Patients with Philadelphia (Ph) chromosome–positive acute lymphoblastic leukemia (ALL) have a rapid disease course and a poor prognosis. Dasatinib, a novel, oral, multitargeted kinase inhibitor of BCR-ABL and SRC family kinases, has previously induced responses in patients with imatinib-resistant or -intolerant Ph-positive ALL. We present the interim results of a phase 2 study designed to further assess the efficacy, safety, and tolerability of dasatinib 140 mg in this patient population (n = 36). With a minimum follow-up of 8 months, treatment with dasatinib resulted in substantial hematologic and cytogenetic response rates. Major hematologic responses were achieved in 42% (15/36) of patients, 67% of whom remained progression-free. Complete cytogenetic responses were attained by 58% (21/36) of patients. The presence of BCR-ABL mutations conferring imatinib resistance did not preclude a response to dasatinib. Dasatinib was also tolerable, with 6% (2/36) of patients discontinuing therapy as a result of study-drug toxicity. Most adverse events (AEs) were grade 1 or 2; febrile neutropenia was the most frequent severe AE, but this and other cytopenias were manageable with dose reduction. Dasatinib represents a safe and effective treatment option and an important therapeutic advance for patients with Ph-positive ALL. This trial was registered at www.clinicaltrials.gov as #CA180015.
Introduction
Presence of the Philadelphia (Ph) chromosome is the most frequent cytogenetic aberration associated with acute lymphoblastic leukemia (ALL). The Ph chromosome, found in approximately 20% of adults with ALL, is the single most significant adverse prognostic marker.1,–3 The Ph chromosome results from a reciprocal translocation between chromosomes 9 and 22, creating the BCR-ABL fusion protein, a constitutively activated form of the ABL tyrosine kinase. The ability of BCR-ABL–transduced marrow cells to induce an ALL-like myeloproliferative disorder in mouse models suggests that the kinase plays a key role in the pathogenesis of Ph-positive ALL.4 Ph-positive ALL is a more aggressive disease, indicating that factors other than BCR-ABL are involved in its development and progression. SRC family kinase (SFK) members LYN, HCK, and FGR, for example, have been shown to be elevated in the hematopoietic cells of mice with Ph-positive ALL, and these SFKs are required for the induction of Ph-positive ALL, but not chronic myeloid leukemia (CML), in mice.5
The involvement of BCR-ABL in Ph-positive ALL, coupled with encouraging results achieved with the selective BCR-ABL inhibitor imatinib mesylate in chronic-phase CML,6,7 prompted studies of imatinib in patients with Ph-positive ALL. In a number of phase 2 studies in patients relapsing after standard induction-remission therapy, complete hematologic responses (CHR) were observed in 19% to 30% of patients.8,9 Unfortunately, imatinib resistance develops rapidly and is quickly followed by disease progression.9 Clinical trials looking at imatinib combined with chemotherapy and used in conjunction with allogeneic stem-cell transplantation (SCT) were ongoing at the time this study was initiated,10,,,–14 leading to a recent approval for the treatment of Ph-positive ALL.
Considerable evidence is available to suggest that BCR-ABL–independent pathways may be driving both the disease and imatinib resistance. Indeed, the SFKs involved in Ph-positive ALL pathogenesis have also been implicated in resistance to imatinib, and in mouse models of Ph-positive ALL, inhibition of BCR-ABL and SFKs afforded greater antileukemic efficacy than BCR-ABL inhibition alone.5 Loss of BCR-ABL–mediated regulation of SFKs has also been cited as a mechanism of resistance to imatinib in CML.15,16 Additional mechanisms of resistance in Ph-positive ALL (and CML) include BCR-ABL point mutations17 ; amplification of the BCR-ABL gene18,19 ; and overexpression of the P-glycoprotein efflux pump.20,21
In light of the aggressive nature of Ph-positive ALL, there is clearly a need for alternative therapies with greater potency and a rapid onset of action. Dasatinib (SPRYCEL, formerly BMS-354825; Bristol-Myers Squibb, New York, NY) is a novel, oral, multitargeted kinase inhibitor of BCR-ABL and SFKs that has been rationally designed for the treatment of Ph-positive malignancies such as ALL and CML. Dasatinib was recently approved in the United States and the European Union for use in patients with chronic, accelerated, or blast phases of CML with resistance or intolerance to imatinib or Ph-positive ALL. Dasatinib has 325-fold greater potency compared with imatinib in cells transduced with unmutated BCR-ABL, is active against all but one BCR-ABL mutation conferring imatinib resistance that has been tested to date, and potently inhibits the SFKs implicated in imatinib resistance.22,23 Unlike imatinib, dasatinib is not a substrate of the P-glycoprotein pump, which is thought to be responsible, at least in part, for imatinib efflux across the blood–brain barrier.24 Studies with dasatinib in mouse models of intracranial CML have shown that dasatinib treatment results in tumor stasis and regression, which parallel survival benefits, whereas imatinib fails to show any activity.25
A phase 1 dose-escalation study demonstrated that dasatinib induced hematologic and cytogenetic responses in Ph-positive ALL at a 70-mg, twice-daily dose and was generally well tolerated.26 These promising phase 1 results prompted initiation of this phase 2, open-label, single-arm, multicenter clinical trial (designated START-L [SRC/ABL Tyrosine kinase inhibition Activity: Research Trials of dasatinib]). The aim was to further evaluate the efficacy and safety of dasatinib in patients with imatinib-resistant or -intolerant Ph-positive ALL and lymphoid blast crisis (LBC) CML. Results from patients with lymphoid blast crisis CML are reported separately. Results from a formal interim analysis conducted with 6 months' follow-up are summarized in tabular format only; data from a subsequent analysis extending the follow-up to a minimum of 8 months form the basis for this report.
Patients, materials, and methods
Patients
The START-L study was open to male and female patients, aged 18 years or older with Ph-positive (or BCR-ABL–positive) imatinib-resistant or -intolerant ALL previously treated with standard induction or consolidation chemotherapy, or imatinib-resistant or -intolerant LBC CML. Patients reported here are those treated with Ph-positive ALL only. Patients were considered imatinib-resistant if they had disease progression or lack of response to treatment with imatinib after a minimum of 4 weeks of therapy at a dose of 600 mg/day or more (or 400 to < 600 mg/day if intolerant of ≥ 600 mg/day). A relapse of blasts while on therapy was considered to be disease progression. Patients were considered imatinib-intolerant if they only tolerated doses less than 400 mg/day or had a toxicity possibly related to imatinib at a dose of 400 mg/day or less that led to discontinuation of therapy.
Adequate hepatic and renal function and an Eastern Cooperative Oncology Group (ECOG) performance status of 2 or fewer were prerequisites for inclusion. Exclusion criteria included prior dasatinib therapy, imatinib therapy within 7 days of initiation, uncontrolled or significant cardiovascular disease, or a history of a significant bleeding disorder unrelated to Ph-positive ALL.
Study design and treatment
This was an open-label, multicenter, international, phase 2 study, conducted in accordance with the ethical principles originating in the current Declaration of Helsinki, and was consistent with International Conference on Harmonization Good Clinical Practice and applicable regulatory requirements. Written, informed consent was obtained for all patients before initiation. The Institutional Review Board/Independent Ethics Committee at each trial center approved the study protocol, amendments, and patients' informed consent before initiation of the study.
The primary objectives of the study were to establish the rates of major hematologic response (MaHR) and overall hematologic response (OHR) to dasatinib in patients with imatinib-resistant, Ph-positive ALL. Secondary variables included rates of MaHR and OHR in patients with imatinib-intolerant disease, duration of all hematologic responses, cytogenetic response (CyR) rates, and the safety and tolerability of dasatinib. Hematologic responses were categorized as major, minor (MiHR), and no response and were determined according to analyses of complete blood counts and bone marrow evaluations (Table 1). A MaHR was defined as a best hematologic response of complete hematologic response (CHR) or no evidence of leukemia (NEL); OHR was defined as a best hematologic response of CHR, NEL, or MiHR. Cytogenetic responses were defined by the prevalence of Ph-positive metaphases in a sample of approximately 20 metaphases (median, 20.0; range, 0-32): complete (CCyR), 0% Ph-positive; partial (PCyR), less than 0% to 35% Ph-positive; minor CyR, more than 35% to 65% Ph-positive; minimal CyR, more than 65% to 95% Ph-positive; or no response, more than 95% to 100% Ph-positive. Major cytogenetic response (MCyR) was defined as the sum of patients achieving a best cytogenetic response of CCyR or PCyR.
Hematologic response criteria
| Major hematologic response |
|
| Minor hematologic response |
|
| Major hematologic response |
|
| Minor hematologic response |
|
WBC indicates white blood cell; ULN, upper limit of normal; and ANC, absolute neutrophil count.
ULNs were as follows: 1 center 8.0, 1 center 8.8, 1 center 9.0, 6 centers 10.0, 2 centers 10.8, 6 centers 11.0, 1 center 11.1.
As specified in the study protocol.
Dasatinib was administered orally, initially at 70 mg twice a day, based on the results of the phase I dose escalation study.26 The dasatinib dose could be adjusted after one cycle of treatment (4 weeks [28 days]). A dose increase to 100 mg twice a day was permitted for patients with any of the following: a rising percentage of blasts on 2 consecutive hematologic assessments at least 1 week apart; no CHR within 1 month of dasatinib initiation; no CCyR after 3 months or more of dasatinib treatment; or loss of a response achieved with dasatinib.
Dose reduction (stepwise to 50 mg twice a day and subsequently to 40 mg twice a day) and interruption were permitted in response to associated toxicity, which was graded according to the National Cancer Institute (NCI) common toxicity criteria (CTC). After a grade 2 to 4 nonhematologic adverse event (AE) considered possibly related to dasatinib, treatment was interrupted until recovery to less than or equal to grade 1 or baseline levels. Dasatinib was reinitiated at the same dose for a first-time grade 2 event, reduced by one dose level for a repeat of the same event, and reduced by a second dose level for yet another event. For grade 3 events, dasatinib was reduced by one dose level for a first-time event and reduced by a second dose level for a repeat event. A decision was made by the investigator and sponsor to reduce the dasatinib dose further or discontinue therapy if patients suffered additional repeat grade 2 to 3 events. Patients with grade 4 nonhematologic toxicity, grade 3 or more organ toxicity related to dasatinib, or corrected QT (QTc) interval greater than or equal to 530 msec, were permanently discontinued from therapy.
Reduction or interruption of the dasatinib dose due to hematologic toxicity was considered on the basis of bone marrow cellularity and presence or absence of blasts, after 14 days of treatment, but only for grade 4 neutropenia (ANC < 500/mm3). Treatment was reinitiated at the same dose for the first event and at one dose level lower for repeat events. A decision on further dasatinib dose reductions or discontinuation from the study was made by the investigator and sponsor if grade 4 neutropenia occurred a fourth time. Patients were required to reinitiate treatment within 21 days of the start of a dose interruption.
Dasatinib was administered until disease progression, intolerable toxicity, withdrawal of consent, or until the investigator and patient agreed that discontinuation of therapy was in the best interests of the patient. Patients were followed for 30 days or more after the last dose of study drug or until recovery from all toxic effects, whichever was longer. Follow-up visits occurred at least every 4 weeks until all study-related toxicities resolved to baseline or less than or equal to grade 1, stabilized, or were considered irreversible.
During the study, treatment other than dasatinib for Ph-positive ALL was not permitted. The only exceptions were anagrelide for platelet counts 700,000/mm3 or higher (although no patient received anagrelide) and hydroxyurea for WBC counts over 50 000/mm3 (administered to 2 patients). Use of hydroxyurea was restricted to approximately 2 weeks. Erythropoietin (1 patient) and colony-stimulating factors (0 patients) were administered at the investigator's discretion. Intrathecal chemotherapy (ARA-C, methotrexate, or dexamethasone) was permitted for 3 patients with evidence of central nervous system (CNS) involvement.
Patient evaluation
Complete blood counts were conducted every 7 days to determine hematologic responses to dasatinib. Cytogenetic responses were assessed by bone marrow aspirates/biopsies conducted every month for the first 3 months and every 3 months thereafter.
Hematologic responses had to be maintained for 4 or more weeks with no anagrelide or hydroxyurea used during the same period; hematologic response duration was measured from the first day the criteria were met until the date treatment was discontinued due to progression or death, or on the date of the last hematologic assessment. For patients who achieved a MaHR or MiHR to dasatinib, progression was defined as failing the criteria for MaHR or MiHR on all measurements over a consecutive 2-week period while on dasatinib. Patients with no decrease from baseline levels in percentage blasts in peripheral blood or bone marrow on all assessments over a 4-week period after starting the maximum dasatinib dose were also classified as having progressed.
Assessment of AEs (including evaluation of skin and mucosa for evidence of bleeding) was carried out every week for the first 8 weeks of treatment and every other week thereafter. Toxicity was assessed according to the definitions of the NCI CTC Version 3.0, with grade 1 to 2 AEs classified as mild-to-moderate and grade 3 to 4 events classified as severe.
Peripheral blood–cell mRNA was collected and analyzed for BCR-ABL gene point mutations by denaturing high-performance liquid chromatography (D-HPLC) and sequencing and for the level of expression by quantitative reverse transcriptase polymerase chain reaction (Q-RT-PCR).
Statistical analysis
Two-sided 95% confidence intervals (CIs) were calculated for the primary endpoints according to the Clopper-Pearson method.27 Kaplan-Meier product limit methodology was used to estimate durations of MaHR and OHR, and 2-sided 95% CIs for median values were calculated using the Brookmeyer and Crowley method.28
Results
Patients and treatment
A total of 36 patients with Ph-positive ALL who were enrolled in this study between January and May 2005 and received at least one dose of study drug were included in this preliminary analysis: 34 patients (94%) with imatinib-resistant disease and 2 patients (6%) with imatinib-intolerant disease. Reasons for imatinib intolerance were gastrointestinal symptoms, liver dysfunction, and leukopenia in one case and hepatotoxicty, nausea, and emesis in the second case. Because of the low number of patients with imatinib-intolerant Ph-positive ALL enrolled, results for this patient cohort are not presented separately but are combined with those for imatinib-resistant patients.
The demographics and baseline characteristics of the treated population were representative of patients with Ph-positive ALL (Table 2). Given the aggressive nature of the disease, patients had a median 20-month duration of leukemia before starting dasatinib, yet all had been extensively pretreated. Fifteen patients (42%) had undergone prior SCT, 32 patients (89%) had received chemotherapy, and 3 (8%) had received interferon (IFN)–α. All patients had previously received ≥ 400 mg/day imatinib, with 17 (47%) having received doses in excess of 600 mg/day. Twenty-five patients (25/32; 78%) had a BCR-ABL kinase domain mutation at baseline. Approximately one third of patients (11/36; 31%) had some extramedullary involvement at baseline; the CNS was the most common site (5/36; 14%).
Patient demographics and baseline characteristics
| . | Ph-positive ALL patients . |
|---|---|
| n | 36 |
| Median age, y (range) | 46 (15-85) |
| Male sex, n (%) | 23 (64) |
| Median duration of ALL, mo (range) | 20 (3-97) |
| Any BCR-ABL domain mutation, n (%)* | 25 (78) |
| Imatinib therapy duration, n (%) | |
| Less than 1 y | 16 (44) |
| 1-3 y | 19 (53) |
| More than 3 y | 1 (3) |
| Highest imatinib dose, n (%) | |
| Less than 400 mg | 0 |
| 400-600 mg | 19 (53) |
| More than 600 mg | 17 (47) |
| Prior chemotherapy, n (%) | 32 (89) |
| Prior interferon, n (%) | 3 (8) |
| Prior SCT, n (%) | 15 (42) |
| Median WBC count/mm3 (range); WBC count at least 20 000/mm3, n (%) | 7.0 (0.7-211.0); 11 (31) |
| Leukopenia grade 1-4, n (%) | 11 (31) |
| Median platelets (range); platelets less than 100 000/mm3, n (%) | 53.5 (8.0-360.0); 25 (69) |
| Median peripheral blasts (range); peripheral blasts at least 30%, n (%) | 32 (0.0-100); 11 (31) |
| Median marrow blasts (range) | 69 (0.0-100) |
| . | Ph-positive ALL patients . |
|---|---|
| n | 36 |
| Median age, y (range) | 46 (15-85) |
| Male sex, n (%) | 23 (64) |
| Median duration of ALL, mo (range) | 20 (3-97) |
| Any BCR-ABL domain mutation, n (%)* | 25 (78) |
| Imatinib therapy duration, n (%) | |
| Less than 1 y | 16 (44) |
| 1-3 y | 19 (53) |
| More than 3 y | 1 (3) |
| Highest imatinib dose, n (%) | |
| Less than 400 mg | 0 |
| 400-600 mg | 19 (53) |
| More than 600 mg | 17 (47) |
| Prior chemotherapy, n (%) | 32 (89) |
| Prior interferon, n (%) | 3 (8) |
| Prior SCT, n (%) | 15 (42) |
| Median WBC count/mm3 (range); WBC count at least 20 000/mm3, n (%) | 7.0 (0.7-211.0); 11 (31) |
| Leukopenia grade 1-4, n (%) | 11 (31) |
| Median platelets (range); platelets less than 100 000/mm3, n (%) | 53.5 (8.0-360.0); 25 (69) |
| Median peripheral blasts (range); peripheral blasts at least 30%, n (%) | 32 (0.0-100); 11 (31) |
| Median marrow blasts (range) | 69 (0.0-100) |
Calculated as a proportion of the 32 patients in the Ph-positive ALL cohort who underwent mutational analysis at baseline.
With a minimum of 8 months of follow-up, 9 patients (25%) remained on study. Primary reasons for discontinuation were disease progression (17 patients, 47%) and death (4 patients, 11%). Two of the 4 patients who died on study demonstrated evidence of disease progression before death. The cause of death for these 4 patients included general status worsening, suspected CNS progression, suspected pulmonary aspergillosis, and pneumonia/pleural effusion, all of which were considered to be unrelated to study therapy by the investigators. Two patients (6%) discontinued treatment because of study drug toxicity, with rash and gastrointestinal toxicity, respectively, given as the reasons. Of the remaining 4 patients, 3 (8%) underwent stem-cell transplantation, while the fourth (3%) experienced deterioration of their condition but without progression. The median duration of therapy was 3.2 months (range, 0.2-11.0) for the total population, and 8.3 months (6.3-11.0) for patients remaining on study.
Efficacy
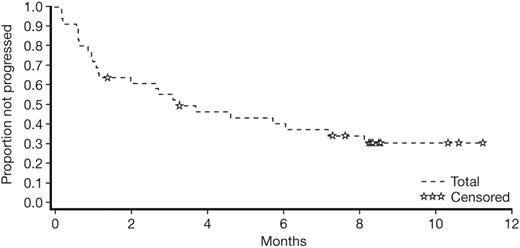
Dasatinib induced rapid hematologic responses in patients with imatinib-resistant or -intolerant Ph-positive ALL. The OHR rate was 50% (18/36 patients); Table 3 summarizes all hematologic responses to dasatinib, sustained for a minimum of 4 weeks, with values representing the best response at any time during the study. Major hematologic responses were observed in 42% (15/36) of these patients and the median time to MaHR was 1.8 months. The median duration of MaHR had not been reached, and among patients who achieved MaHR, response duration ranged from 1.9 to over 8.7 months (Figure 1). Ten of the 15 patients (67%) who achieved a MaHR had not progressed at the 8-month follow-up.
Best confirmed hematologic response to dasatinib
| . | No. (%) of patients . | |
|---|---|---|
| 6 months' follow-up | 8 months' follow-up | |
| Overall hematologic response | 17 (47) | 18 (50) |
| Major hematologic response | 15 (42) | 15 (42) |
| CHR | 11 (31) | 12 (33) |
| NEL | 4 (11) | 3 (8) |
| Minor hematologic response | 2 (6) | 3 (8) |
| No response | 19 (53) | 18 (50) |
| . | No. (%) of patients . | |
|---|---|---|
| 6 months' follow-up | 8 months' follow-up | |
| Overall hematologic response | 17 (47) | 18 (50) |
| Major hematologic response | 15 (42) | 15 (42) |
| CHR | 11 (31) | 12 (33) |
| NEL | 4 (11) | 3 (8) |
| Minor hematologic response | 2 (6) | 3 (8) |
| No response | 19 (53) | 18 (50) |
Number of patients in each column was 36.
Duration of major hematologic response to dasatinib at 8 months' follow-up. Only 15 patients with a MaHR are included in this plot (2 of whom were censored with the same duration of response [195 days]).
Duration of major hematologic response to dasatinib at 8 months' follow-up. Only 15 patients with a MaHR are included in this plot (2 of whom were censored with the same duration of response [195 days]).
Marked rates of CyR were also observed with dasatinib therapy (Table 4). Major cytogenetic responses were evident in 58% (21/36) of patients. This is of clinical importance given the advanced nature of the disease and considering that with prior imatinib the MCyR rate was only 50%. Treatment was discontinued for 39% (14/36) of patients before the first on-study cytogenetic assessment, ie, within the first month of therapy.
Best cytogenetic response to dasatinib
| . | No. (%) of patients . | |
|---|---|---|
| 6 months' follow-up | 8 months' follow-up | |
| CCyR | 21 (58) | 21 (58) |
| PCyR | 0 | 0 |
| Minor cytogenetic response | 1 (3) | 1 (3) |
| Minimal cytogenetic response | 0 | 0 |
| No cytogenetic response | 0 | 0 |
| Not evaluable* | 14 (39) | 14 (39) |
| . | No. (%) of patients . | |
|---|---|---|
| 6 months' follow-up | 8 months' follow-up | |
| CCyR | 21 (58) | 21 (58) |
| PCyR | 0 | 0 |
| Minor cytogenetic response | 1 (3) | 1 (3) |
| Minimal cytogenetic response | 0 | 0 |
| No cytogenetic response | 0 | 0 |
| Not evaluable* | 14 (39) | 14 (39) |
Number of patients in each column was 36.
Not evaluable represents patients who discontinued therapy before the first cytogenetic assessment.
The majority of hematologic and cytogenetic responses to dasatinib in patients who were resistant or intolerant to imatinib were similar to or exceeded responses achieved by these same patients when previously receiving treatment with imatinib.
The median duration of progression-free survival was 3.3 months (Figure 2).
Progression-free survival associated with dasatinib therapy at 8 months' follow-up.
Progression-free survival associated with dasatinib therapy at 8 months' follow-up.
Efficacy by baseline BCR-ABL mutational status
Thirty-two patients (89%) underwent mutational analysis at baseline (31 imatinib-resistant and 1 imatinib-intolerant). Eighteen imatinib-resistant BCR-ABL mutations were detected among 25 of these patients (24 imatinib-resistant and 1 imatinib-intolerant) throughout the BCR-ABL kinase domain. The T315I mutation, which was found in 6 patients, was the most common. Eleven patients (34%) had mutations in the P-loop and 3 patients (9%) had mutations in the A-loop (Table 5).
Response by baseline BCR-ABL mutation analysis
| Imatinib-resistant mutation . | No. (%) of patients at 8 months' follow-up . | ||
|---|---|---|---|
| Total* . | MaHR . | MCyR . | |
| None | 7 (22) | 2/7 (29) | 4/7 (57) |
| Any | 25 (78) | 11/25 (44) | 14/25 (56) |
| P-loop; amino acids 248-255 | 11 (34) | 4/11 (36) | 5/11 (45) |
| A-loop; amino acids 379-398 | 3 (9) | 3/3 (100) | 3/3 (100) |
| Other regions | 11 (34) | 4/11 (36) | 6/11 (55) |
| Specific mutations† | |||
| M244V | 2 | 1 | 1 |
| G250E | 4 | 1 | 2 |
| Y253H | 3 | 3 | 2 |
| E255K | 4 | 0 | 1 |
| D276G | 2 | 2 | 2 |
| T315I | 6 | 0 | 0 |
| E355G | 2 | 0 | 0 |
| H396P | 2 | 2 | 2 |
| F486S | 2 | 1 | 2 |
| Imatinib-resistant mutation . | No. (%) of patients at 8 months' follow-up . | ||
|---|---|---|---|
| Total* . | MaHR . | MCyR . | |
| None | 7 (22) | 2/7 (29) | 4/7 (57) |
| Any | 25 (78) | 11/25 (44) | 14/25 (56) |
| P-loop; amino acids 248-255 | 11 (34) | 4/11 (36) | 5/11 (45) |
| A-loop; amino acids 379-398 | 3 (9) | 3/3 (100) | 3/3 (100) |
| Other regions | 11 (34) | 4/11 (36) | 6/11 (55) |
| Specific mutations† | |||
| M244V | 2 | 1 | 1 |
| G250E | 4 | 1 | 2 |
| Y253H | 3 | 3 | 2 |
| E255K | 4 | 0 | 1 |
| D276G | 2 | 2 | 2 |
| T315I | 6 | 0 | 0 |
| E355G | 2 | 0 | 0 |
| H396P | 2 | 2 | 2 |
| F486S | 2 | 1 | 2 |
n = 32. Four patients had no mutational analysis performed at baseline.
Reported in at least 2 patients.
Response data by mutational status at baseline are reported for patients at the 8-month efficacy assessment. Analysis of response rates among patients bearing BCR-ABL mutations revealed that, despite the poor prognosis associated with many of the identified genotypes, the response rates to dasatinib therapy remained high (Table 5): MaHR and MCyR were achieved by 44% (11/25) and 56% (14/25) of patients with any mutation, respectively, similar to those achieved by the total population (42% [15/16] and 58% [21/36]). A marked proportion of patients with mutations in the P- and A-loops of the ABL domain also achieved MaHR and MCyR. Moreover, MaHR and MCyR rates were 31% (5/16) and 38% (6/16), respectively, for patients with a mutation known to cause moderate-to-very high resistance to imatinib (≥ 5-fold increase in IC50 compared with unmutated BCR-ABL).
Safety
The overall safety profile for patients with Ph-positive ALL was representative of the advanced stage of the disease. The most frequent events, irrespective of grade, were gastrointestinal disorders (diarrhea, nausea) and pyrexia. AEs related to study drug experienced by 10% or more of patients in the total population are shown in Table 6. The incidence of severe nonhematologic AEs related to dasatinib therapy was low. Although severe febrile neutropenia was documented for 4 patients (11%), this event was manageable with dasatinib dose interruptions or reductions and/or appropriate medical intervention. Only 2 patients (6%), both with imatinib-resistant disease, discontinued therapy due to toxicity.
Adverse events associated with dasatinib experienced by at least 10% of patients
| . | No. (%) of patients at 8 months' follow-up . | |
|---|---|---|
| Grades 1-4 . | Grades 3-4 . | |
| Diarrhea | 11 (31) | 3 (8) |
| Pyrexia | 9 (25) | 1 (3) |
| Nausea | 8 (22) | 0 |
| Asthenia | 7 (19) | 3 (8) |
| Pleural effusion | 7 (19) | 1 (3) |
| Rash | 6 (17) | 1 (3) |
| Weight decrease | 6 (17) | 0 |
| Peripheral edema | 6 (17) | 0 |
| Dyspnea | 5 (14) | 1 (3) |
| Headache | 5 (14) | 0 |
| Febrile neutropenia | 4 (11) | 4 (11) |
| Fatigue | 4 (11) | 0 |
| Vomiting | 4 (11) | 0 |
| . | No. (%) of patients at 8 months' follow-up . | |
|---|---|---|
| Grades 1-4 . | Grades 3-4 . | |
| Diarrhea | 11 (31) | 3 (8) |
| Pyrexia | 9 (25) | 1 (3) |
| Nausea | 8 (22) | 0 |
| Asthenia | 7 (19) | 3 (8) |
| Pleural effusion | 7 (19) | 1 (3) |
| Rash | 6 (17) | 1 (3) |
| Weight decrease | 6 (17) | 0 |
| Peripheral edema | 6 (17) | 0 |
| Dyspnea | 5 (14) | 1 (3) |
| Headache | 5 (14) | 0 |
| Febrile neutropenia | 4 (11) | 4 (11) |
| Fatigue | 4 (11) | 0 |
| Vomiting | 4 (11) | 0 |
As expected, myelosuppression was reported in a number of patients at baseline before receiving dasatinib. Myelosuppression is part of the natural history of the disease and the level of hematologic toxicity may partly be the result of the underlying leukemic diagnosis coupled with the extensive prior regimens received. Baseline and on-study cytopenias are summarized in Table 7. At baseline, 5 patients (14%) had severe leukopenia, 7 (19%) had severe neutropenia, and 18 (50%) had severe thrombocytopenia. The majority of on-study cytopenias were reversible and could be managed by dose interruption or reduction.
Baseline and on-study grade 3-4 cytopenias associated with dasatinib
| . | No. (%) of patients . | |
|---|---|---|
| Baseline . | 8 months' follow-up . | |
| Leukocytopenia | 5 (14) | 23 (64) |
| Neutropenia | 7 (19) | 26 (72) |
| Thrombocytopenia | 18 (50) | 28 (78) |
| Anemia | 0 | 17 (47) |
| . | No. (%) of patients . | |
|---|---|---|
| Baseline . | 8 months' follow-up . | |
| Leukocytopenia | 5 (14) | 23 (64) |
| Neutropenia | 7 (19) | 26 (72) |
| Thrombocytopenia | 18 (50) | 28 (78) |
| Anemia | 0 | 17 (47) |
Discussion
Treatment of patients with Ph-positive ALL represents a considerable clinical challenge. These patients are often refractory to currently available treatment options, and their prognosis is poor. The results of this phase 2 study demonstrate that dasatinib is active in adults with Ph-positive ALL: 42% of patients achieved a MaHR, including 33% who achieved a CHR. It is of significance that the onset of MaHR was rapid, given the aggressive nature of Ph-positive ALL. The median time to MaHR was 1.8 months, indicating the rapid achievement of disease control in patients responding to dasatinib therapy. Furthermore, among patients who achieved MaHR, only 33% had progressed at 8 months' follow-up, and the median duration of MaHR had yet to be reached. Major cytogenetic responses were achieved by 58% of patients, all of which were CCyR. Dasatinib therapy was also well tolerated; only 2 patients (6%) had discontinued therapy due to study drug toxicity at 8 months' follow-up.
It is of note that rates of MCyR with dasatinib were higher than rates of MaHR. This observation was attributed to instances where a cytogenetic response was achieved but residual cytopenias were present, thus precluding such patients from being classified as having attained MaHR. We believe that in these cases, the cytogenetic response reflects a more valuable end point.
The response rates reported in this study represent a substantial augmentation over those reported for other treatment modalities; these results were seen in a population that was either resistant or intolerant to imatinib and where the majority of patients had also received other treatment options.
The responses seen with dasatinib may, at least in part, be due to the increased potency of dasatinib against unmutated BCR-ABL and the ability of dasatinib to inhibit a spectrum of mutants of BCR-ABL.22,23 In addition, the ability of dasatinib to target SFKs may also have contributed to the efficacy of this drug in this patient population, in light of evidence that suggests a role for these SFKs in the pathophysiology of Ph-positive ALL.5 The role of BCR-ABL–independent mechanisms of imatinib resistance is supported by the observation in this study that 9 of the 25 patients tested in the imatinib-resistant Ph-positive ALL cohort had no detectable BCR-ABL mutations at baseline. This suggests that mechanisms other than BCR-ABL mutations were responsible for resistance in these patients.
Dasatinib was effective in patients with imatinib-resistant disease with a range of mutations in the BCR-ABL kinase domain, including those known to cause moderate-to-very high levels of resistance to imatinib (≥ 5-fold increase of the IC50). Mutations in the BCR-ABL P-loop are thought to contribute to increased oncogenicity of the kinase and are associated with a lower life expectancy among patients treated with imatinib.29 However, in the present study, hematologic and cytogenetic responses were very similar between patients with P-loop mutations and patients with other imatinib-resistant mutations. This observation is in agreement with the less stringent requirements of dasatinib for binding BCR-ABL compared with imatinib.17,30 Two mutants identified in at least 2 patients, T315I and E355G, were associated with a failure to achieve a MaHR or MCyR to dasatinib. In other dasatinib phase 2 studies of patients with imatinib-resistant or -intolerant CML, as well as in the LBC CML cohort of patients also enrolled in this START-L trial, the presence of E355G did not preclude a response to dasatinib, suggesting that this substitution alone is not responsible for resistance to dasatinib. However, no response to dasatinib has been observed for patients identified with T315I, confirming the results of previous preclinical studies and highlighting again the importance of BCR-ABL residue 315 for binding of ATP-competitive kinase inhibitors.23
The finding that 14 patients progressed rapidly is not surprising in view of the extensive prior treatment received and the high frequency of mutations conferring absolute or high-level resistance to ABL kinase inhibitors (eg, the T315I mutation was present in 6 patients).
Cytopenia developed frequently in many patients but could be controlled by dose interruption or reduction and adequate medical intervention. Febrile neutropenia was the only severe AE related to dasatinib that occurred in more than 10% of patients. It is worth noting that the majority of patients in the study population were heavily pretreated: approximately half of all patients had been treated with imatinib for over 1 year, a similar proportion had received a dose greater than 600 mg per day, and 42% had undergone prior SCT. Consequently, a marked proportion of patients entered the study with hematologic and nonhematologic toxicities likely to be associated with their condition and with prior treatment, including various degrees of cytopenia.
The rates of nonhematologic toxicity associated with dasatinib were low, and the majority of nonhematologic AEs were mild to moderate in severity. These results are in contrast to AEs observed with conventional cytotoxic agents and highlight the benefit of targeted molecular therapy.
The results presented in this report show that potent, multitargeted kinase inhibition of BCR-ABL and SFKs with dasatinib rapidly induces hematologic and cytogenetic responses in patients with relapsed Ph-positive ALL. These data are highly significant given the refractory nature of patients enrolled in this trial to current treatment modalities, including imatinib. Stem-cell transplantation remains the only potentially curative option in this population, though the likelihood of success is low31 ; however, long-term survival rates with SCT are markedly increased when patients with Ph-positive ALL are in complete remission.32 Therefore, incorporation of dasatinib into induction and consolidation regimens before SCT should be considered. It may also be of value to evaluate the optimal dasatinib dose and dosing regimen in patients with Ph-positive ALL destined to undergo SCT.
In the additional phase 2 trials in the START program, dasatinib has been demonstrated to be effective and well tolerated in patients with imatinib-resistant and -intolerant CML in all phases.33,–35 The results of this study also suggest that dasatinib represents an important therapeutic option in Ph-positive ALL, able to induce rapid and relatively durable responses in a substantial proportion of patients. As such, this novel agent has the potential to significantly improve existing treatment options for this aggressive disease.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by research funding from Bristol-Myers Squibb. S.C. was supported by a grant from the G. Williard Miller Foundation.
Authorship
Contribution: O.O. performed research and was principal investigator; H.D. performed research; G.M. analyzed data; B.S. performed research, was a national principal investigator, and contributed to aspects of the manuscript; F.G. performed research; R.A.L. enrolled and treated subjects, evaluated responses and toxicities, and edited and approved the final manuscript; G.R.C. performed research; J.R. was involved in early team discussions on studies and performed molecular studies of BCR-ABL; A.H. designed and performed research, analyzed data, and substantially revised the manuscript; A.M.A. analyzed data; A.G. designed research and analyzed data; and S.C. was principal investigator at one site, reviewed primary data, and contributed to the final manuscript.
Conflict-of-interest disclosure: One of the authors (R.A.L.) holds stock in and has received research grants from competitors of Bristol-Myers Squibb. A.M.A. and A.G. are employees of Bristol-Myers Squibb.
Correspondence: Oliver Ottmann, Medizinische Klinik II/Abteilung Haematologie, Johann Wolfgang Goethe Universität, Theodor-Stern-Kai 7, Frankfurt 60590, Germany; e-mail:ottmann@em.uni-frankfurt.de.

![Figure 1. Duration of major hematologic response to dasatinib at 8 months' follow-up. Only 15 patients with a MaHR are included in this plot (2 of whom were censored with the same duration of response [195 days]).](https://ash.silverchair-cdn.com/ash/content_public/journal/blood/110/7/10.1182_blood-2007-02-073528/2/m_zh80160706190001.jpeg?Expires=1769149296&Signature=ASV2JQ7h~CVgk7xzCmNEkjRWjc4tuslCTEi0x7Z~JCFda0pOMD~FKDXd7gsih4q9Dwtrdz6OiJjdByvYsqILiGbEHarQ7GpIEKVVA53T9Oz-z0t2tYp7Vgu5cidlO5MftsiIJb2-vXIUhpwmj6GnQ2tTWNSOpwEZVtY16s0drwPxyOz0T-i-5kJ~ywM6~Mby15~fIHAf30OwJHfd3w~sjCAH-9na68Y9bmYAyxIIxQVDHDpEitZRnpYTPy7yzbkqQjxDgMyWN03RB19Z2rVOzXtIfrBdSIgql8myppg2Fp0SjFWJQCfyQC8yWsvapH6vWAjbEbebzoZGCTgnlLYpNQ__&Key-Pair-Id=APKAIE5G5CRDK6RD3PGA)