They reflect a population of detergent-free membrane endovesicles derived from erythrocytes with raft properties, suggesting that raft domains can easily be induced in this terminally differentiated cell by mechanical destabilization and in the absence of detergent. Lipid analyses reveal that they concentrate PIP2, yet are depleted in PG and PE. This sorting does not appear to be overly influenced by fatty acid chain length. In summary, both protein and lipid analyses confirm that even without detergents, erythrocyte rafts can enrich in vesicular membranes in an ordered fashion (Figure 5A). Further, this enrichment into compositionally defined raft endovesicles can be stimulated simply by chemicals that destabilize the bilayer, albeit in an energy-dependent manner.43,44 It suggests that any active process of erythrocyte membrane invagination may involve mechanically stimulated recruitment of rafts to the site of deformation, although the precise mechanism by which this is achieved is unclear.
Model of lipid and protein uptake into the erythrocyte DIV and the malarial vacuole. (A) The erythrocyte membrane contains a variety of phospholipids (PS, PI/PIP2, PE, PG, etc) and raft (stomatin, flotillins) and nonraft (actin, spectrin) proteins, most of which are taken up into DIV membranes. However, PE- and PG-type phospholipids are specifically excluded from these DIVs. Triangles represent membrane raft proteins flotillin-1 and flotillin-2 (light blue) and stomatin (black). Red bars and blue curved bars represent cytoskeletal actin and spectrin, respectively. Circles represent phospholipids PS (purple), PIP2 (green), PE (yellow), or PG (black). (B) Model of lipid and protein uptake into the malarial vacuolar membrane. Although the merozoite-induced indentation of the nascent vacuole may be remarkably similar to that of DIVs, the fully formed vacuole appears specifically enriched in flotillins, some additional raft proteins (not shown), and host PS. Unlike DIVs, the malarial vacuolar membrane is devoid of cytoskeleton, PIP2, and membrane raft stomatin; arrows depict the selective departure of actin, PIP2, stomatin, and spectrin from the forming malarial vacuole. This selectivity may reflect the combined contribution of erythrocyte-dependent molecular sorting on the basis of membrane curvature and lipid composition as well as potential contributions from parasite-encoded factors that serve to modify the vacuolar membrane during malarial invasion. PE and PG are not shown in panel B. In panel A, a flippase activity is implicated to keep PS cytoplasmically oriented in erythrocytes and DIVs. In malarial invasion (panel B), PS is not exposed at the infected erythrocyte membrane during PVM formation; it remains unknown whether PS is lumenal in the PVM. Our result that PS is detected on the cytoplasmic face of the vacuole is the first clear evidence of PS distribution in the PVM.
Model of lipid and protein uptake into the erythrocyte DIV and the malarial vacuole. (A) The erythrocyte membrane contains a variety of phospholipids (PS, PI/PIP2, PE, PG, etc) and raft (stomatin, flotillins) and nonraft (actin, spectrin) proteins, most of which are taken up into DIV membranes. However, PE- and PG-type phospholipids are specifically excluded from these DIVs. Triangles represent membrane raft proteins flotillin-1 and flotillin-2 (light blue) and stomatin (black). Red bars and blue curved bars represent cytoskeletal actin and spectrin, respectively. Circles represent phospholipids PS (purple), PIP2 (green), PE (yellow), or PG (black). (B) Model of lipid and protein uptake into the malarial vacuolar membrane. Although the merozoite-induced indentation of the nascent vacuole may be remarkably similar to that of DIVs, the fully formed vacuole appears specifically enriched in flotillins, some additional raft proteins (not shown), and host PS. Unlike DIVs, the malarial vacuolar membrane is devoid of cytoskeleton, PIP2, and membrane raft stomatin; arrows depict the selective departure of actin, PIP2, stomatin, and spectrin from the forming malarial vacuole. This selectivity may reflect the combined contribution of erythrocyte-dependent molecular sorting on the basis of membrane curvature and lipid composition as well as potential contributions from parasite-encoded factors that serve to modify the vacuolar membrane during malarial invasion. PE and PG are not shown in panel B. In panel A, a flippase activity is implicated to keep PS cytoplasmically oriented in erythrocytes and DIVs. In malarial invasion (panel B), PS is not exposed at the infected erythrocyte membrane during PVM formation; it remains unknown whether PS is lumenal in the PVM. Our result that PS is detected on the cytoplasmic face of the vacuole is the first clear evidence of PS distribution in the PVM.
Our previous studies established that a subset of raft proteins is enriched in the malarial vacuole, which is also dependent on signaling via host raft Gs.16,45 This led to a model that invasion was due to active vacuolar recruitment of raft proteins. However, our results herein now lead us to consider that malarial invasion may involve rafts by another distinct mechanism. Recruitment of raft components could alternatively be due to mechanical perturbations initiated by extracellular merozoite deformation and destabilization of the membrane (Figure 5B). This may be sufficient to nucleate host rafts at the parasite-host junction. Subsequent exclusion of specific raft (ie, PIP2, stomatin) and cytoskeletal components may occur during parasite invasion.
We are just beginning to understand sorting processes that direct host proteins into or out of the PVM. Lipid trafficking is even less well understood. One earlier study showed that exogenous, chemically labeled PE lipids introduced into erythrocytes were excluded from the PVM.46 Our work here on PIP2 provides the first report of an intrinsic erythrocyte raft lipid excluded from the PVM, and was made possible by a new ghost-loading approach16 that allowed experimental access to cytoplasmic PIP2 and PS. Since PIP2 is cytoplasmically oriented, sorting/remodeling signals must be transduced to the cytoplasmic leaflet of the erythrocyte bilayer. Whether exclusion is linked to signaling via an erythrocyte Gs known to be harnessed by malaria parasites or to additional lipid signaling pathways needs to be investigated further.
What is the function of PIP2 in malarial entry? This molecule is critical for endocytic processes in many cell types. PIs interact with the actin cytoskeleton, regulate ion channels, direct PI-binding proteins to the membrane, and act on effectors following head group hydrolysis.47 In particular, PIP2 regulates protein 4.1–containing junctional complexes in mature erythrocytes,41,42 affects shape change in sheep erythrocytes,48 and directs actin remodeling in phagocytes.49 PIP2 also associates with cytoskeleton via actin binding proteins (eg, cofilin). Therefore, loss of PIP2 from the vacuole (by phosphatases, kinases, or lipases of either host or parasite origin) may enable active clearance of the erythrocyte skeleton to propagate the early vacuole and ensure infection. This could also remove other major raft proteins known to have strong links to the erythrocyte skeleton (eg, stomatin50 ). The continued presence of PIP2 on budding membranes can also inhibit membranefission processes required for endosomes to pinch off from membranes,47 and thus PIP2 removal from the nascent vacuole may serve to circumvent this possibility. PIP2 removal may be needed to create a larger vacuole by the malaria parasite (as compared with drug-induced vesicles) and thus be critical to establishing erythrocytic infection.
Thus, our work suggests that protein and lipid distribution in the erythrocyte membrane may be significantly more ordered than previously expected, such that simple mechanical perturbation may enable highly specialized domains to energetically separate from the rest of the erythrocyte membrane. The addition of the malarial merozoite adds a layer of complexity since the invading parasite encodes molecules that may further modify and/or regulate the membrane internalization process to propagate malarial infection.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
We would like to thank Theodore Steck (University of Chicago) for his helpful insight and advice.
This work was supported by an American Heart Association Pre-doctoral Fellowship (0315210Z to S.C.M.), a Northwestern University Presidential Fellowship (S.C.M.), and National Institutes of Health grants (P01 HL078826 to K.H., J.W.L., and N.M.; AI39071 and HL079397 to K.H.; U54 GM069338 to H.A.B.; HL55591 and HL03961 to J.W.L.; and DK32094 to N.M.).
National Institutes of Health
Authorship
Contribution: S.C.M. designed and performed the research, developed the ghosting methodology, analyzed the data, and wrote the paper. S.F., P.H.C., and S.P.M. performed research and analyzed data. S.B.M. and H.A.B. performed mass spectrometry analyses and analyzed the structural lipid data. M.S. performed membrane stability studies. J.W.L. helped design research and analyzed the data. N.M. conducted membrane stability studies and analyzed the data. K.H. designed the research, analyzed the data, and helped write the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Kasturi Haldar, Ward 3-240; Northwestern University Feinberg School of Medicine, 303 E Chicago Ave, Chicago, IL 60611; e-mail: k-haldar@northwestern.edu.

![Figure 2. The GFP-PLCδ1 PH domain preferentially binds PIP2 on the cytoplasmic face of the plasma membrane of loaded-erythrocyte ghosts. (A) Single optical sections of fluorescence micrographs (60×/1.42 oil objective [Olympus]) of erythrocyte ghosts loaded with recombinant GFP-PLCδ1 PH domain (5 μM) protein. Scale bar equals approximately 5 μm. (B) Membrane association of GFP-PLCδ1 PH domain in loaded ghosts. Ghosts loaded with GFP-PLCδ1 PH domain were lysed and fractionated into membrane and cytoplasmic fractions by centrifugation, separated by SDS-PAGE, and analyzed for recombinant protein by anti-GFP immunoblotting. T, total PLCδ1 PH domain–loaded ghost lysate; C, cytoplasmic fraction; M, membrane fraction; 5 × 107 cells per lane; protein loading equal by Ponceau staining. Purified GFP-PLCδ1 standard is shown at right; nanogram scale. (C) Protease protection assay of GFP-PLCδ1 PH domain in loaded ghosts. Ghosts loaded with GFP-PLCδ1 PH domain were incubated in buffer with or without proteinase K and/or 1% Triton X-100 detergent, then separated and analyzed as in panel B. In addition to anti-GFP immunoblotting, control antibodies to erythrocyte flotillin-1 (flot1; completely protease protected) were also used. C, control; P, proteinase K; T, Triton X-100; P/T, proteinase K and Triton X-100; 2 × 107 cells per lane; protein loading equal by Ponceau staining. (D) In vitro liposome association of GFP PLCδ1 PH domains. Recombinant GFP PLCδ1 PH domain fusion protein was incubated with purified liposomes containing PIP2, PI4P, or PA, separated into a liposome-containing pellet (P) and liposome-depleted supernatant (S) fractions by ultracentrifugation, and immunoblotted for GFP as described in the other panels. Numbers represent the relative ratios of DMPC to PIP2, PI4P, or PA in liposomes (0.1 = 1:20; 0.5 = 1:40; 0.025 = 1:80). *Lane with most PH domain binding of PIP2-containing liposomes.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/6/10.1182_blood-2007-04-083873/7/m_zh80180706600002.jpeg?Expires=1765892285&Signature=2rDYbxJw4hgvoJRvfglVa69VrsQN-6yo4JrA2TSF-BKqHzb2Uwx0uD971VQftqdLOVVjEP0D1wV-Af6RLB0u8LBuIuTdhRjcBfYC35VGksQ1lM0jOElFspDTSFfv9e86oNwPFDtSKWLDnWulRrZJni6BtaRi8~XXn2RAV9MBwbSRf4CszK8kCxFfCcHF3uL4QVWvpNxFmx~d7DMqeOGjaiA2R6TnkhjnsNHg8bGCcf0U~jFwdYgSDLtt~G-hHHGymRXVPBbrh46mDCX0sKG8ayXFgeFxUPUGp3JFePwMAU2fvm0yzFyUPNzKyDnNAPFefX2xuFVxrfcIdh2IscqfzA__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)
![Figure 3. The GFP-PLCδ1 PH domain accumulates on drug-induced endovesicles. (A-D) Fluorescence micrographs (60×/1.42 oil objective [Olympus]) of erythrocyte ghosts loaded with GFP- PLCδ1 PH domain (panel B; green) and treated with primaquine in the presence of 70-kDa rhodamine dextran (panel C; red). Rhodamine dextran accumulated inside endovesicles, and the GFP-PLCδ1 PH domain colocalized to the same region as rhodamine dextran. Merge shown in panels A (bar equals 10 μm) and D. Dashed box in panel A denotes cell magnified in panels B through D. Scale bar equals 3 μm (panels B-D). (E) Model of erythrocyte lipid uptake and exclusion in drug-induced vesicles (DIVs). Based on mass spectrometry and immunofluorescence results, DIVs appear to undergo complex but ordered lipid sorting during their primaquine-induced formation. In particular, whereas PE and PG are absent from isolated endovesicles, PS and especially PI phospholipids traffic to the endovesicle membranes. With respect to PIP2, this is quantitatively apparent by mass spectrometry and fluorescence microscopy. DIVs may consist of smaller, dynamic raft microdomains containing PIP2 and PS. The extent to which the ratio of cytoskeletal proteins such as spectrin and actin are changed in DIV as well as the presence of junctional complex proteins like 4.1 in DIV have not yet been established.](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/6/10.1182_blood-2007-04-083873/7/m_zh80180706600003.jpeg?Expires=1765892285&Signature=EJZ0hbONJK61FynsIZFyaSlPMZqmE3Eo8T6NPmEffypd0~j35xYTdpY9nVvV69tkerpVxqbNrxb4iRZI4aQTkF5Rl1Oj86Tijx463VlbBTYqD~ugIqIAP9ksbmIswSmmywE8vmKAh0-nE9Av0JtqpAsTEjHOeoC~-9KdWv1S9fQS6o0lJGKBcIB2AO-D0Zh8g10inSai7x0rV0iYa3TZnax~483KYdCKXdj-Zw6mbh65YJtzfS1hdj10Gqs0yrW1UUVWUtZ-ROh1BEkkkPp0Daa6AnmAG-Sclz6IpbFAS~QVmNOFCLgDlrjpVtHNxWJjFmauYkYY6UFwtcyRvZDVbg__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal